Abstract
Although epidemiologic studies have shown an inverse association between isoflavones and breast cancer risk, little evidence for a dose–response relation is available. We conducted hospital-based case–control studies of patients aged 20–74 years with primary, incident, histologically confirmed invasive breast cancer, and matched controls from medical checkup examinees in Nagano, Japan and from cancer-free patients in São Paulo, Brazil. A total of 850 pairs (390 Japanese, 81 Japanese Brazilians and 379 non-Japanese Brazilians) completed validated food frequency questionnaires. The odds ratio of breast cancer according to isoflavone intake was estimated using a conditional logistic regression model. We found a statistically significant inverse association between isoflavone intake and the risk of breast cancer for Japanese Brazilians and non-Japanese Brazilians. For Japanese, a non-significant inverse association was limited to postmenopausal women. In the three populations combined, breast cancer risk linearly decreased from ‘no’ to ‘moderate’ isoflavone intake and thereafter leveled off. Compared to non-consumers, adjusted odds ratios (95% confidence interval) for consumers in increasing quintile intake categories (median intake in each category: 8.7, 23.1, 33.8, 45.7, and 71.3 mg/day) were 0.69 (0.44–1.09), 0.54 (0.31–0.94), 0.45 (0.26–0.77), 0.34 (0.19–0.62), and 0.43 (0.24–0.76), respectively. Overall, we found an inverse association between dietary isoflavone intake and risk of breast cancer. Our finding suggests a risk-reducing rather than risk-enhancing effect of isoflavones on breast cancer within the range achievable from dietary intake alone. In addition, women may benefit from risk reduction if they consume at least moderate amounts of isoflavones.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soy foods, which are rich in isoflavones, are habitually consumed by Asian populations in large amounts. Isoflavones, of which genistein and daidzein are major examples, are classified as phytoestrogens, which are plant-derived non-steroidal compounds with estrogen-like biological properties. A high intake of isoflavones has therefore been hypothesized to contribute to the lower incidence of breast cancer in Asia than Western countries [1]. This hypothesis is supported by not only in vitro studies at high genistein concentrations and the majority of animal studies [2, 3] but also epidemiological studies [4–10]. In particular, a recent meta-analysis showed a small decrease in risk of breast cancer with higher soy intake [11] while a more recent meta-analysis indicated that risk reduction was limited to Asian populations [12]. In apparent contradiction to potential protective effects, however, genistein exhibits estrogenic properties at low concentrations, which could theoretically enhance breast cancer risk [2, 3], and some animal studies have in fact reported that genistein stimulates tumor development and growth [13, 14].
Although research remains insufficient for any comprehensive determination of whether isoflavones are protective or harmful for breast cancer, interest in soy foods and isoflavones is nevertheless increasing. This increase may reflect an expectation of potential benefits in a wide variety of medical conditions, including cancer of the endometrium and prostate as well as breast, cardiovascular diseases, osteoporosis, and menopausal symptoms. In fact, consumption of soy foods in the United States has increased over the past ten years, against fairly constant intake in Japan over the past four decades [15]. Moreover, phytoestrogen supplements are commercially marketed for use by postmenopausal women as natural and safe alternatives to hormone replacement therapy. A dose–response pattern, in particular the effect of relatively high-dose isoflavones on breast cancer risk, is thus now of concern. Nevertheless, little evidence of any dose–response relationship is available—indeed, we do not know the answer to ‘how much isoflavones is needed?’ This is partly because few studies have estimated isoflavone intake using a validated food-frequency questionnaire (FFQ) [4–6, 16, 17], and also because most studies in Western countries have involved only a small variation in isoflavone intake [6, 7, 16–20].
Here, to evaluate the dose–response relationship between isoflavone intake and the risk of breast cancer, ranging from zero to the relatively high levels achievable from dietary intake only, we conducted hospital-based case–control studies in Nagano, Japan and São Paulo, Brazil, areas with a low and middle incidence of breast cancer, respectively (age-standardized rate per 100,000 world population, 32.7 and 46.0 in 2002, respectively) [21], using validated FFQs with relatively high validity in three populations: Japanese living in Japan, Japanese Brazilians living in São Paulo, and non-Japanese Brazilians living in São Paulo. The mortality of breast cancer among these three populations has increased over the last 20 years, with that in Japanese Brazilians intermediate between that in Japanese and Brazilians [22]. In addition, because amounts and variations in isoflavone intake are expected to be high and large for Japanese, intermediate and relatively large for Japanese Brazilians, and low and small for non-Japanese Brazilians, respectively, these populations serve as suitable venues for studies of the effect of dose–response relations.
Materials and methods
Study subjects
These multicenter, hospital-based case–control studies of breast cancer were designed to determine lifestyle factors and genetic susceptibility to the risk of breast cancer and to compare potential risk factors among Japanese living in Nagano, Japan, and Japanese Brazilians and non-Japanese Brazilians living in São Paulo, Brazil. Eligible cases were a consecutive series of female patients aged 20–74 years with newly diagnosed and histologically confirmed invasive breast cancer. Cases were recruited between 2001 and 2005 at four hospitals in Nagano, and between 2001 and 2006 at eight hospitals in São Paulo. A total of 405 cases (98%) participated in Nagano, and 83 Japanese Brazilians (91%) and 389 non-Japanese Brazilians (99%) in São Paulo. In the study in Nagano, eligible controls were selected from medical checkup examinees in two of the four hospitals and confirmed not to have cancer. One control was matched for each case by age (within 3 years) and residential area during the study period. Among potential controls, one examinee refused to participate and two refused to provide blood samples. Consequently, we obtained written informed consent from 405 matched pairs. In the study in São Paulo, eligible controls were preferentially selected from cancer-free patients who visited the same hospital as the index cases. One control was matched for each case by age (within 5 years) and ethnicity during the study period. Among potential controls, 22 patients refused to participate (participation rate = 96%). Consequently, we obtained written informed consent from 472 matched pairs (83 for Japanese Brazilians and 389 for non-Japanese Brazilians). The study protocol was approved by CONEP (Comissão Nacional de Ética em Pesquisa), Brasília, Brazil and by the institutional review board of the National Cancer Center, Tokyo, Japan.
Data collection
Participants in Nagano were asked to complete a self-administered questionnaire, while in-person interviews were conducted by trained interviewers using a structured questionnaire in São Paulo. The two questionnaires contained closely similar questions concerning demographic characteristics, medical history, family history of cancer, menstrual and reproductive history, anthropometric factors, physical activity, and smoking habits. For dietary habits, we used a semi-quantitative FFQ (136 items for the Japanese version and 118 items for the Brazilian version) which was developed and validated in each population [23, 24]. Information on estrogen receptor (ER) and progesterone receptor (PR) status was obtained from medial records. Hormone receptor status was determined by either enzyme-linked immunoassay or immunohistochemical assay. Hormone receptor positivity values were determined either as specified by the laboratory that performed the assay, or in accordance with the laboratory’s written interpretation thereof, or both.
Dietary assessment
In the FFQ, participants were questioned on how often they consumed the individual food items (frequency of consumption), as well as relative sizes compared to standard portions. Response choices for frequency were never or less than once/month, 1–3 times/month, 1–2 times/week, 3–4 times/week, 5–6 times/week, once/day, 2–3 times/day, 4–6 times/day, and 7 times/day or more, and relative sizes to a standard portion were small (50% smaller than standard), medium (same as standard), and large (50% larger). For the Japanese version, white rice intake was determined in terms of the relative size of the rice bowl used and the frequency of intake, with the nine choices of less than 1–10 bowls per day. Frequency for miso soup intake was given in the six choices of almost never, 1–3 times/month, 1–2 times/week, 3–4 times/week, 5–6 times/week, or daily, while amount was given in nine categories ranging from less than 1–10 bowls per day, without reference to the relative size of the bowl used. Daily food intake was calculated by multiplying frequency by standard portion and relative size for each food item in the FFQ. Daily intakes of genistein and daidzein were calculated using a food composition table of isoflavones developed previously [25, 26]. Isoflavone intake was defined for this study as the sum of genistein and daidzein intake. Other nutrients were calculated using the Japanese Standard Tables of Food Composition, 5th ed. for the Japanese version [27] and the United States Department of Agriculture (USDA) food composition tables for the Brazilian version [28]. For some Japanese-specific foods in the Brazilian version, the Japanese Standard Tables of Food Composition, 5th ed. was used.
The validity of isoflavone intake estimated from the Japanese version of the FFQ was evaluated in a subsample of the Japan Public Health Center-based Prospective Study, which includes Nagano as one of the study areas. The estimated intake according to the FFQ was compared to that in four consecutive 7-day dietary records, one conducted in each the four seasons. Spearman’s correlation coefficients between energy-adjusted genistein and daidzein intake estimated from the FFQ and from dietary records were 0.59 for genistein and 0.60 for daidzein [24]. For the Brazilian version, the validity of isoflavone intake estimated from the FFQ was evaluated in a subsample of the control group in this case–control study by comparing the estimated intake according to the FFQ to that in two consecutive 4-day dietary records, one each in two seasons. Spearman’s correlation coefficients between energy-adjusted genistein and daidzein intake estimated from the FFQ and from dietary records were 0.76 for genistein and 0.76 for daidzein (unpublished data).
Statistical analysis
We excluded subjects who reported extremely low or high total energy intake (<500 or ≥ 4000 Kcal), leaving 390 pairs of Japanese, 81 pairs of Japanese Brazilians and 379 pairs of non-Japanese Brazilians for use in the present analyses. Comparison of baseline characteristics between cases and controls was evaluated by the Mantel-Haenszel test using matched-pair strata in each population. Dietary intake of isoflavones was adjusted for total energy intake by the residual method and divided into median or tertile categories based on control distribution for Japanese and Japanese Brazilians, respectively. Because of the small proportion of consumers, non-Japanese Brazilians were categorized into non-consumers and consumers of isoflavones. Using a conditional logistic regression model, we calculated odds ratios (ORs) and 95% confidence intervals (CIs) of breast cancer for isoflavone intake. An unconditional logistic regression model was used for stratified analyses according to menopausal status. Associations between isoflavone intake and hormone receptor-defined breast cancer were assessed by an unconditional polytomous logistic regression model. Linear trends for ORs were tested in the logistic regression model using the exposure categories as ordinal variables. The following variables, which were mainly selected based on comparison of baseline characteristics between cases and controls, were adjusted for as potential confounders: menopausal status, number of births, family history of breast cancer, smoking status, moderate physical activity in the past 5 years, and vitamin supplement use. We did not include a history of benign breast disease as a covariate since we regarded it as an intermediate variable in the causal pathway between isoflavone intake and breast cancer. All p values reported are two-sided, and significance level was set at P < 0.05. All statistical analyses were performed with SAS software version 9.1 (SAS Institute, Inc., Cary, NC).
Results
Characteristics of cases and controls and isoflavone intake (Table 1)
For Japanese, the proportion of premenopausal women, current smokers, and vitamin supplement users was higher in cases than in controls, and cases tended to have a family history of breast cancer and history of benign breast disease. Cases were less likely than controls to breast-feed, be physically active, and eat vegetables. For Japanese Brazilians, cases were less likely than controls to give birth and be physically active and more likely to eat vegetables and fruits. For non-Japanese Brazilians, the proportion of premenopausal women and current smokers was higher in cases than controls while the proportion of physically active women and vitamin supplement users was lower. Isoflavone intake substantially varied among populations, with mean intakes (mg/day) in control subjects of 46.1 for Japanese, 24.9 for Japanese Brazilians, and 4.4 for non-Japanese Brazilians. Because genistein and daidzein intakes were highly correlated, with a Spearman’s correlation coefficient for the three populations of 0.99, only isoflavone intake was used for the following analyses.
ORs in the three populations (Table 2)
We found a statistically significant inverse association between isoflavone intake and the risk of breast cancer for Japanese Brazilians and non-Japanese Brazilians but not for Japanese. Adjusted OR for the highest versus lowest tertile of isoflavone intake was 0.25 (95% CI 0.09–0.68; P for trend <0.01) for Japanese Brazilians. For non-Japanese Brazilians, adjusted OR for consumers versus non-consumers of isoflavones was 0.56 (95% CI 0.35–0.90). No substantial change was seen after further adjustment for other potential confounders, such as age at menarche, age at menopause, age at first birth, history of breast feeding, body mass index, alcohol drinking, or vegetable and fruit intake.
A stratified analysis according to menopausal status revealed that an inverse association was limited to postmenopausal women in Japan although it was not statistically significant. Adjusted OR for the highest versus lowest tertile of isoflavone intake was 0.62 (95% CI 0.38–1.01; P for trend = 0.06) for postmenopausal women, but 1.35 (95% CI 0.72–2.54; P for trend = 0.41) for premenopausal women. The inverse association was stronger in premenopausal than postmenopausal women for Japanese Brazilians but no remarkable difference between the two strata was seen for non-Japanese Brazilians.
ORs of hormone receptor-defined breast cancer (Table 3)
Information on the combined ER and PR status of the breast tumor was available for 387 (99%) Japanese, 61 (75%) Japanese Brazilians, and 264 (70%) non-Japanese Brazilians cases. The following subtypes were used for modeling in an unconditional polytomous logistic regression model: positive for both receptors (ER+/PR+), ER-positive and PR-negative (ER+/PR−), and negative for both receptors (ER−/PR−) for Japanese, and ER+/PR+, ER+/PR−, ER−/PR−, and unknown for Japanese Brazilians and non-Japanese Brazilians. Overall, we found no remarkable difference in risk by hormone receptor-defined subtype.
Dose–response pattern (Table 4; Fig. 1)
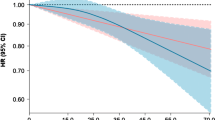
To evaluate dose–response relations using a wide range of isoflavone intake, we combined individual study data from three populations and categorized the subjects into six groups, namely non-consumers and quintiles among isoflavone consumers based on the combined control distribution. Compared to non-consumers, adjusted ORs (95% CI) for consumers in increasing quintile categories (median intake in each category: 8.7, 23.1, 33.8, 45.7, and 71.3 mg/day) based on a conditional logistic regression model were 0.69 (0.44–1.09), 0.54 (0.31–0.94), 0.45 (0.26–0.77), 0.34 (0.19–0.62), and 0.43 (0.24–0.76), respectively. A stratified analysis according to menopausal status based on an unconditional logistic regression model revealed that this inverse association was more prominent in postmenopausal than premenopausal women. To clarify the effect of high isoflavone intake in detail, subjects were further categorized into 11 groups, namely non-consumers and deciles of isoflavone consumers. We found a linear decrease in breast cancer risk from zero to moderate intake (20–30 mg/day) and a leveling-off thereafter based on a conditional logistic regression model (Fig. 1). No increasing trend was found for relatively high intake.
Odds ratios (ORs) and 95% confidence intervals of breast cancer according to dietary isoflavone intake based on combined individual data from three populations. Subjects were categorized into 11 groups: non-consumers and deciles of isoflavone consumers based on the control distribution. ORs were estimated using matching pairs with adjustment for menopausal status (premenopausal women, postmenopausal women), number of births (0, 1, 2, 3, 4, 5+), family history of breast cancer (yes, no), smoking status (never, past, current smokers), moderate physical activity in the past 5 years (no, less than 3 days/month, 1–4 days/week, more than 5 days/week), and vitamin supplement use (yes, no)
Discussion
In these case–control studies of Japanese, Japanese Brazilians, and non-Japanese Brazilians, overall, we found an inverse association between dietary isoflavone intake and the risk of breast cancer. Our finding is in general agreement with those of a recent meta-analysis [11] and in five of the ten previous studies examining the association between isoflavone intake as estimated by FFQ and breast cancer risk [4–8]. It is noteworthy that, although several experimental studies have suggested adverse effects from soy constituents [2, 3, 13, 14], no epidemiological study estimating isoflavone intake by FFQ has reported an increased risk of breast cancer. Our study also suggests a risk-reducing rather than risk-enhancing effect of isoflavones on breast cancer within the range achievable from dietary intake alone. It remains unclear, however, whether isoflavone exposure other than dietary intake is associated with the risk of breast cancer.
We found a linear decrease in breast cancer risk from zero to moderate intake (20–30 mg/day) and thereafter a leveling-off. This dose–responses pattern might imply the presence of a ceiling effect and suggests that women may benefit from risk reduction if they consume at least a moderate amount of isoflavones. Alternatively, it might merely reflect differences in measurement errors due to the use of different FFQs, selection bias, and residual confounding among the three populations, notwithstanding that it clearly reflected the results of separate analyses. Specifically, consumers had lower risk than non-consumers in non-Japanese Brazilians, whose average intake of isoflavone was 4.4 mg/day among the control group; the risk of breast cancer decreased with increasing intake of isoflavone in Japanese Brazilians, whose average intake of isoflavone was 24.9 mg/day among the control group; while higher intake of isoflavone was not associated with further risk reduction in Japanese, whose average intake of isoflavone was 46.1 mg/day among the control group. Confirmation of this pattern would require further prospective cohort studies using blood or urine samples as an exposure assessment, because these could minimize the measurement errors and selection bias mentioned above.
Our stratified analysis by menopausal status using data from the three populations combined showed that an inverse association was more prominent among postmenopausal than premenopausal women. In addition, our separate analyses showed somewhat different patterns in the three populations: the inverse association was limited to postmenopausal women in Japanese; it was stronger in premenopausal than postmenopausal women in Japanese Brazilians; and no remarkable difference was found in non-Japanese Brazilians. These findings are inconsistent with a recent meta-analysis showing an inverse association regardless of menopausal status [11]. Moreover, findings to date on the association of isoflavone intake and the risk of breast cancer stratified by menopausal status have been inconsistent, with one prospective cohort study in Japan [4] and one case–control study in the United States [8] reporting that an inverse association was limited to postmenopausal women; one case–control study in Japan [5] showing it was limited to premenopausal women; and one prospective cohort study in the United States [16] and three case–control studies [6, 17, 18] finding no difference between the two strata.
Several mechanisms by which isoflavones may reduce the risk of breast cancer have been proposed [2, 3]. The most prominent and thoroughly investigated mechanisms are mediated via estrogen receptors, arising due to the similar chemical structure of isoflavones to the human estrogen hormone and their binding affinity to estrogen receptors [3, 29]. Given that the action of estrogen on breast cell proliferation appears to be mediated by estrogen receptors, therefore, any association between isoflavone intake and breast cancer risk might differ by hormone receptor-defined subtype. The present study did not support this hypothesis, however, showing no apparent difference in risk by subtype. Moreover, results for the few studies to date have been inconsistent [7, 16, 18, 19]. Although our findings might merely be explained by a lack of statistical power, they suggest that the anti-cancer effects of isoflavones might be evoked not only by mechanisms mediated by estrogen receptors but also by other mechanisms, such as the modulation of endogenous hormones via inhibition of the key enzyme involved in estrogen biosynthesis and metabolism; the arrest of cell cycle progression; induction of apoptosis; inhibition of tyrosine kinase activity, topoisomerase II activity, and angiogenesis; and antioxidant activity [2, 3].
Our study has several methodological advantages over previous studies of isoflavones and the risk of breast cancer. First, isoflavone intake differed considerably among the three populations, with median levels (interquartile rage) in the control group (mg/day) of 40.6 (25.9–61.2) among Japanese, 13.4 (8.1–35.0) among Japanese Brazilians, and 0 (0–0) among non-Japanese Brazilians. This range allowed the detailed evaluation of dose–response relations, ranging from zero to a relatively high level achievable from dietary intake only, and is unique to the present study. Second, the overall consistency of findings in the three populations allowed for the greater generalizability of results as compared to those from a single population.
Several limitations of this study warrant mention. First, dietary intake of isoflavone was assessed after the diagnosis of breast cancer and is therefore sensitive to recall bias. Second, although the substantially high participation rates among both eligible cases and controls minimized potential biases related to control selection, the use of controls from medical checkup examinees and cancer-free patients, whose dietary habits may differ from the general population due to health consciousness or disease, might have lead to selection bias. Third, stratified analyses were performed based on a relatively small number of cases. The interpretability of our results might therefore be limited.
Allowing for these methodological issues, we found an inverse association between dietary isoflavone intake and the risk of breast cancer in case–control studies of Japanese, Japanese Brazilians, and non-Japanese Brazilians. Our findings suggest a risk-reducing rather than risk-enhancing effect of isoflavones on breast cancer within the range achievable from dietary intake alone. In addition, women may benefit from risk reduction if they consume at least moderate amounts of isoflavones.
Abbreviations
- CI:
-
Confidence interval
- ER:
-
Estrogen receptor
- FFQ:
-
Food-frequency questionnaire
- OR:
-
Odds ratio
- PR:
-
Progesterone receptor
References
Adlercreutz H (1998) Epidemiology of phytoestrogens. Baillieres Clin Endocrinol Metab 12:605–623. doi:10.1016/S0950-351X(98)80007-4
Magee PJ, Rowland IR (2004) Phyto-oestrogens, their mechanism of action: current evidence for a role in breast and prostate cancer. Br J Nutr 91:513–531. doi:10.1079/BJN20031075
Limer JL, Speirs V (2004) Phyto-oestrogens and breast cancer chemoprevention. Breast Cancer Res 6:119–127. doi:10.1186/bcr781
Yamamoto S, Sobue T, Kobayashi M et al (2003) Soy, isoflavones, and breast cancer risk in Japan. J Natl Cancer Inst 95:906–913
Hirose K, Imaeda N, Tokudome Y et al (2005) Soybean products and reduction of breast cancer risk: a case–control study in Japan. Br J Cancer 93:15–22. doi:10.1038/sj.bjc.6602659
Santos Silva I, Mangtani P, McCormack V et al (2004) Phyto-oestrogen intake and breast cancer risk in South Asian women in England: findings from a population-based case–control study. Cancer Causes Control 15:805–818. doi:10.1023/B:CACO.0000043431.85706.d8
Linseisen J, Piller R, Hermann S et al (2004) Dietary phytoestrogen intake and premenopausal breast cancer risk in a German case–control study. Int J Cancer 110:284–290. doi:10.1002/ijc.20119
Wu AH, Wan P, Hankin J et al (2002) Adolescent and adult soy intake and risk of breast cancer in Asian-Americans. Carcinogenesis 23:1491–1496. doi:10.1093/carcin/23.9.1491
Verheus M, van Gils CH, Keinan-Boker L et al (2007) Plasma phytoestrogens and subsequent breast cancer risk. J Clin Oncol 25:648–655. doi:10.1200/JCO.2006.06.0244
Iwasaki M, Inoue M, Otani T et al (2008) Plasma isoflavone level and subsequent risk of breast cancer among Japanese women: a nested case–control study from the Japan public health center-based prospective study group. J Clin Oncol 26:1677–1683. doi:10.1200/JCO.2007.13.9964
Trock BJ, Hilakivi Clarke L, Clarke R (2006) Meta-analysis of soy intake and breast cancer risk. J Natl Cancer Inst 98:459–471
Wu AH, Yu MC, Tseng CC et al (2008) Epidemiology of soy exposures and breast cancer risk. Br J Cancer 98:9–14. doi:10.1038/sj.bjc.6604145
Day JK, Besch Williford C, McMann TR et al (2001) Dietary genistein increased DMBA-induced mammary adenocarcinoma in wild-type, but not ER alpha KO, mice. Nutr Cancer 39:226–232. doi:10.1207/S15327914nc392_11
Ju YH, Allred KF, Allred CD et al (2006) Genistein stimulates growth of human breast cancer cells in a novel, postmenopausal animal model, with low plasma estradiol concentrations. Carcinogenesis 27:1292–1299. doi:10.1093/carcin/bgi370
Messina M, Nagata C, Wu AH (2006) Estimated Asian adult soy protein and isoflavone intakes. Nutr Cancer 55:1–12. doi:10.1207/s15327914nc5501_1
Horn Ross PL, Hoggatt KJ, West DW et al (2002) Recent diet and breast cancer risk: the California Teachers Study (USA). Cancer Cause Control 13:407–415. doi:10.1023/A:1015786030864
Horn Ross PL, John EM, Lee M et al (2001) Phytoestrogen consumption and breast cancer risk in a multiethnic population: the Bay Area Breast Cancer Study. Am J Epidemiol 154:434–441. doi:10.1093/aje/154.5.434
Fink BN, Steck SE, Wolff MS et al (2007) Dietary flavonoid intake and breast cancer risk among women on Long Island. Am J Epidemiol 165:514–523. doi:10.1093/aje/kwk033
Touillaud MS, Thiebaut AC, Niravong M et al (2006) No association between dietary phytoestrogens and risk of premenopausal breast cancer in a French cohort study. Cancer Epidemiol Biomarkers Prev 15:2574–2576. doi:10.1158/1055-9965.EPI-06-0543
Keinan Boker L, Van Der Schouw YT, Grobbee DE et al (2004) Dietary phytoestrogens and breast cancer risk. Am J Clin Nutr 79:282–288
Ferlay J, Bray F, Pisani P et al (2004) GLOBOCAN 2002 Cancer Incidence, Mortality and Prevalence Worldwide, IARC CancerBase No. 5, version 2.0. IARCPress, Lyon
Iwasaki M, Mameri CP, Hamada GS et al (2008) Secular trends in cancer mortality among Japanese Immigrants in the State of São Paulo, Brazil, 1979–2001. Eur J Cancer Prev 17:1–8
Tsubono Y, Takamori S, Kobayashi M et al (1996) A data-based approach for designing a semiquantitative food frequency questionnaire for a population-based prospective study in Japan. J Epidemiol 6:45–53
Yamamoto S, Sobue T, Sasaki S et al (2001) Validity and reproducibility of a self-administered food-frequency questionnaire to assess isoflavone intake in a Japanese population in comparison with dietary records and blood and urine isoflavones. J Nutr 131:2741–2747
Kimira M, Arai Y, Shimoi K et al (1998) Japanese intake of flavonoids and isoflavonoids from foods. J Epidemiol 8:168–175
Arai Y, Watanabe S, Kimira M et al (2000) Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J Nutr 130:2243–2250
The Council for Science, Technology Ministry of Education C, Sports, Science, Technology, Japan (2005) Standard Tables of Food Composition in Japan, the fifth revised and enlarged edition. National Printing Bureau, Tokyo
U.S. Department of Agriculture, Agricultural Research Service, USDA Nutrient Data Laboratory (2006) USDA National Nutrient Database for Standard Reference Release 18
Kuiper GG, Lemmen JG, Carlsson B et al (1998) Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 139:4252–4263. doi:10.1210/en.139.10.4252
Acknowledgments
This study was supported by a Grant-in-Aid for Research on Risk of Chemical Substances from the Ministry of Health, Labour and Welfare of Japan, and Grants-in-Aid for Scientific Research on Priority Areas (17015049) and for Young Scientists (B) (17790378 and 19790415) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and the Japan Society for the Promotion of Science. We are grateful to the participants in the “São Paulo-Japan Breast Cancer Study Group”: C. I. Yamaguchi, C. M. Kunieda, and S. S. Sugama (Nikkei Disease Prevention Center, São Paulo); C. K. Taniguchi and J. A. Marques (Departamento de Ginecologia, Hospital Pérola Byington, São Paulo); M. R. Eichhorn (Departamento de Nutrição, Hospital Pérola Byington, São Paulo); H. Iyeyasu, M. S. Maciel, S. M. T. Carvalho, J. B. D. Collins, and C. E. M. Fontes (Departamento de Mastologia, Hospital A.C. Camargo, São Paulo); L. P. Kowalski and J. M. F. Toyota (Departamento de Cirurgia de Cabeça e Pescoço e Otorrinolaringologia, A. C. Camargo Hospital, São Paulo); E. M. Barbosa (Departamento de Mastologia, Instituto Brasileiro de Controle ao Câncer, São Paulo); O. Ferraro (Departamento de Mastologia, Hospital do Servidor Público Estadual Francisco Morato de Oliveira, São Paulo); R. Anzai (Departamento de Mastologia, Hospital Santa Cruz); E. H. Hotta and D. A. Petti (Instituto de Ginecologia e Mastologia, Hospital Beneficencia Portuguesa); S. Mendes (Instituto Brasileiro de Mastologia e Ginecologia, Hospital Beneficencia Portuguesa).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iwasaki, M., Hamada, G.S., Nishimoto, I.N. et al. Dietary isoflavone intake and breast cancer risk in case–control studies in Japanese, Japanese Brazilians, and non-Japanese Brazilians. Breast Cancer Res Treat 116, 401–411 (2009). https://doi.org/10.1007/s10549-008-0168-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-008-0168-1