Abstract
So far, studies on dietary antioxidant intake, including β-carotene, vitamin C and vitamin E, and breast cancer risk are inconclusive. Thus, we addressed this question in the European Prospective Investigation into Cancer and Nutrition. During a median follow-up time of 8.8 years, 7,502 primary invasive breast cancer cases were identified. Cox proportional hazard models were used to estimate hazard ratios (HR) and 95% confidence intervals (CI). All analyses were run stratified by menopausal status at recruitment and, additionally, by smoking status, alcohol intake, use of exogenous hormones and use of dietary supplements. In the multivariate analyses, dietary intake of β-carotene, vitamin C and E was not associated with breast cancer risk in premenopausal [highest vs. lowest quintile: HR, 1.04 (95% CI, 0.85–1.27), 1.12 (0.92–1.36) and 1.11 (0.84–1.46), respectively] and postmenopausal women [0.93 (0.82–1.04), 0.98 (0.87–1.11) and 0.92 (0.77–1.11), respectively]. However, in postmenopausal women using exogenous hormones, high intake of β-carotene [highest vs. lowest quintile; HR 0.79 (95% CI, 0.66–0.96), P trend 0.06] and vitamin C [0.88 (0.72–1.07), P trend 0.05] was associated with reduced breast cancer risk. In addition, dietary β-carotene was associated with a decreased risk in postmenopausal women with high alcohol intake. Overall, dietary intake of β-carotene, vitamin C and E was not related to breast cancer risk in neither pre- nor postmenopausal women. However, in subgroups of postmenopausal women, a weak protective effect between β-carotene and vitamin E from food and breast cancer risk cannot be excluded.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The role of antioxidants in the aetiology of breast cancer is still unclear [1–3, 17]. In an early analysis of the EPIC-cohort, no association between fruit and vegetable intake and breast cancer risk was found [4]. However, it is biologically plausible that diets high in antioxidants protect against cancer. Dietary antioxidants such as carotenoids, vitamins C and E can neutralise free radicals and may protect DNA from oxidative damage [5, 6]. In order to account for huge differences in antioxidant content of the variety of fruit and vegetables, it is worthwhile to perform studies at the nutrient level, i.e. to test for the association between dietary antioxidant intake (derived from food consumption data) and cancer risk. However, it is acknowledged that interpretation of the results in terms of dietary intake recommendations shall be done at the food level again.
Findings from epidemiologic studies on dietary intake of carotenoids (with pro-vitamin-A activity) and retinol, as biologically active metabolite, and breast cancer risk have been inconclusive [3]. Mostly, the results of cohort studies showed no statistically significant association between β-carotene intake and breast cancer risk [7–10]. In contrast, in one cohort study (the Nurses Health Study), a significantly inverse association between β-carotene intake and breast cancer risk has been observed in premenopausal but not in postmenopausal women [11]. In addition, β-carotene supplementation did not affect cancer incidence in women [12]. Most prospective studies showed no association between vitamin C intake and breast cancer risk [9, 13–15]. However, in a meta-analysis of nine case-control studies on vitamin C intake and breast cancer, an inverse association has been reported [16]. Another meta-analysis reported reduced breast cancer risk for higher dietary β-carotene and vitamin C levels [17]. Further investigation of biomarkers of antioxidants did not clarify the relationships [18–21].
Previous work indicates a modest protective effect of antioxidants in populations with potentially higher oxidative stress [11, 22]: high intakes of vitamin C and E in premenopausal female smokers were associated with lower breast cancer risk [22]. Also, an inverse association between β-carotene and breast cancer risk in premenopausal women consuming ≥15 g/day alcohol was reported [11]. In addition, there is evidence from a gene–environment study that oxidative stress is involved in the association between hormone therapy and breast cancer risk in postmenopausal women [23].
The objectives of our study were to explore the associations between dietary intake of β-carotene, vitamin C and vitamin E and breast cancer risk by menopausal status and to investigate subgroups with potentially higher oxidative stress.
Methods
Study population
European Prospective Investigation into Cancer and Nutrition (EPIC) is a multicentre prospective cohort study designed to primarily investigate the associations of diet and lifestyle factors with the incidence of cancer. Between 1992 and 2000, approximately 520,000 individuals were recruited in 23 research centres in 10 European countries: Denmark, France, Germany, Greece, Italy, the Netherlands, Norway, Spain, Sweden and the United Kingdom (UK). The study design is described in detail elsewhere [24, 25]. In brief, most study subjects were recruited from the general population in a given geographical area. Eligible subjects were invited to participate in the studies, and those who accepted gave informed consent and completed questionnaires on their diet, lifestyle and medical history. The French EPIC cohort (based on female members of the health insurance for state school employees), part of the Oxford cohort, UK (based on vegetarians and healthy eaters), the Utrecht cohort, the Netherlands and the Florence cohort, Italy (both based on women attending breast cancer screening), the Ragusa cohort, Italy and most of the Spanish cohorts (both based on blood donors and their spouses) are not based on a random selection of the underlying population.
The EPIC-cohort consists of 345,995 women mostly aged between 35 and 70 years of which participants were excluded due to missing nondietary data or due to not filling in the dietary questionnaire or due to being in the top or bottom 1% ratio of the energy intake and energy requirement (N = 10,127). We further excluded women due to missing values reporting on breast cancer occurrence or non-invasive breast cancer or missing information on behaviour of the tumour (N = 1,375) resulting in 334,493 women including 7,502 cases with invasive breast cancer. The analytic cohort with full set of covariates comprised 288,776 women including 6,478 cases of invasive breast cancer covering in total 2,518,722 person-years.
Diet
Habitual diet over the previous 12 months was measured by country-specific validated questionnaires designed to capture local dietary habits as previously described in detail [24, 26]. In Greece, Spain and Ragusa, a face-to-face dietary interview was performed. Questionnaires in France, Northern Italy, Spain, the Netherlands and Greece were quantitative, estimating individual average portion sizes systematically. Those in Denmark, Germany, Norway, Naples in Italy and Umea in Sweden were semiquantitative, with the same standard portion assigned to all subjects. In Malmö and in the United Kingdom, the dietary questionnaire was combined with food records. Rough data on the supplementation of vitamin or multivitamin preparations in general (yes, no) were collected by dietary questionnaire for about 80% of the cohort. The algorithm by which women were allocated to menopausal status at enrolment has been described in detail before [27]. In brief, information on menstrual history, type of menopause (natural, surgical), use of oral contraceptives and menopausal hormones has been used to classify women according to menopausal status at enrolment.
Covariates
Height and weight were measured in all EPIC centres except in France, Norway and Oxford, for which self-rated anthropometric measures were assessed by questionnaires. Extensive, standardised information about medical history including medication and reproductive history, physical activity, smoking, alcohol consumption, and other lifestyle factors was collected by a separate questionnaire.
Endpoint
Cases of cancer occurring after recruitment are identified through local and national cancer registries in seven of the ten countries (Denmark, Italy, Norway, Spain, Sweden, the Netherlands and United Kingdom). Data on mortality were also obtained from either cancer registries or mortality registries. In France, Germany and Greece, active follow-up was carried out applying a combination of contacts with national health insurances and/or active follow-up through the study subjects or their next-of-kin. In all EPIC centres, cancer diagnosis was confirmed by review of pathology reports. We used the Tenth Revision International Classification of Diseases, Injury and Causes of Death (ICD-10), and invasive breast cancer was defined as C50.0–50.9.
Statistical analysis
We calculated the number of person-years for each person under observation. Participants were censored as follows: December 1999 (Turin), June 2000 (Bilthoven), December 2000 (Asturias, Murcia, Cambridge), December 2001 (Florence, Varese, Ragusa, Granada, Navarra, San Sebastian, Oxford, Malmo, Norway), June 2002 (France), December 2002 (Umea, Aarhus, Copenhagen, Naples) and June 2003 (Utrecht). For Germany and Greece, the end of the follow-up was considered to be the last-known contact, the date of diagnosis or the date of death, whichever came first. We used Cox proportional hazard method to estimate the hazard ratio (HR) and 95% confidence intervals (CI) of each quintile compared to the lowest quintile of antioxidant intake. The age at recruitment was used as entry time or t0, and exit time or t1 was the age at breast cancer incidence or censoring. The variable centre was used as stratum in order to control for differences in the questionnaire design and follow-up procedures.
Dietary intake of β-carotene, vitamin C and vitamin E were estimated from dietary questionnaires, calculated based on country-specific food composition tables and expressed as mg/day. The nutrient intake levels were analysed using variables as categorical, by EPIC-wide quintiles or as continuous (β-carotene per 2 mg/day, vitamin C per 100 mg/day and vitamin E per 10 mg/day) in the so-called ‘observed’ models.
All multivariate models were controlled for estimated energy from protein and carbohydrates (kcal/day, continuous), saturated fatty acids (SFA, g/day, continuous), monounsaturated fatty acids (MUFA, g/day, continuous), polyunsaturated fatty acids (PUFA, g/day, continuous), alcohol intake (g/day, continuous), weight (kg, continuous), height (m, continuous), age at menarche (≤12, 13–14, ≥15 years), parity (yes/no), age at first full-term pregnancy (nulliparous, <20, 20–30, >30 years), use of hormone therapy at recruitment (yes, no, missing), smoking status (current, former, never, unknown), overall physical activity [metabolic equivalent of energy expenditure score (MET) classified as inactive, moderately inactive, moderately active, active] [28] and education (none, primary school, technical/professional school, secondary school, university). All models were calculated stratified by menopausal status at recruitment (pre-, peri-, postmenopausal as defined in [13]). In addition, stratified analyses were run by smoking status, alcohol consumption levels, the use of exogenous hormones and the use of supplementary vitamins. Heterogeneity between centres (P heterogeneity) of effect was tested using Wald statistics [29].The median values for antioxidants within each quintile was entered in a regression model, and the significance was tested by the Wald’s test (P trend). Interaction was investigated by including a cross-product term in the equation, and P for interaction was calculated with the log-likelihood test. (P interaction). Two-sided P-values are reported. All analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, NC, USA).
Calibration
To correct for systematic over- or underreporting, dietary intakes were calibrated by means of data obtained from a detailed computerised 24-h diet recall (24HR) as second dietary assessment in a random sample of the cohort (N = 21,522 women in the present study) [30]. Country-specific calibration models were used to predict dietary exposures for all participants [31]. The 24HR values were regressed on the intake values for β-carotene, vitamin C, vitamin E, SFA, MUFA, PUFA, alcohol and energy from carbohydrates and proteins. Disease models were then run with the calibrated value of the nutrient of interest applying also the calibrated values of the adjustment variables.
Results
The number of breast cancer cases by country and age at diagnosis is shown in Table 1. During median follow-up time of 8.8 years, 7,502 cases with invasive breast cancer occurred. The median intake of β-carotene ranged between 1.6 mg/day in Spain and 4.5 mg/day in Greece and for vitamin C between 80 mg/day in Norway and 201 mg/day in Greece. Median intake of vitamin E was lowest in Sweden (7.28 mg/day) and highest in France (13.33 mg/day). Basic characteristics of women according to consumption levels of antioxidants are shown in Table 2. In comparison with the low consumption category (Q1), women with higher intakes of antioxidants (Q5), smoked currently less, were more physically active, drank more alcohol and reported higher levels of education.
Table 3 shows hazard ratios and 95% confidence intervals for the risk of breast cancer by dietary β-carotene, vitamin C and vitamin E intake among premenopausal women. Consistently for different exposure models, dietary β-carotene and vitamin C intake from food was not associated with breast cancer risk. For vitamin E intake, the trend test indicated a positive association with breast cancer risk in premenopausal women overall and in subgroups with moderate alcohol consumption and no use of supplements. Further adjustment for dietary supplement use did not substantially affect the estimates (please see supplementary Tables 2, 3). Alcohol intake did not modify the association between antioxidants and breast cancer risk in premenopausal women.
In Table 4, hazard ratios and 95% confidence intervals for the risk of breast cancer by dietary β-carotene, vitamin C and vitamin E intake among postmenopausal women are shown. Overall, also in postmenopausal women, no associations were found for dietary intake of β-carotene (highest vs. lowest quintile: HR, 0.93; 95% CI (0.82–1.04); P heterogeneity = 0.093), vitamin C [0.98 (0.87–1.10); P heterogeneity = 0.065] and vitamin E [0.92 (0.77–1.11); P heterogeneity = 0.029]. Exclusion of Norway and Italy in order to increase homogeneity between centres did not substantially influence the estimates for vitamin E intake [0.92 (0.75–1.12); P heterogeneity = 0.226]. Neither adjustment for supplementary vitamin use did influence the estimates nor do the results of a sub-analysis in non-users indicate effect modification by supplement use (supplementary Tables 1, 2). No substantial differences in the risk estimates emerged by exclusion of breast cancer cases diagnosed within the first 2 years of follow-up in order to explore whether cancers diagnosed closely to recruitment date influenced the findings. When analysing all perimenopausal women (N = 62,808), no relationship between β-carotene, vitamin E and vitamin C emerged (data not shown).
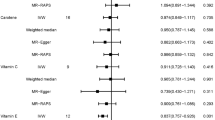
Stratification for the use of exogenous hormones, revealed that among postmenopausal women using exogenous hormones, high intake of β-carotene when compared to low intake was related to lower breast cancer risk [0.79 (0.66–0.96); Ptrend = 0.06]. High vitamin C intake was also associated with lower breast cancer risk [0.88 (0.72–1.07)], but reached statistical significance in the continuous model with observed intake data [0.989 (0.979–0.999); Ptrend = 0.05]. For vitamin E, no association was found. Only the interaction between exogenous hormone use and vitamin C intake was statistically significant [Pinteraction = 0.016]. Figure 1a–d shows the combined and country-specific HRs for postmenopausal breast cancer risk in relation to β-carotene and vitamin C intake and exogenous hormone use. Since the number of cases is relatively low in Greece and Norway and the follow-up time is short, these results should be interpreted with caution.
Stratification by levels of alcohol consumption revealed an inverse association between breast cancer risk and dietary β-carotene intake among women with high (≥10 g/day) alcohol consumption [0.75 (0.62–0.92)]. For vitamin C and E intake, no clear associations were found. None of the interactions with alcohol consumption was statistically significant (β-carotene Pinteraction = 0.967, vitamin C Pinteraction = 0.875 and vitamin E Pinteraction = 0.894).
Stratification by smoking status (supplementary Table 1) revealed to none of the subgroups a remarkable association between the intake of β-carotene, vitamin C and E from food and breast cancer risk, except for associations of higher vitamin C with higher breast cancer risk in former and never smokers among premenopausal women and increasing vitamin E and breast cancer risk in current smokers among postmenopausal women. Overall, none of the interaction terms between antioxidant intake and smoking status was statistically significant (β-carotene Pinteraction = 0.446, vitamin C Pinteraction = 0.582 and vitamin E Pinteraction = 0.346).
Discussion
In this large prospective study, no consistent relationships between dietary intake of β-carotene, vitamins C or E and breast cancer risk were found among pre- and postmenopausal women. Also in perimenopausal women, no association was found (data not shown). However, in subgroup analyses, we found some evidence of an association between β-carotene and vitamin C intake and lower breast cancer risk among postmenopausal women using hormones. A lower risk was also observed for high β-carotene intake among postmenopausal women consuming more than 10 g alcohol per day. In premenopausal women, vitamin E intake from food was in trend positively associated with breast cancer risk.
Our results are in agreement with former research [1, 3, 7–11, 13] showing no association between β-carotene, vitamin C and E intake and breast cancer risk. However, one cohort study reported a weak protective effect of dietary β-carotene on breast cancer risk in premenopausal women [11]. Discrepancies in the associations between dietary antioxidants and breast cancer risk could be related to differences in the adjustment variables or the selection of subgroups and study design. Protective effects of antioxidants were predominantly found in case-control studies [16, 17], which are susceptible to recall bias. Differences in the associations could also be related to the hormone receptor status of the tumour [32–34]. Unfortunately, this information was not available for our study, nor was family history of breast cancer available for all centres. However, for some centres (France, Spain, Cambridge and Norway) with information on familiar breast cancer, a sensitivity analyses revealed no substantial differences of the risk estimates. Few studies also reported positive associations, e.g. an increased breast cancer risk has been found for obese [10] postmenopausal women with high vitamin C intake [35]. In line with our findings, vitamin E intake from foods and supplements was positively related to breast cancer risk in premenopausal women, whereas in postmenopausal women an inverse association was found [11].
Alcohol consumption leads to oxidative stress and free radical damage [36]. Stratification by levels of alcohol consumption revealed an inverse association between β-carotene intake and breast cancer risk among postmenopausal women who consumed more than 10 g alcohol per day. In contrast to Zhang et al. [11], we found no clear relationship among premenopausal women consuming alcohol. However, in their analyses, 15 g/day of alcohol was used as cut-point, by which we would have excluded some centres. No associations were found for vitamin C and E in alcohol consumers, independent of menopausal status.
Our finding of no apparent association between β-carotene and breast cancer risk for smokers among both pre- and postmenopausal women is consistent with previous results concerning various carotenoids [37], although high vitamin A intake was associated with reduced breast cancer risk among premenopausal smokers in another study [22]. Between β-carotene intake and tobacco-related cancers a positive association among smokers and an inverse association among non-smokers have been reported, but no association for non-tobacco-related cancers, including breast cancer [38]. However, our results suggest an association of higher vitamin C with higher breast cancer risk in former and current smoker among premenopausal women, which are in line with another study [35]. In postmenopausal smokers, vitamin E intake was positively associated with breast cancer risk. However, since the interaction term between vitamin E intake and smoking status was not statistically significant, and no association existed after calibration of the vitamin E intake data, this result should be interpreted with caution.
Our observation of a protective association of β-carotene with vitamin C intake among postmenopausal current hormone users could also be attributed to bioactive compounds, which are highly correlated with the vitamins C and E and not yet identified or listed in common food tables. However, some support comes from in vitro studies showing a link between oestrogen-induced oxidative stress and breast cancer [39, 40]. Epidemiological studies with stratification by menopausal status and hormone therapy are scarce. However, the results of a case-control study on β-carotene intake suggest effect modification by HRT-use [23]. Further research including biomarker measurements is warranted to clarify the mechanisms. Given the large number of subgroups analysed in this study, chance remains a plausible explanation of these results.
In the present study, we were interested in the effects of dietary antioxidants from foods, since users of vitamin supplements may differ from the general population [41, 42]. Previous analyses of dietary supplementation with specific dietary micronutrients showed mixed results [3], and recent results from a prospective study do not support an association between dietary multivitamin supplement use and breast cancer risk [43]. In our study, no substantial change of the risk estimates was observed by additional adjustment for supplement use (Supplementary Tables 2, 3).
Among the limitations of our study, measurement error in questionnaire-based dietary assessment has to be considered. In order to explore the exposure–disease relationship, individuals were ranked according to their intake levels, thus systematic over- or underestimation is of less relevance. In addition, models using calibrated dietary values were used to minimise measurement error. Our observations could be due to mixed effects of vitamins or other bioactive compounds such as α-carotene or lycopene, which may be more important for cancer prevention [34, 44, 45], but intake data in EPIC were not available so far. In this analysis, we conducted multiple comparisons, and some significant associations could have emerged by chance.
Strengths of the investigation are the prospective study design, the large sample size, standardised assessment methods and the verification of the endpoint data. In the EPIC calibration subsample, vitamins C and E were among the most frequent components of supplements used [41]; thus, considering overall supplement use is likely to reflect vitamin C and E supplementation.
In conclusion, we found no evidence for an overall association between intake of β-carotene, vitamin C and E and breast cancer risk in pre- and postmenopausal women in this prospective cohort study. Thus, our results are in line with the conclusions of the WCRF report of 2007 [1]. Results of subgroup analyses suggest a protective effect from high intake of vitamin C or β-carotene in some subgroups of postmenopausal women, notably those using exogenous hormones or consuming moderate to high amounts of alcohol. These results, however, have to be interpreted with caution, and further research applying biomarker measurements is needed to clarify these observations.
References
World Cancer Research Fund & American Institute for Cancer Research (2007) Food, nutrition, physical activity, and the prevention of cancer. A global perspective. American Institute for Cancer Research, Washington, DC
Smith-Warner SA, Spiegelman D, Yaun SS, Adami HO, Beeson WL, van den Brandt PA, Folsom AR, Fraser GE, Freudenheim JL, Goldbohm RA, Graham S, Miller AB, Potter JD, Rohan TE, Speizer FE, Toniolo P, Willett WC, Wolk A, Zeleniuch-Jacquotte A, Hunter DJ (2001) Intake of fruits and vegetables and risk of breast cancer: a pooled analysis of cohort studies. JAMA 285(6):769–776
Michels KB, Mohllajee AP, Roset-Bahmanyar E, Beehler GP, Moysich KB (2007) Diet and breast cancer: a review of the prospective observational studies. Cancer 109(12 Suppl):2712–2749 Review
van Gils CH, Peeters PH, Bueno-de-Mesquita HB, Boshuizen HC, Lahmann PH, Clavel-Chapelon F, Thiebaut A, Kesse E, Sieri S, Palli D, Tumino R, Panico S, Vineis P, Gonzalez CA, Ardanaz E, Sanchez MJ, Amiano P, Navarro C, Quiros JR, Key TJ, Allen N, Khaw KT, Bingham SA, Psaltopoulou T, Koliva M, Trichopoulou A, Nagel G, Linseisen J, Boeing H, Berglund G, Wirfalt E, Hallmans G, Lenner P, Overvad K, Tjonneland A, Olsen A, Lund E, Engeset D, Alsaker E, Norat T, Kaaks R, Slimani N, Riboli E (2005) Consumption of vegetables and fruits and risk of breast cancer. JAMA 293(2):183–193
Lanchance PA, Nakat Z, Jeong W-S (2001) Antioxidants: an integrative approach. Nutrition 17:835–838
Rock CL (2000) Nutrition in the prevention of disease: current issues and concepts. Am J Prev Med 18(4):351–353
Kushi LH, Fee RM, Sellers TA, Zheng W, Folsom AR (1996) Intake of vitamins A, C, and E and postmenopausal breast cancer. The Iowa Women’s Health Study. Am J Epidemiol 144(2):165–174
Jarvinen R, Knekt P, Seppanen R, Teppo L (1997) Diet and breast cancer risk in a cohort of Finnish women. Cancer Lett 114(1–2):251–253
Verhoeven DT, Assen N, Goldbohm RA, Dorant E, van ‘t Veer P, Sturmans F, Hermus RJ, van den Brandt PA (1997) Vitamins C and E, retinol, beta-carotene and dietary fibre in relation to breast cancer risk: a prospective cohort study. Br J Cancer 75(1):149–155
Michels KB, Holmberg L, Bergkvist L, Ljung H, Bruce A, Wolk A (2001) Dietary antioxidant vitamins, retinol, and breast cancer incidence in a cohort of Swedish women. Int J Cancer 91(4):563–567
Zhang S, Hunter DJ, Forman MR, Rosner BA, Speizer FE, Colditz GA, Manson JE, Hankinson SE, Willett WC (1999) Dietary carotenoids and vitamins A, C, and E and risk of breast cancer. J Natl Cancer Inst 91(6):547–556
Lee IM, Cook NR, Manson JE, Buring JE, Hennekens CH (1999) Beta-carotene supplementation and incidence of cancer and cardiovascular disease: the Women’s Health Study. J Natl Cancer Inst 91(24):2102–2106
Graham S, Zielezny M, Marshall J, Priore R, Freudenheim J, Brasure J, Haughey B, Nasca P, Zdeb M (1992) Diet in the epidemiology of postmenopausal breast cancer in the New York State Cohort. Am J Epidemiol 136(11):1327–1337
Hunter DJ, Manson JE, Colditz GA, Stampfer MJ, Rosner B, Hennekens CH, Speizer FE, Willett WC (1993) A prospective study of the intake of vitamins C, E, and A and the risk of breast cancer. N Engl J Med 329(4):234–240
Lin J, Cook NR, Albert C, Zaharris E, Gaziano JM, Van Denburgh M, Buring JE, Manson JE (2009) Vitamins C and E and beta carotene supplementation and cancer risk: a randomized controlled trial. J Natl Cancer Inst 101(1):14–23
Howe GR, Hirohata T, Hislop TG, Iscovich JM, Yuan JM, Katsouyanni K, Lubin F, Marubini E, Modan B, Rohan T et al (1990) Dietary factors and risk of breast cancer: combined analysis of 12 case-control studies. J Natl Cancer Inst 82(7):561–569
Gandini S, Merzenich H, Robertson C, Boyle P (2000) Meta-analysis of studies on breast cancer risk and diet: the role of fruit and vegetable consumption and the intake of associated micronutrients. Eur J Cancer 36(5):636–646
Hulten K, Van Kappel AL, Winkvist A, Kaaks R, Hallmans G, Lenner P, Riboli E (2001) Carotenoids, alphatocopherols, and retinol in plasma and breast cancer risk in, northern Sweden. Cancer Causes Control 12(6):529–537
Toniolo P, Van Kappel AL, Akhmedkhanov A, Ferrari P, Kato I, Shore RE, Riboli E (2001) Serum carotenoids and breast cancer. Am J Epidemiol 153(12):1142–1147
Ching S, Ingram D, Hahnel R, Beilby J, Rossi E (2002) Serum levels of micronutrients, antioxidants and total antioxidant status predict risk of breast cancer in a case control study. J Nutr 132(2):303–306
Sato R, Helzlsouer KJ, Alberg AJ, Hoffman SC, Norkus EP, Comstock GW (2002) Prospective study of carotenoids, tocopherols, and retinoid concentrations and the risk of breast cancer. Cancer Epidemiol Biomarkers Prev 11(5):451–457
Cho E, Spiegelman D, Hunter DJ, Chen WY, Zhang SM, Colditz GA, Willett WC (2003) Premenopausal intakes of vitamins A, C, and E, folate, and carotenoids, and risk of breast cancer. Cancer Epidemiol Biomarkers Prev 12(8):713–720
Quick SK, Shields PG, Nie J, Platek ME, McCann SE, Hutson AD, Trevisan M, Vito D, Modali R, Lehman TA, Seddon M, Edge SB, Marian C, Muti P, Freudenheim JL (2008) Effect modification by catalase genotype suggests a role for oxidative stress in the association of hormone replacement therapy with postmenopausal breast cancer risk. Cancer Epidemiol Biomarkers Prev 17(5):1082–1087
Riboli E, Hunt KJ, Slimani N, Ferrari P, Norat T, Fahey M, Charrondiere UR, Hemon B, Casagrande C, Vignat J, Overvad K, Tjonneland A, Clavel-Chapelon F, Thiebaut A, Wahrendorf J, Boeing H, Trichopoulos D, Trichopoulou A, Vineis P, Palli D, Bueno-De-Mesquita HB, Peeters PH, Lund E, Engeset D, Gonzalez CA, Barricarte A, Berglund G, Hallmans G, Day NE, Key TJ, Kaaks R, Saracci R (2002) European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr 5(6B):1113–1124
Bingham S, Riboli E (2004) Diet and cancer—the European Prospective Investigation into Cancer and Nutrition. Nat Rev Cancer 4(3):206–215
Riboli E, Kaaks R (1997) The EPIC Project: rationale and study design. European Prospective Investigation into Cancer and Nutrition. Int J Epidemiol 26(Suppl 1):S6–S14
Lahmann PH, Hoffmann K, Allen N, van Gils CH, Khaw KT, Tehard B, Berrino F, Tjønneland A, Bigaard J, Olsen A, Overvad K, Clavel-Chapelon F, Nagel G, Boeing H, Trichopoulos D, Economou G, Bellos G, Palli D, Tumino R, Panico S, Sacerdote C, Krogh V, Peeters PH, Bueno-de-Mesquita HB, Lund E, Ardanaz E, Amiano P, Pera G, Quirós JR, Martínez C, Tormo MJ, Wirfält E, Berglund G, Hallmans G, Key TJ, Reeves G, Bingham S, Norat T, Biessy C, Kaaks R, Riboli E (2004) Body size and breast cancer risk: findings from the European Prospective Investigation into Cancer and Nutrition (EPIC). Int J Cancer 111(5):762–771
Wareham NJ, Jakes RW, Rennie KL, Schuit J, Mitchell J, Hennings S, Day NE (2003) Validity and repeatability of a simple index derived from the short physical activity questionnaire used in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Public Health Nutr 6:407–413
Greenland S, Rothman KJ (1998) Introduction to stratified analysis. In: Rothman KJ, Greenland S (eds) Modern epidemiology, 2nd edn. Lippincott-Raven, Philadelphia (PA), pp 53–79
Slimani N, Kaaks R, Ferrari P, Casagrande C, Clavel-Chapelon F, Lotze G, Kroke A, Trichopoulos D, Trichopoulou A, Lauria C, Bellegotti M, Ocke MC, Peeters PH, Engeset D, Lund E, Agudo A, Larranaga N, Mattisson I, Andren C, Johansson I, Davey G, Welch AA, Overvad K, Tjonneland A, Van Staveren WA, Saracci R, Riboli E (2002) European Prospective Investigation into Cancer and Nutrition (EPIC) calibration study: rationale, design and population characteristics. Public Health Nutr 5(6B):1125–1145
Ferrari P, Day NE, Boshuizen HC, Roddam A, Hoffmann K, Thiébaut A, Pera G, Overvad K, Lund E, Trichopoulou A, Tumino R, Gullberg B, Norat T, Slimani N, Kaaks R, Riboli E (2008) The evaluation of the diet/disease relation in the EPIC study: considerations for the calibration and the disease models. Int J Epidemiol, Jan 6 (Epub ahead of print)
Gaudet MM, Britton JA, Kabat GC, Steck-Scott S, Eng SM, Teitelbaum SL, Terry MB, Neugut AI, Gammon MD (2004) Fruits, vegetables, and micronutrients in relation to breast cancer modified by menopause and hormone receptor status. Cancer Epidemiol Biomarkers Prev 13(9):1485–1494
Olsen A, Tjonneland A, Thomsen BL, Loft S, Stripp C, Overvad K, Moller S, Olsen JH (2003) Fruits and vegetables intake differentially affects estrogen receptor negative and positive breast cancer incidence rates. J Nutr 133(7):2342–2347
Cui Y, Shikany JM, Liu S, Shagufta Y, Rohan TE (2008) Selected antioxidants and risk of hormone receptor defined invasive breast cancers among postmenopausal women in the Women’s Health Initiative Observational Study. Am J Clin Nutr 87(4):1009–1018
Nissen SB, Tjonneland A, Stripp C, Olsen A, Christensen J, Overvad K, Dragsted LO, Thomsen B (2003) Intake of vitamins A, C, and E from diet and supplements and breast cancer in postmenopausal women. Cancer Causes Control 14(8):695–704
Albano E (2006) Alcohol, oxidative stress and free radical damage. Proc Nutr Soc 65(3):278–290
Terry P, Jain M, Miller AB, Howe GR, Rohan TE (2002) Dietary carotenoids and risk of breast cancer. Am J Clin Nutr 76:883–888
Touvier M, Kesse E, Clavel-Chapelon F, Boutron-Ruault MC (2005) Dual association of beta-carotene with risk of tobacco-related cancers in a cohort of French women. J Natl Cancer Inst 97(18):1338–1344
Yager JD (2000) Endogenous estrogens as carcinogens through metabolic activation. J Natl Cancer Inst Monogr (27):67–73
Jefcoate CR, Liehr JG, Santen RJ, Sutter TR, Yager JD, Yue W, Santner SJ, Tekmal R, Demers L, Pauley R, Naftolin F, Mor G, Berstein L (2000) Tissue-specific synthesis and oxidative metabolism of estrogens. J Natl Cancer Inst Monogr (27):95–112
Skeie G, Braaten T, Hjartåker A, Lentjes M, Amiano P, Jakszyn P et al. (2009) Use of dietary supplements in the European Prospective Investigation into Cancer and Nutrition (EPIC) calibration study (in preparation)
Reinert A, Rohrmann S, Becker N, Linseisen J (2007) Lifestyle and diet in people using dietary supplements: a German cohort study. Eur J Nutr 46(3):165–173
Ishitani K, Lin J, Manson JE, Buring JE, Zhang SM (2008) A prospective study of multivitamin supplement use and risk of breast cancer. Am J Epidemiol 167(10):1197–1206
Nishino H, Tokuda H, Murakoshi M, Satomi Y, Masuda M, Onozuka M, Yamaguchi S, Takayasu J, Tsuruta J, Okuda M, Khachik F, Narisawa T, Takasuka N, Yano M (2000) Cancer prevention by natural carotenoids. Biofactors 13(1–4):89–94
Al-Delaimy WK, Ferrari P, Slimani N, Pala V, Johansson I, Nilsson S, Mattisson I, Wirfalt E, Galasso R, Palli D, Vineis P, Tumino R, Dorronsoro M, Pera G, Ocké MC, Bueno-de-Mesquita HB, Overvad K, Chirlaque M, Trichopoulou A, Naska A, Tjønneland A, Olsen A, Lund E, Alsaker EH, Barricarte A, Kesse E, Boutron-Ruault MC, Clavel-Chapelon F, Key TJ, Spencer E, Bingham S, Welch AA, Sanchez-Perez MJ, Nagel G, Linseisen J, Quirós JR, Peeters PH, van Gils CH, Boeing H, van Kappel AL, Steghens JP, Riboli E (2005) Plasma carotenoids as biomarkers of intake of fruits and vegetables: individual-level correlations in the European Prospective Investigation into Cancer and Nutrition (EPIC). Eur J Clin Nutr 59(12):1387–1396
Acknowledgments
The EPIC study was funded by “Europe Against Cancer” Programme of the European Commission (SANCO); Ligue contre le Cancer (France); Société 3M (France); Mutuelle Générale de l’Education Nationale; Institut National de la Santé et de la Recherche Médicale (INSERM); German Cancer Aid; German Cancer Research Center; German Federal Ministry of Education and Research; Danish Cancer Society; Health Research Fund (FIS) of the Spanish Ministry of Health (RCESP-C03/09); the participating regional governments and institutions of Spain; Cancer Research UK; Medical Research Council, UK; the Stroke Association, UK; British Heart Foundation; Department of Health, UK; Food Standards Agency, UK; the Wellcome Trust, UK; Greek Ministry of Education; Greek Ministry of Health and Social Solidarity; Hellenic Health Foundation and Stavros Niarchos Foundation; Italian Association for Research on Cancer; Italian National Research Council; Dutch Ministry of Public Health, Welfare and Sports; Dutch Ministry of Health; Dutch Prevention Funds; LK Research Funds; Dutch ZON (Zorg Onderzoek Nederland); World Cancer Research Fund (WCRF); Swedish Cancer Society; Swedish Scientific Council; Regional Government of Skane, Sweden; Norwegian Cancer Society.
Conflict of interest statement
None declared.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nagel, G., Linseisen, J., van Gils, C.H. et al. Dietary β-carotene, vitamin C and E intake and breast cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC). Breast Cancer Res Treat 119, 753–765 (2010). https://doi.org/10.1007/s10549-009-0444-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-009-0444-8