Abstract
Drug resistance in breast cancer remains a major cause for the failure of chemotherapy. Glucosylceramide synthase (GCS) plays an important role in multidrug resistance (MDR) in breast cancer. P-glycoprotein (P-gp) also confers a cross-resistance of many unrelated drugs. In this study, we studied the MDR effect and potential mechanisms of breast cancer after constructing permanent breast cancer cell lines with GCS knockout by using recombinant vectors targeting GCS (pSUPER-GCSshRNAs). The GCSshRNA stably transfected cells were successfully established and significant lower levels of GCS mRNA and protein expression were confirmed. In in vitro experiments, the GCSshRNA stably transfected cells showed a significantly reduced level of MDR1 and P-gp expression and decreased drug efflux ability. Reduced level of GCS expression conveyed a significant reversal of drug resistance by MTT assay and increased caspase-3 activity. In in vivo experiments by using nude mice with xenograft tumors, a significant inhibition of tumor growth was observed after comparing with the control group. Furthermore, enhanced response of chemotherapy was acquired by reduced expression of GCS as well as MDR1 in vivo. In conclusion, GCSshRNA could efficiently suppress GCS and MDR1 expression in vitro and in vivo and these findings may be used as one of the methods to reverse MDR in breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemo-resistance is one of the major causes of failure in cancer management [1]. Glucosylceramide synthase (GCS) is a glycosyltransferase in sphingolipid metabolism. This enzyme transfers a glucose residue from UDP-glucose to ceramide for synthesis of glucosylceramide. In addition to many vital biologic functions in cell growth and apoptosis [2], GCS also plays an important role in multidrug resistance (MDR) [3–5]. Targeting glycolipid metabolism has proven useful in altering chemotherapy responses in human tumor cell lines.
P-glycoprotein (P-gp), a membrane-resident glycoprotein encoded by the MDR1 gene, confers a cross-resistance of many unrelated drugs [6–8]. P-gp acts as an energy-dependent efflux pump that efficiently enhances outward drugs transport and prevents entry of compounds, thereby resulting in reduced intracellular accumulation and decreased cytotoxicity of anticancer drugs [9, 10].
RNA interference (RNAi) represents a sequence-specific, post-transcriptional gene silencing process in many organisms. Initiated by a double-stranded RNA (dsRNA), RNAi induces degradation of mRNA of homologous sequences to dsRNA [11–13]. The mediators for the degradation of sequence-specific messenger RNA are usually siRNAs with 21- and 22-nucleotides, which are generated by ribonuclease III-like enzyme, Dicer. These siRNAs are then incorporated into a protein complex, which is also called RNA-induced silencing complex. This protein complex is able to degrade homologous mRNA and then to inhibit the targeting gene(s) at the post-transcriptional levels [14, 15]. RNAi constitutes a promising source for new therapeutic approaches. In mammalian systems, sequence-specific RNAi effects can easily be achieved by introducing synthetic small interfering RNA (siRNA) via transfection [16, 17]. Furthermore, vectors expressing short hairpin RNAs (shRNAs) have been developed for long-term cellular expression of siRNA [18–20]. RNAi-induced suppression of endogenous gene expression is an attractive approach of gene therapy since multiple targets may be manipulated simultaneously. Thus, we planned to explore whether shRNAs targeting GCS can induce gene silencing in vitro and in vivo.
In this report, by using a retroviral vector we constructed recombinant vectors targeting GCS to study their multidrug reversal effect and potential mechanisms in breast cancer and to explore a novel strategy to increase the sensitivity of chemotherapy in breast cancer.
Materials and methods
Cell lines and culture conditions
Human breast carcinoma cells, MCF-7/ADR, which is resistant to adriamycin, was kindly provided by National Cancer Institute in the United States. It was maintained in the culture medium RPMI supplemented with heat-inactivated fetal bovine serum (10%), l-glutamine (1%) and penicillin/streptomycin (0.1%). Cells were incubated at 37°C in a humidified atmosphere with 5% CO2.
Recombinant vectors construction
shRNAi constructs corresponding to GCS gene were designed in our laboratory by using RNAi software (Ambion, Inc., Austin, TX, USA) and were synthesized in Integrated DNA Technologies (Coralville, IA, USA). The detailed information of these shRNAi is listed in Table 1. The constructs were then cloned into pSUPER.retro vector to generate GCSshRNA expression vectors, which were defined as pSUPER-GCSshRNA1 and pSUPER-GCSshRNA2, respectively.
Transfection
Human breast carcinoma cells (MCF-7/ADR) were transfected with those recombinant pSUPER vectors using lipofectamineTM (Invitrogen, CA, USA) according to the manufacturer’s recommendations. After selection with G418 at 400 μg/ml, resistant clones were randomly isolated for cell number expansion.
Immunoblot analysis
The immunoblot method has been described in detail elsewhere [21]. Briefly, untransfected MCF-7/ADR cells and stably transfected cells were collected and lysed in a lysis buffer (150 mM Nacl, 0.1% Nonidet P-40, 50 mM Tris–HCl, 0.1% SDS, 0.2% sodium azide and 0.5% sodium deoxycholate), respectively. Lysates were cleared by centrifugation at 12,000×g for 10 min at 4°C. Total proteins from tumor tissues were extracted using another lysis buffer (8 mol/l urea, 2 mol/l sulfocarbamide, 2% NP-40, 1% Triton X-100, 100 mmol/l DTT, 4% CHAPS, 0.5 mmol/l EDTA, 5 mmol/lPMSF and 40 mmol/l Tris). The proteins were boiled at 100°C for 5 min and then electrophoresed in SDS-polyacrylamide gel. Following transfer of the separated proteins to a polyvinylidene difluoride membrane using a semi-dry electroblotter, the membrane was blocked with 5% skimmed milk powder in TBST for 2 h. Then the blot was incubated with monoclonal antibody diluted in 5% skim milk in 1× TBST (1:1,000) overnight at 4°C. Antibodies used were GCS (Kindly provided by Dr. D. Marks in Mayo Clinic Center), P-gp, caspase-3 and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The membrane was washed three times with TBST and incubated with a horseradish peroxidase (HRP)-conjugated IgG secondary antibody (1:4,000, Invitrogen, Carlsbad, CA, USA) at room temperature for 1 h. The resultant bands on the membrane were visualized by enhanced chemiluminescence development kit (Amersham, Pharmacia Biotech, Piscataway, NJ).
RT-PCR
Total RNAs were extracted from untransfected or stably transfected MCF-7/ADR cells using the RNA isolation kit from Oiagen, Inc. (Valencia, CA, USA), respectively. While from cancer tissue, total RNAs were extracted using sepasol-RNA I super (NacalaiTesque, Kyoto, Japan). Purified RNA was reverse-transcribed using the one-step RT-PCR system (TaKaRa, Kyoto, Japan) according to the manufacturer’s protocols in 20 μl of final volume. Subsequently, the resultant cDNAs were PCR-amplified using TaqDNA polymerase (TaKaRa, Kyoto, Japan) for 30 cycles using GCS or MDR1 specific primers, for which sequences are listed in Table 2. Each cycle comprised of denaturation at 95°C for 1 min, annealing at 55°C for 45 s and elongation at 72°C for 1 min. RT-PCR products were electrophoresed through a 1% agarose gel with ethidium bromide. The house keeping gene β-actin was also amplified as an internal standard.
Measurement of caspase-3 activity
Cells were lysed for 1 h as reported in the manufacturer’s instructions and centrifuged at 12,000×g for 3 min at 4°C. Cytosolic protein was mixed with caspase-3-specific substrate DEVD-para-mitroanilide and incubated at 37°C. Substrate cleavage was monitored at 400 nm using a micro ELISA reader (Sigma, St. Louis, MO, USA). To confirm that substrate cleavage was due to caspase activity, extracts were incubated in the presence of caspase-3-specific inhibitor DEVD-CHO for 30 min at 37°C before the addition of substrate.
Flow cytometric analyses of P-gp function
For detection of P-gp function as a transporter, Rhodamine 123 (Rhd 123, Sigma, St. Louis, MO, USA) was used as a fluorescence tracer. The cells were incubated in RPMI1640 medium containing 5 μg/ml Rhd 123 at 37°C in a humidified atmosphere of 5% CO2 for 30 min to allow uptake. The cells were then centrifuged, washed twice with PBS, and re-suspended in fresh medium at 37°C in a humidified atmosphere of 5% CO2 for 45 min. Green fluorescence, indicating cellular Rhd 123 accumulation, was analyzed using a flow cytometer (Becton Dickinson, San Jose, CA, USA).
Cytotoxicity assay
Cells were plated at a density of 5,000 cells per well in a 96-well plate. After culturing for 24 h, drug in various concentrations was added to each well. Incubations were continued for an additional 48 h, and then MTT (Sigma, St. Louis, MO, USA) solution 15 μl (5 mg/ml) was added to each well and the plates was further incubated for 4 h at 37°C. The absorbance in individual wells was determined at 570 and 630 nm by a microplate reader (Bio-Tek, Vernon Hills, Illinois, USA). MTT assay is based on the conversion of the yellow tetrazdium salt MTT to purple formazan crystals by metabolically active cells. Thus, this method provides a quantitative determination of viable cells. The inhibition of cell growth was evaluated by the MTT assay using triplicate assay. The concentrations required to inhibit growth by 50% (IC50 values) were calculated from cytotoxicity curves.
In vivo tumor model
Female Balb/c nude mice (6 weeks old), obtained from the center for Experimental Animals in shanghai of China, were maintained in a pathogen-free condition. The GCSshRNA stably transfected cells were subcutaneously injected into the back of nude mice with 5 × 106 cells per injection. Meanwhile, the pSUPER.retro stably transfected cells were also injected as controls. The formation of subcutaneous tumors was monitored and measured with a vernier caliper. The tumor volume formed was calculated using the following formula 0.523 (L × W 2), where L was the length and W the width of tumor. After sacrificing the mice, we dissected tumors from subcutaneous area and immediately preserved in freezer for further analysis. The rate of inhibition (IR) was calculated by the formula: IR = 1 − [Mean tumor volume of experimental group/Mean tumor volume of control group] × 100%. Animal experiment was performed in accordance with institutional policy for biomedical research in College of Medicine, Shandong University.
Statistic analysis
The data are expressed as Mean ± standard deviation (SD) and the statistical significance of differences was assessed by one-way analysis of variance (ANOVA). Differences were considered significant when P < 0.05.
Results
Stable expression of GCSshRNAs in MCF-7/ADR cells
shRNAs targeting GCS were cloned into pSUPER.retro vectors. The recombinant vectors were validated by restriction enzyme digestion and the inserted sequences were verified by DNA sequencing. After transfection and selection, the stably transfected cells were named as MA/PS1 (transfected with pSUPER-GCSshRNA1), MA/PS2 (transfected with pSUPER-GCSshRNA2) and MA/CON (transfected with parental vector pSUPER.retro), respectively.
GCS and MDR1 expression efficiently suppressed by pSUPER-GCSshRNAs in vitro
After GCSshRNA stably transfected cells (MA/PS1 and MA/PS2) were generated, GCS and MDR1 expression in both mRNA and protein levels were evaluated by RT-PCR and Western blot analysis. In comparison with control cells, the levels of GCS mRNA decreased 87.8 and 89.7% in MA/PS1 and MA/PS2 cells, respectively (P < 0.01; Fig. 1a). The total protein levels of GCS were also reduced by 81.9 and 85.6% in both MA/PS cells, respectively (P < 0.01; Fig. 1b). Moreover, as shown in Fig. 2a and b, MDR1 mRNA expression was reduced by 79.7 and 82.8% and P-gp (protein encoded by MDR1) decreased by 74.3 and 77.6% in MA/PS1 and MA/PS2 cells, respectively (P < 0.01). In addition, no effects of RNAi were observed on the expression of β-actin used as an internal control. These results suggest that GCSshRNA can efficiently downregulate GCS and MDR1 mRNA as well as protein levels and can be used to target GCS for breast cancer therapy.
GCS mRNA and protein expression in MCF-7/ADR cells and stably transfected cells (MA/CON, MA/PS1 and MA/PS2 cells). a Semi-quantitative RT-PCR showed that GCS mRNA expression significantly reduced in MA/PS1 and MA/PS2 cells compared to MCF-7/CON cells. b Western blot showing obviously reduced protein levels of GCS in MA/PS1 and MA/PS2 cells. β-actin was used as an internal normalization standard
MDR1 mRNA and protein expression in MCF-7/ADR cells and stably transfected cells (MA/CON, MA/PS1 and MA/PS2 cells). a When compared to MA/CON cells, MDR1 mRNA in MA/PS1 and MA/PS2 cells decreased by 79.7 and 82.8% respectively detected by semi-quantitative RT-PCR (P < 0.01). b The protein levels of P-glycoprotein in MA/PS1 and MA/PS2 cells significantly reduced by 74.3 and 77.6% respectively by Western blot analysis (P < 0.01). β-actin was considered as an internal normalization standard
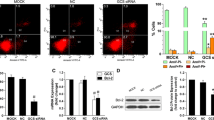
Enhancing the accumulation of Rhodamine 123 in GCSshRNA transfected breast cancer cells
In order to evaluate whether GCSshRNA could inhibit the function of P-gp, we conducted a Rhodamine 123 accumulation test. The results revealed that the fluorescence intensity of Rhodamine 123 was 2.5–2.6 fold higher in MA/PS1 (59.1 ± 3.0%) and MA/PS2 cells (62.3 ± 2.7%) than that of in MCF-7/ADR control cells (23.9 ± 1.1%, P < 0.05; Fig. 3). These data indicate that GCSshRNA inhibited the cellular efflux function of P-gp, and thus may be able to increase the intracellular accumulation of anticancer drugs.
Reversal of multidrug resistance by pSUPER-GCSshRNAs
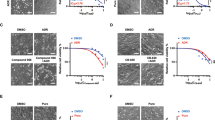
To examine whether downregulation of GCS expression by shRNAi constructs in stably transfected breast cancer cells is able to change the sensitivity of anti-cancer drugs, we compared the MA/PS and control cells after treating them with vinblastin and paclitaxel. Cell viability was assessed by comparison of IC50 values using MTT assay. As shown in Table 3, the resistance factor to vinblastin was decreased from 225 to 13.4- and 10.9, equivalent to a 16.8- and 20.6-fold reduction of resistance for vinblastine in MA/PS1 and MA/PS2 cells, respectively after normalization with controls (P < 0.01). Furthermore, the drug resistance to paclitaxel was significantly dropped from 201 to 15.2- and 13.4, equivalent to a 13.2- and 15-fold reduction of resistance for paclitaxel in MA/PS1 and MA/PS2 cells, respectively (P < 0.01). However, there was no difference of drug resistance between MCF-7/ADR cells and MA/CON cells after treatment with those anti-cancer drugs (P > 0.05). Taken together, these results indicate that GCSshRNA may be able to reverse MDR in MCF-7/ADR cells by down-regulating GCS expression.
Increased caspase-3 expression and activity by shRNAi constructs
In mammalian cells, ceramide induces apoptosis directly through effector caspases, such as caspase-3. To identify whether an alteration in ceramide metabolism in MA/PS cells is related to drug sensitivity via signal cascades, we investigated caspase-3 expression by Western blot analysis and examined caspase-3 activity on stably transfected cells (MA/CON, MA/PS1 and MA/PS2). As shown in Fig. 4a, caspase-3 expression in MA/PS cells increased dramatically compared to MA/CON cells. In addition, caspase-3 activity in MA/PS1 and MA/PS2 cells was 27- and 31-fold greater than that of in MA/CON cells (P < 0.01; Fig. 4b). The data demonstrate that suppressed GCS expression permits cell to maintain high levels of ceramide, activating caspase-3 for progression of apoptosis.
Effect of GCSshRNA on the activation of caspase-3. a Immunoblot of MCF-7/ADR cells and stably transfected cells (MA/CON, MA/PS1 and MA/PS2 cells). The protein levels of caspase-3 in MA/PS1 and MA/PS2 cells enhanced compared to MCF-7/CON cells. Equal loading was confirmed by showing equal β-actin levels. b Caspase-3 activity in MA/PS1 and MA/PS2 cells increased by 27- and 31-fold respectively in comparison with MA/CON cells. ** P < 0.01
GCSshRNAs inhibit tumor growth in vivo
Since pSUPER-GCSshRNAs exhibited great GCS-suppressive effect, we explored their possible effect in vivo by injecting them into nude mice. As seen in Figs. 5 and 6, compared to controls, tumor volume was greatly reduced in mice treated with pSUPER-GCS shRNAs. The average tumor volume of the control group (MA/CON) was 2,622 ± 411 mm3, while those of MA/PS1 and MA/PS2 group were 423 ± 65 and 361 ± 53 mm3, respectively. Thereby mice bearing tumor xenograft showed tumor growth inhibition of 84 and 86%, respectively (P < 0.01) in response to pSUPER-GCSshRNA treatment. These findings suggest that RNAi-mediated suppression of GCS expression had strong inhibitory effects on MCF-7/ADR cells in vivo.
Down-regulation of GCS expression inhibited tumor growth in vivo. Tumor volume in the mice injected with stably expressing GCSshRNA cells (MA/PS1 and MA/PS2) or stably expressing pSUPER.retro cells (MA/CON). There was significant difference between MA/PS mouse group and MA/CON mouse group. ** P < 0.01
GCSshRNAs downregulate GCS and MDR1 expression in vivo
To investigate the significance of GCS downregulation in vivo, we assessed GCS and MDR1 expression in tumor tissues. As shown in Fig. 7a and b, RT-PCR and Western blot demonstrated that treatment with pSUPER-GCSshRNA1 and pSUPER-GCSshRNA2 resulted in 65.7–71.8% GCSmRNA and 61.9–64.5% protein reduction (P < 0.05), respectively. Moreover, downregulation of MDR1 expression by pSUPER-GCSshRNAs was observed in MA/PS2 mouse group with 50.6% MDR1 and 47.9% P-gp reduction (P < 0.05), respectively (Fig. 8). These data indicate that GCSshRNA inhibits the expression of both GCS and MDR1 in vivo.
Stable downregulation of GCS expression in nude mice. a RNA samples extracted from tumors of per group were analyzed using semi-quantitative RT-PCR for GCS mRNA expression. GCS mRNA in MA/PS1 and MA/PS2 tumor tissues obviously reduced 65.7 and 71.8% respectively compared to MA/CON tumor tissues (P < 0.01). b Protein samples extracted from tumors of animals were detected by Western blot analysis for GCS expression levels. The level of GCS protein in MA/PS1 and MA/PS2 mice significantly reduced by 61.9 and 64.5% compared to MA/CON mice (P < 0.01). β-actin was used as a loading control. a1–a5, mice in MA/CON group; b1–b5, mice in MA/PS1 group; c1–c5, mice in MA/PS2 group
Stable downregulation of MDR1 expression in nude mice. a Semi-quantitative RT-PCR showing reduced expression of MDR1 mRNA in MA/PS2 tumor tissues compared to MA/CON tumor tissues. b Western blot analysis showing reduced protein levels of P-glycoprotein in MA/PS2 tumor tissues. β-actin was used as an internal normalization standard. a1–a5, mice in MA/CON group; c1–c5, mice in MA/PS2 group
Discussion
Drug-resistance in breast cancer remains a major cause for the failure of chemotherapy. Multiple factors and/or mechanisms may contribute the drug resistance such as increased effect of drug efflux pump, alteration of drug transport and metabolism, impaired function of repair after drug-induced damage [22]. P-gp, the product of the MDR1 gene, plays an important role in cancer chemotherapy and is the most common mechanism for MDR in cancer cells [23].
Several reports have demonstrated an association between GCS and MDR in multidrug-resistant cancer cells. Accumulation of glucosylceramide and elevated GCS activity is a characteristic finding [24, 25]. It has recently been shown that, in breast cancer cells, drug resistance was related to an enhanced expression of GCS secondary to an increased capacity of ceramide glycosylation [26]. Several studies have showed that GCS inhibition could enhance chemotherapy response. PDMP (the specific inhibitor of GCS) treatment has been shown to enhance the chemosensitivity of MCF-7/ADR cells and P-gp overexpressing MDA-MB-435 cells to vincristin and paclitaxel [27]. Liu et al. [28] have demonstrated that GCS suppression by antisense oligodeoxynucleotides reversed drug resistance in human breast cancer cells. However, the effect induced by antisense oligodeoxynucleotides is transient. Therefore, by using a new retroviral pSUPER.retro vector system, we successfully generated permanent cell lines that constitutively express specific siRNA and examine if MDR in these cells could be reversed. First, we successfully established stably transfected cells MA/PS1, MA/PS2 and MA/CON. GCS and MDR1 mRNA and protein levels were significantly reduced in MA/PS1 and MA/PS2 cells compared to MA/CON cells. In addition to reduced GCS expression, these cells also demonstrated a significantly reduced level of MDR1 and P-gp expression. The later resulted in an abnormally high intracellular accumulation of Rhodamine 123 due to reduction of drug efflux. These molecular changes in transfected breast cancer cells are considered due to the reduction of P-gp expression on the cell surface after shRNA mediated RNAi.
One of the significant findings of our work was that suppressing GCS expression significantly inhibited the expression of MDR1 and its product, P-gp. This effect can be explained by the relationship among gangliosides, NF–kB and MDR1. Previous studies showed that MDR1 promoter can be activated by NF–kB. NF–kB complexes are able to bind at a consensus NF–kB binding site in the first intron of the human MDR1 gene and transactivate MDR1 promoter luciferase construct, thus regulate the MDR1 gene expression in cancer cells [29]. Gangliosides, synthesized by glucosylceramide, are able to enhance MDR1 expression by activating NF–kB [30]. Therefore, the finding of significantly suppressed MDR1 expression in our study is likely due to the reduced level of GCS expression. This is also supported by the work that high level of glucosylceramide enhanced expression of MDR1 gene and GCS inhibition down-regulated MDR1 expression in cancer cells [26]. Overall, our findings suggest that GCS plays an important role in the expression and function of the multidrug resistant phenotype.
Reduced level of GCS expression conveyed a significant reversal of drug resistance. This was further supported by a significant lower IC50 and resistance factor in transfected breast cancer cells demonstrated in MTT assay. It is not fully understood why reduced GCS expression resulted in a reversed drug resistance. We speculate that it may be related to an activated apoptosis pathway. This is supported by the presence of increased caspase-3 activity in our transfected breast cancer cells. Caspase-3 belongs to one of the effector caspases in the stress-activated protein kinase apoptotic signaling pathway, which can be activated by ceramide in response to extracellular stimuli [31]. In this study, the results demonstrate that shRNA targeting GCS is able to activate caspase-3 activity in transfected breast cancer cells. Therefore, the diminished capacity for ceramide glycosylation may promote cellular cytotoxicity via ceramide-linked caspase-3 activation, which finally resulted in reversed drug resistance.
After observing the reversal of drug resistance in vitro, we further examined this phenomenon in a nude mice model. To our knowledge, the present work is the first showing that GCS suppression by RNAi is able to inhibit tumor growth and down-regulate MDR1 expression in vivo. Compared to the control group, tumors with reduced GCS level after shRNA treatment showed a significantly reduced tumor size. Several other studies other than in breast cancer showed a similar effect. Deng et al. [32] demonstrated that transfection of GCS antisense inhibited melanoma growth in mice. In addition, GCS inhibition using N-butyldeoxynojirimycin delayed tumor development in a murine melanoma model [33]. These findings in animal models indicate that GCS inhibition could suppress cell proliferation, enhance apoptosis and inhibit tumor formation. In molecular level, MDR1 and P-gp were similarly reduced in tumor tissues in the study group in comparison with control group. Our results in this study demonstrate that GCSshRNA is able to efficiently down-regulate GCS and MDR1 expression and thereby reverse MDR in vivo.
In conclusion, the present study has demonstrated an effective RNA technology based approach for suppression of GCS and MDR1 gene both in vitro and in vivo to reverse MDR in breast cancer. Our study provides a novel strategy to enhance the chemotherapy sensitivity and ultimately benefit the patients with breast cancer. In addition to targeting GCS gene, simultaneously knock-down cell cycle-regulating and apoptosis-related genes may provide a better strategy for the management of various human cancers.
Abbreviations
- GCS:
-
Glucosylceramide synthase
- MDR:
-
Multidrug resistance
- P-gp:
-
P-glycoprotein
- RNAi:
-
RNA interference
- siRNA:
-
Small interfering RNA
- shRNA:
-
Short hairpin RNA
References
Takara K, Sakaeda T, Okumura K (2006) An update on overcoming MDR1-mediated multidrug resistance in cancer chemotherapy. Curr Pharm Des 12:273–286
Kohyama-Koganeya A, Sasamura T, Oshima E et al (2004) Drosophila glucosylceramide synthase: a negative regulator of cell death mediated by proapoptotic factors. J Biol Chem 279:35995–36002
Lavie Y, Cao H, Bursten SL et al (1996) Accumulation of gulcosylceramide in multidrug-resistant cancer cells. J Biol Chem 271:19530–19536
Liu YY, Han TY, Giuliano AE et al (1999) Expression of glucosylceramide synthase, converting ceramide to glucosylceramide, confers adriamycin resistance in human breast cancer cells. J Biol Chem 274:1140–1146
Cai Z, Bettaieb A, Mahdani NE et al (1997) Alteration of the sphingomyelin/ceramide pathway is associated with resistance of human breast carcinoma MCF-7 cells to tumor necrosis factor-α-mediated cytotoxicity. J Biol Chem 272:6918–6926
Volm M (1998) Multidrug resistance and its reversal. Anticancer Res 18:2905–2909
Zrieki A, Farinotti R, Buyse M (2008) Cyclooxygenase inhibitors down regulate P-glycoprotein in human colorectal Caco-2 cell line. Pharm Res 25:1991–2001
Kitagawa S (2006) Inhibitory effects of polyphenols on p-glycoprotein-mediated transport. Biol Pharm Bull 29:1–6
Saeki T, Tsuruo T, Sato W et al (2005) Drug resistance in chemotherapy for breast cancer. Cancer Chemother Pharmacol 56:84–89
Holland ML, Panetta JA, Hoskins JM et al (2006) The effects of cannabinoids on P-glycoprotein transport and expression in multidrug resistant cells. Biochem Pharmacol 71:1146–1154
Mungall BA, Schopman NC, Lambeth LS et al (2008) Inhibition of Henipavirus infection by RNA interference. Antiviral Res 80:324–331
Chu CY, Rana TM (2008) Potent RNAi by short RNA triggers. RNA 14:1714–1719
Burkhardt BR, Lyle R, Qian K et al (2006) Efficient delivery of siRNA into cytokine-stimulated insulinoma cells silences Fas expression and inhibits Fas-mediated apoptosis. FEBS Lett 580:553–560
Delgado R, Regueiro BJ (2005) The future of HIV infection: gene therapy and RNA interference. Enferm Infecc Microbiol Clin 23:76–83
Devi GR (2006) siRNA-based approaches in cancer therapy. Cancer Gene Ther 13:819–829
Amarzguioui M, Rossi JJ, Kim D (2005) Approaches for chemically synthesized siRNA and vector-mediated RNAi. FEBS Lett 579:5974–5981
Kumar P, Lee SK, Shankar P et al (2006) A single siRNA suppresses fatal encephalitis induced by two different flaviviruses. PLoS Med 3:e96
Stein U, Walther W, Stege A et al (2008) Complete in vivo reversal of the multidrug resistance phenotype by jet-injection of anti-MDR1 short hairpin RNA-encoding plasmid DNA. Mol Ther 16:178–186
Matters GL, Harms JF, McGovern CO et al (2009) Growth of human pancreatic cancer is inhibited by down-regulation of gastrin gene expression. Pancreas 38:151–161
Chen Y, Chen H, Hoffmann A et al (2006) Adenovirus-mediated small-interference RNA for in vivo silencing of angiotensin AT1a receptors in mouse brain. Hypertension 47:145–146
Brezinsky SC, Chiang GG, Szilvasi A et al (2003) A simple method for enriching populations of transfected CHO cells for cells of higher specific productivity. J Immunol Methods 277:141–155
Volm M, Kästel M, Mattern J et al (1993) Expression of resistance factors (P-glycoprotein, glutathione S-transferase-pi, and topoisomerase II) and their interrelationship to proto-oncogene products in renal cell carcinomas. Cancer Cell 71:3981–3987
Marzolini C, Paus E, Buclin T et al (2004) Polymorphisms in human MDR1 (P-glycoprotein): recent advances and clinical relevance. Clin Pharmacol 75:13–33
Kok JW, Veldman RJ, Klappe K et al (2000) Differential expression of sphingolipids in MRP1 overexpressing HT29 cells. Int J Cancer 87:172–178
Nicholson KM, Quinn DM, Kellett GL et al (1999) Preferential killing of multidrug-resistant KB cells by inhibitors of glucosylceramide synthase. Br J Cancer 81:423–430
Gouazé-Andersson VYJ, Kreitenberg AJ et al (2007) Ceramide and glucosylceramide upregulate expression of the multidrug resistance gene MDR1 in cancer cells. Biochim Biophys Acta 1771:1407–1417
Shabbits JA, Mayer LD (2002) P-glycoprotein modulates ceramide-mediated sensitivity of human breast cancer cells to tubulin-binding anticancer drugs. Mol Cancer Ther 1:205–213
Liu YY, Han TY, Giuliano AE et al (2000) Uncoupling ceramide glycosylation by transfection of glucosylceramide synthase antisense reverses adriamycin resistance. J Biol Chem 275:138–143
Bentires-Alj M, Barbu V, Fillet M et al (2003) NF-kappaB transcription factor induces drug resistance through MDR1 expression in cancer cells. Oncogene 22:90–97
Kanto T, Kalinski P, Hunter OC et al (2001) Ceramide mediates tumor-induced dendritic cell apoptosis. J Immunol 167:3773–3784
Hara S, Nakashima S, Kiyono T et al (2004) Ceramide triggers caspase activation during gamma-radiation-induced apoptosis of human glioma cells lacking functional p53. Oncol Rep 12:119–123
Deng W, Li R, Guerrera M et al (2002) Transfection of glucosylceramide synthase antisense inhibits mouse melanoma formation. Glycobiology 12:145–152
Guerrera M, Ladisch S (2003) N-butyldeoxynojirimycin inhibits murine melanoma cell ganglioside metabolism and delays tumor onset. Cancer Lett 201:31–40
Acknowledgments
This work was supported in part by National Nature Science Foundation of China (No. 30300124); Nature Science Foundation of Shandong Province of China (No. Y2007C112, Y2007C15).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Dr. Yanlin Sun and Dr. Gengyin Zhou contributed equally to this study.
Rights and permissions
About this article
Cite this article
Sun, Y., Zhang, T., Gao, P. et al. Targeting glucosylceramide synthase downregulates expression of the multidrug resistance gene MDR1 and sensitizes breast carcinoma cells to anticancer drugs. Breast Cancer Res Treat 121, 591–599 (2010). https://doi.org/10.1007/s10549-009-0513-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-009-0513-z