Abstract
Purpose
We investigated the association between isoflavone (ISF) intake and hereditary breast cancer (BC) risk, particularly by molecular subtype, in East-Asian BRCA1/2 mutation carriers and non-carriers at a high risk of hereditary breast cancer (i.e., family history of BC (FHBC) and early-onset BC [EOBC, age < 40 years]).
Methods
The association between ISF intake and BC risk by molecular subtypes was assessed in 1709 participants (407 BRCA1/2 carriers, 585 FHBC non-carriers, 586 EOBC non-carriers, and 131 unaffected non-carriers) from the Korean Hereditary Breast Cancer Study using hazard ratios (HRs) and 95% confidence intervals (CIs) in weighted Cox regression models. Daily ISF intake was assessed using a validated food frequency questionnaire. We evaluated gene-environment interactions between BRCA1/2 mutation and ISF intake in 1604 BC cases by calculating the case-only odds ratios (CORs) and 95% CIs in logistic regression models.
Results
ISF intake was inversely associated with luminal A BC risk in BRCA2 mutation carriers and FHBC non-carriers (HR = 0.14, 95% CI = 0.04–0.50 for high intake [ISF intake ≥ 15.50 mg/day]; HR = 0.27, 95% CI = 0.11–0.69 for high intake, respectively). We observed a reduced risk of triple negative BC (TNBC) in BRCA1 carriers and FHBC non-carriers (HR = 0.09, 95% CI = 0.02–0.40 for high intake; HR = 0.19, 95% CI = 0.05–0.69 for high intake, respectively). In the case-only design, an interaction between BRCA1 mutation carrier status and ISF intake emerged in TNBC patients (COR = 0.39, 95% CI = 0.16–0.95).
Conclusions
This study suggests that ISF intake is inversely associated with BC risk in women at high risk of hereditary BC and that the effect could differ by molecular subtypes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer (BC) is a heterogeneous disease with a varying risk of disease progression and therapeutic resistance. Approximately 5%–10% of BC cases are hereditary and are classified into high risk BC groups. Furthermore, 25%–40% of these hereditary BC cases can be attributed to the BC susceptibility genes, BRCA1 and BRCA2 [1]. Non-BRCA mutated BC accounting for the majority of hereditary BC cases, exhibits distinct differences from sporadic BC in the general population in terms of clinical features, molecular biology, and outcomes [2]. Such genes and prevention targets of associated mutations will likely play a critical role in preventing hereditary BC in the future. Previous studies have found a higher incidence of BRCA1/2-associated BC in carriers in more recent birth cohorts [3, 4], suggesting that non-genetic factors may modify the inherited risk of BRCA-mutated BC. Further studies of probable non-surgical factors associated with BC risk will help in developing preventive strategies for high-risk women who would consider a prophylactic mastectomy as a preventive intervention.
Many studies have substantiated the protective effect of soy-derived isoflavones (ISFs) on BC risk in the general population—particularly in the Asian population because soy foods are common in traditional Asian foods [5,6,7,8,9,10]. Soy-based foods contain high ISF concentrations, including mainly genistein and daidzein, and these have a similar structure to estradiol. Previous experimental studies have reported the potential biological mechanisms of the anti-carcinogenic effects of ISFs in the context of BC, including the regulation of estrogenic effects, apoptosis, cell proliferation and survival, inhibition of angiogenesis, and antioxidant effects [11]. However, whether ISF intake has a similar effect on BC patients with a high familial risk remains unclear. Our preliminary study suggested an inverse association between soy product consumption and the risk of hereditary BC in the Korean Hereditary BC (KOHBRA) study [12]. However, this study assessed the protective effect of soy intake only by counting the number of soy products consumed more than once a week (0–1, 2, 3, and 4–5 soy products), which has limited utility in demonstrating the effect of ISFs as a putative chemopreventive agent on BC risk. Furthermore, it did not consider this association by BC molecular subtype.
Thus, we aimed to investigate the association between ISF intake and BC risk in women at high risk of hereditary BC, such as BRCA1/2 mutation carriers and non-carriers meeting the high risk criteria of BRCA mutations, including non-carriers with a BC family history (FHBC non-carriers) and non-carriers with early-onset BC (EOBC non-carriers, diagnosed with BC before age 40), particularly by BC molecular subtypes.
Participants and methods
Study population and design
The KOHBRA study is a nationwide, multicenter cohort study that was conducted from 2007 to 2014 to estimate the prevalence of BRCA1/2 mutations among women at high risk of hereditary BC, and to identify clinical characteristics and prognostic factors of BRCA1/2-related BC. The eligibility criteria for participation were the following: familial BC patients with first- or second-degree relatives and non-familial BC patients at a high risk of hereditary BC, such as male BC, early-onset BC, bilateral BC patients, or BC patients with another primary malignancy. Patients underwent genetic testing for BRCA mutations—those who were positive were advised to recruit family members who were at least 20 years old and who agreed to participate in the study. The details of the KOHBRA study are described elsewhere [13].
Of the 2962 KOHBRA participants at baseline, our study only included females (n = 2810) (Fig. 1). We excluded subjects with no information on the date of genetic testing or interview (n = 157). We also excluded participants with ovarian cancer or first- or second-degree relatives with ovarian cancer for the analysis in BRCA non-carriers (n = 213), those with insufficient dietary information collected using a food frequency questionnaire (FFQ) during the interview (n = 96), 484 affected participants who participated in the study more than six months after BC diagnosis, and 151 affected non-carriers older than 40 years who did not have a family history of BC. Finally, we included 1709 cohort participants: 407 BRCA1/2 mutation carriers (153 BRCA1 mutation carriers and 254 BRCA2 mutation carriers) and 1302 non-carriers (585 FHBC non-carriers, 586 EOBC non-carriers, and 131 unaffected non-carriers).
Flow chart of study subjects selection in the Korean Hereditary BC Study, 2007–2014. 1These patients were used in the case-only gene-environment interaction study. 2Same unaffected non-carriers were used in comparing with both of BC patients with family history and early-onset BC patients. BC Breast cancer
Data collection and definition
Data on general lifestyle, reproductive factors, family history of malignancies, and diet were collected using a structured questionnaire. Dietary information was collected using a semi-quantitative FFQ that was developed and validated for the Korean Genome Epidemiology Study [14, 15]. The FFQ included 103 food items to assess the usual dietary intake during the 12 months prior to enrollment in the KOHBRA study. The frequency of intake of each food item was classified into nine levels: “never or little”, “once a month”, “two to three times a month”, “one to two times a week”, “three to four times a week”, “five to six times a week”, “once a day”, “twice a day”, and “three times or more a day”. The portion size of each food was classified as “less than standard”, “standard”, and “more than standard”. The daily ISF intake was estimated by multiplying the frequency of consumption of each food, the portion size, and the ISF content obtained from the standardized food and nutrient composition database published by the Korean Nutrition Society [16]; the intake was summed across all food items. We grouped the participants into three groups, with the optimal ISF intake cut-points determined using a restricted spline survival analysis [17]: low intake, 0–7.99 mg/day; intermediate intake, 8.00–15.49 mg/day; and high intake, ≥ 15.50 mg/day. Clinical information regarding patient characteristics, diagnosis, and treatment was collected from a medical record review. Molecular subtypes of BC were defined by the 2011 St. Gallen Consensus based on immunohistochemistry results for estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), and Ki-67 and the FISH results for HER2: luminal A (ER+ and/or PR+ , and HER2− and low Ki-67), luminal B (ER+ and/or PR+ , HER2− and/or high Ki-67), HER2−enriched (ER− and PR− and HER2 +) and triple negative BC (TNBC) (ER− and PR− and HER2−) [18]. According to a population-based case–control study in Korea, the distribution of molecular subtypes of BC in the general population are different from that of women in high risk of hereditary BC, including luminal A (30.8%), luminal B (22.0%), HER2-enriched (11.5%), TNBC (15.9%), and unclassified BC (19.8%) [19].
We collected blood samples at baseline and used them for BRCA1/2 mutation testing within 24 h of sampling. BRCA genetic testing was performed using genomic DNA from peripheral blood via Sanger’s sequencing. The BRCA1/2 mutation carriers were defined as those who had the protein-truncating mutations or the missense mutation on the BRCA1/2 genes [13]. A review of 14 studies on non-BRCA familial BC suggests well-defined criteria, including the following for mutation-negative “BRCA X” cases: early-onset diagnosis of BC or having one or more first- or second-degree relatives with BC [20]. According to a recent study addressing tumor heterogeneity between these two traits in non-carriers [21], we defined two separate groups in the same way: non-carriers having one or more first- or second-degree relatives with BC at any age and non-carriers diagnosed with BC before 40 years of age. We confined this study to women with no personal or family history of ovarian cancer because ovarian cancer may have other traits distinct from those two groups.
Retrospective cohort analysis
We conducted a retrospective cohort analysis as an optimal study design to investigate the association between ISF intake and BRCA1/2-related BC risk, as shown in previous studies [22, 23]. Because the BRCA1/2 gene testing guidelines in Korea had not been fully established when the study began, we could not find the number of affected BRCA1/2 mutation carriers and were unable to collect data on the unaffected carriers for the prospective cohort. Additionally, women determined to contain a BRCA1/2 mutation may consider preventive measures or lifestyle changes to lower the risk of developing BC, which also complicates a prospective cohort study. In this study, we modeled time to first BC diagnosis from birth, censoring at the age at baseline interview or genetic testing or at the last follow-up at the end of 2014, whichever occurred first. All participants were unaware that they carried the BRCA1/2 mutation during the retrospective follow-up. BRCA1/2 mutation carriers were not randomly selected with respect to BC status. To correct for this potential testing bias, all analyses of the retrospective cohort were conducted using the weighted cohort approach developed by Antoniou et al. because a standard Cox proportional hazard model can lead to a biased estimate of the HR in this study design [24]. This method involves assigning relative weights reflecting their sampling probabilities to all person-years of each study participant. We computed the weights by obtaining the age-specific penetrance of BRCA1 and BRCA2 mutations estimated by the meta-analysis of Antoniou et al. for carriers [3] and the age-specific incidence of BC in the Korean population from the Korean Central Cancer Registry in 2010 for non-carriers (Supplemental Table 1).
Statistical analysis
We compared the baseline characteristics for hereditary BC between affected and unaffected participants, using the chi-square test or Fisher’s exact test for categorical variables, and Student’s t-test for continuous variables. The association between ISF intake and BC risk in women at high risk of hereditary BC across the four groups (BRCA1/2 mutation carriers, FHBC non-carriers, and EOBC non-carriers) was assessed using a weighted Cox proportional hazards model with age as the timescale by estimating the HRs and 95% CIs. No major violation of the proportional hazard assumption was identified, suggesting that the HRs did not vary with the exposure (ISF intake) over time in a Cox proportional regression model, in which one-year dietary information can be extrapolated to lifetime dietary information. A robust variance–covariance estimation method was used to correct for potential correlations of related individuals from the same family [25]. A dose–response relationship for ISF intake was estimated by entering intake amount as a continuous variable in the model. The weighted Cox proportional regression models were intrinsically stratified for birth cohort groups (< 1963, 1964–1971, 1972–1976, and 1977+), clustered to 154 families to correct for potential within-family correlations in risk factors, and adjusted for marriage, parity, family history of ovarian cancer, alcohol consumption, regular physical activity, and total energy intake.
We assessed the effect of ISF intake on the risk of BC by three major molecular subtypes of BC: luminal A BC, luminal B BC, and TNBC. We have assessed the association with luminal BC by combining luminal A BC and luminal B BC, but could not investigate this association with HER2-overexpressed BC due to the small sample size. We employed a case-only study design to assess potential gene-environment interactions between ISF intake and BRCA1/2 gene mutations in the affected participants by estimating the case-only odds ratios (CORs) and 95% CIs from multiple logistic regression models, assuming that genetic and environmental factors are independent [26]. Statistical analyses were conducted using SAS 9.4 software (SAS Institute, Cary, NC).
Results
We evaluated the effect of ISF consumption on the risk of BC in a cohort of 1709 East-Asian females at high risk of hereditary BC (153/254 BRCA1/2 carriers, 585 affected FHBC non-carriers, 586 EOBC non-carriers, and 131 unaffected non-carriers). The affected BRCA2 carriers and FHBC non-carriers were older than the unaffected participants, whereas the EOBC non-carriers were younger than the unaffected non-carriers, as shown in Supplemental Table 2. The proportion of patients with a history of marriage and pregnancy was higher among the affected BRCA1/2 mutation carriers and affected non-carriers compared to the unaffected non-carriers. A higher proportion of postmenopausal women comprised affected BRCA2 carriers, whereas a relatively lower proportion of EOBC non-carriers had gone through menopause than unaffected non-carriers.
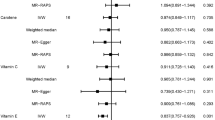
Among all participants, the ISF intake was significantly associated with a lower risk of BC in BRCA2 mutation carriers and FHBC non-carriers (Tables 1 and 2) (p-trend < 0.01, HR = 0.23, 95% CI = 0.08–0.68 for high intake [ISF intake ≥ 15.50 mg/day]; p-trend = 0.02, HR = 0.42, 95% CI = 0.19–0.97 for high intake, respectively).
In terms of BC molecular subtypes, ISF intake was inversely associated with the risk of TNBC among BRCA1 mutation carriers and FHBC non-carriers (p-trend = 0.01, HR = 0.09, 95% CI = 0.02–0.40 for high intake; p-trend = 0.01, HR = 0.19, 95% CI = 0.05–0.69 for high intake, respectively), as shown in Tables 1 and 2 and Fig. 2a. A significant luminal A BC risk reduction according to ISF intake was found in BRCA2 mutation carriers and FHBC non-carriers (p-trend < 0.01, HR = 0.14, 95% CI = 0.04–0.50 for high intake; p-trend < 0.01, HR = 0.27, 95% CI = 0.11–0.69 for high intake, respectively). Similar inverse trends were shown in both BRCA1 and BRCA2 mutation carriers for luminal BC risk in which luminal A BC and luminal B BC were combined. We additionally observed a similar inverse association on HR-negative BC in BRCA1 carriers and HR-positive BC in BRCA2 carriers in terms of the expression of HRs (HR = 0.14, 95% CI = 0.03–0.54 for high intake; HR = 0.18, 95% CI = 0.06–0.62 for high intake, respectively) (Fig. 2b; Supplemental Table 3). For the expression of HER2, high ISF intake was also correlated with a lower risk of HER2-negative BC in BRCA1 carriers, BRCA2 carriers, and FHBC non-carriers (HR = 0.24, 95% CI = 0.07–0.90; HR = 0.15, 95% CI = 0.04–0.52; HR = 0.27, 95% CI = 0.11–0.66, respectively).
In a case-only design using 1604 BC cases, high ISF intake was inversely associated with the risk of BRCA-related BC (COR = 0.66, 95% CI = 0.44–0.98), particularly with BRCA-related TNBC (COR = 0.42, 95% CI = 0.19–0.95), as shown in Table 3. The effect of the interaction between the gene (BRCA) and environment (ISF) was persistent in the BRCA1-associated TNBC risk (COR = 0.39, 95% CI = 0.16–0.95 for high intake). Because both an inverse association of ISF intake and an interactive effect of ISF intake on BRCA1-related BC were identified, we further investigated which sources of ISF could explain this association, as shown in Supplemental Table 4. We found that soy-derived ISF intake could significantly affect BRCA1-related TNBC risk compared with ISFs derived from vegetables (HR = 0.47 for soy product intake two to three times per week; HR = 0.17 for soy product intake more than four times per week).
Discussion
In this study, a high ISF intake was found to be beneficial for BC risk in BRCA2 mutation carriers and non-carriers with FHBC in the total population. Since BC is known to have different clinical prognoses depending on the molecular subtype, we wanted to observe whether the preventive effect of ISFs from BC was stronger in certain BC molecular subtypes. In the analysis of BC by molecular subtypes, a high ISF intake was significantly associated with a reduced risk of BRCA1-associated TNBC and BRCA2-associated luminal A BC. For FHBC non-carriers, a reduced risk of BC was observed with increased ISF intake in both luminal A BC and TNBC. In the case-only analysis, a high ISF intake was negatively associated with the BRCA mutation in the overall BC cases, and the presence of a negative interaction was identified with the BRCA1 mutant gene with respect to TNBC.
The favorable associations between soy food or ISF intake and overall BC risk in Asian women were summarized in two recent meta-analyses [27, 28]. By contrast, ISF intake was not correlated with BC risk in studies conducted in Western women consuming relatively low levels of ISF (median highest ISF intake ≥ 0.8 mg/day). The results of our study for Korean women at high risk of hereditary BC are generally in accordance with epidemiological studies conducted in Asian women.
Several epidemiological studies have assessed the association between soy or ISF intake and BC risk stratified by HR and HER2 status in the general population; however, these studies have yielded inconsistent findings. The Shanghai Breast Cancer Study found a greater reduction in risk of BC for ER+/PR+ status than those with other ER/PR status [5]. In other case–control studies, the protective effect of soy products against BC risk was similar across all subtypes of ER/PR status [9, 10, 29]. Two cohort studies also reported similar association across all subtypes of ER/PR status [8, 30]. Among these studies, Baglia et al. observed a significantly decreased risk of ER-/PR- BC only in premenopausal women and that of ER+/PR+ BC only in postmenopausal women [30]. However, another case–control study found no significant association according to HR status [31]. With respect to the HER2 status, in a case–control study in Japan, high levels of intake of soybean products significantly reduced the risk (21%) of HER2-negative BC [6]; however, the cohort study of Baglia et al. found no significant association according to HER2 status [30]. To date, only Suzuki et al. have investigated the impact of intake of soybean products on BC risk according to joint receptor status; they observed a beneficial effect in HER+/HER2− BC (top tertile OR = 0.73, 95% CI = 0.54–0.97). In our study, ISF intake was associated with decreased risk of HR-positive and HR-negative BC; however, the association differed by BRCA1/2 mutation status and in subgroups of non-carriers who are at high risk of hereditary BC. Our results are, to some extent, inconsistent with the findings from other observational studies conducted in the general population; this may be attributable to the fact that the other studies did not account for the BRCA1/2 status.
Several biologically plausible mechanisms may explain the protective effect of ISF intake against hereditary BC observed in our study. Soy ISFs have structural similarities to estradiol; previous studies suggest that these may mediate biological phenomenon such as cell proliferation, differentiation, or apoptosis by competitively binding to ERs with endogenous estrogen through the modulation of estrogen-signaling pathways [11]. Other molecular mechanisms of action of ISFs may also explain the protective effect of ISF intake against hereditary BC, particularly HR-independent BC; these include apoptosis induction, anti-proliferative and anti-inflammatory effects, induction of cell cycle arrest via inhibition of the activity of tyrosine protein kinase, mitogen-activated kinase, or DNA topoisomerase II, and inhibition of angiogenesis [11, 32].
In terms of BRCA1/2 mutation status, the effect of ISF intake on BRCA1-mutated TNBC observed in our study is consistent with the results of a previous experimental study in which genistein treatment was shown to inhibit the proliferation and growth of TNBC cells by targeting G protein-coupled receptor 30 (GPR3), which led to downregulation of Akt and Cyclin B1 expression in cell cycle progression in the G2/M phase naly the BRCA1 mutated condition [33]. This particular study also observed similar gene expression in ER-positive BRCA1 mutant cell lines, although BRCA1 mutant TNBC cells were apparently more sensitive to genistein, which suggested that ISF intake also plays a role in BRCA1-mutated HR-positive BC. However, we could not demonstrate a significant association in our study due to the small sample size. The beneficial effect of ISF intake against BRCA2-mutated BC may also be explained by upregulating BRCA1/2 genes and inducing apoptosis under BRCA2 knockdown conditions, according to another previous experimental study [34]. Many studies have investigated the biologically plausible nalyzing of the protective effect of ISF against BC; however, the precise molecular mechanisms are yet to be fully elucidated, particularly for hereditary BC related BRCA1/2 mutation status.
One of the limitations of this study is the relatively small sample size of subgroups according to the presence of BRCA1/2 mutations and molecular subtypes of BC, which may limit the study power and hamper the generalizability to women at high risk of hereditary BC. We could not obtain significant results for HR-positive BRCA1 mutant BC and HR-negative BRCA2-mutant BC owing to the small sample size due to BC heterogeneity, including the finding that BRCA1 mutation carriers are closely linked to ER-negative BC and TNBC, whereas BRCA2 mutated tumors are linked to ER-positive BC [35]. In our cohort, patients with HR-positive BRCA1 mutated BC accounted for only 17% of all patients with BRCA1-mutated BC. Women in Korea have a higher prevalence of BRCA2 rather than BRCA1 mutations, which is similar result to that in most Asian countries [36]. Due to its heterogeneity, however, we could not perform combined analyses with BRCA1/2 mutation carriers and molecular subtypes to increase the statistical power. Further large-scale investigations are required to understand the relation between ISF intake and BC in these groups. To the best of our knowledge, however, no prior epidemiological studies have assessed the potential benefits of ISF against the risk of BRCA1/2-mutated BC according to molecular subtypes. Second, the FFQ, used to collect dietary information, reflects only dietary intake in the year before study enrollment. Some of the affected individuals may have modified their dietary behavior after BC diagnosis, which may have caused a temporary bias. To avoid this bias, we excluded BC patients who participated in the study more than six months after their BC diagnosis. Finally, we could not consider equol-producing status in relation to BC risk. Equol, a metabolite of daidzein, is produced differently depending on human intestinal bacteria. Since equol has been reported to bind with greater affinity to the ERβ protein, which may lead to a lower BC risk for equol producers, assessing equol-producing status might contribute to better findings for this study [37]. Our study, however, is one of few observational studies supporting several experimental studies on the association between ISF intake and hereditary BC risk in BRCA1/2 carriers and high-risk non-carriers. We also examined whether this association would be modified by different types of molecular BC in women at high risk of hereditary BC.
Based on our findings, we propose that high ISF intake in women at high risk of hereditary BC can act as a preventive factor against BC, particularly in BRCA2-mutated luminal A type BC and BRCA1-mutated TNBC. We also suggest that ISF intake may interact with BRCA1 mutations to decrease the risk of TNBC for whom the chemotherapeutic regimen has not yet been established. However, our findings warrant further investigation, including large-scale perspective cohort studies and intervention studies, to evaluate the novel preventive or therapeutic approach of ISF intake on developing BC risk to women at high risk of hereditary BC and to clarify the mechanistic interaction by which ISF intake can alter the genetic risk of BRCA1 mutated genes on TNBC.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BC:
-
Breast cancer
- COR:
-
Case-only odds ratio
- EOBC:
-
Early-onset breast cancer
- ER:
-
Estrogen receptor
- FFQ:
-
Food frequency questionnaire
- FHBC:
-
Family history of breast cancer
- HER2:
-
Human epidermal growth factor receptor 2
- HR:
-
Hormone receptor
- IKK:
-
IkB kinase
- ISF:
-
Isoflavone
- KOHBRA:
-
Korean Hereditary Breast Cancer Study
- NF-kB:
-
Nuclear factor kappa B
- PR:
-
Progesterone receptor
References
Claus EB, Schildkraut JM, Thompson WD, Risch NJ (1996) The genetic attributable risk of breast and ovarian cancer. Cancer 77(11):2318–2324. https://doi.org/10.1002/(SICI)1097-0142(19960601)77:11<2318:AID-CNCR21>3.0.CO;2-Z
Arpino G, Pensabene M, Condello C, Ruocco R, Cerillo I, Lauria R, Forestieri V, Giuliano M, De Angelis C, Montella M, Crispo A, De Placido S (2016) Tumor characteristics and prognosis in familial breast cancer. BMC Cancer 16(1):924. https://doi.org/10.1186/s12885-016-2962-1
Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, Loman N, Olsson H, Johannsson O, Borg A, Pasini B, Radice P, Manoukian S, Eccles DM, Tang N, Olah E, Anton-Culver H, Warner E, Lubinski J, Gronwald J, Gorski B, Tulinius H, Thorlacius S, Eerola H, Nevanlinna H, Syrjakoski K, Kallioniemi OP, Thompson D, Evans C, Peto J, Lalloo F, Evans DG, Easton DF (2003) Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet 72(5):1117–1130. https://doi.org/10.1086/375033
Narod S, Lynch H, Conway T, Watson P, Feunteun J, Lenoir G (1993) Increasing incidence of breast cancer in family with BRCA1 mutation. Lancet 341(8852):1101–1102
Dai Q, Shu XO, Jin F, Potter JD, Kushi LH, Teas J, Gao YT, Zheng W (2001) Population-based case-control study of soyfood intake and breast cancer risk in Shanghai. Br J Cancer 85(3):372–378. https://doi.org/10.1054/bjoc.2001.1873
Suzuki T, Matsuo K, Tsunoda N, Hirose K, Hiraki A, Kawase T, Yamashita T, Iwata H, Tanaka H, Tajima K (2008) Effect of soybean on breast cancer according to receptor status: a case-control study in Japan. Int J Cancer 123(7):1674–1680. https://doi.org/10.1002/ijc.23644
Wada K, Nakamura K, Tamai Y, Tsuji M, Kawachi T, Hori A, Takeyama N, Tanabashi S, Matsushita S, Tokimitsu N, Nagata C (2013) Soy isoflavone intake and breast cancer risk in Japan: from the Takayama study. Int J Cancer 133(4):952–960. https://doi.org/10.1002/ijc.28088
Wu AH, Koh WP, Wang R, Lee HP, Yu MC (2008) Soy intake and breast cancer risk in Singapore Chinese Health Study. Br J Cancer 99(1):196–200. https://doi.org/10.1038/sj.bjc.6604448
Zhang C, Ho SC, Lin F, Cheng S, Fu J, Chen Y (2010) Soy product and isoflavone intake and breast cancer risk defined by hormone receptor status. Cancer Sci 101(2):501–507. https://doi.org/10.1111/j.1349-7006.2009.01376.x
Zhang M, Yang H, Holman CD (2009) Dietary intake of isoflavones and breast cancer risk by estrogen and progesterone receptor status. Breast Cancer Res Treat 118(3):553–563. https://doi.org/10.1007/s10549-009-0354-9
Uifalean A, Schneider S, Ionescu C, Lalk M, Iuga CA (2015) Soy isoflavones and breast cancer cell lines: molecular mechanisms and future perspectives. Molecules 21(1):E13. https://doi.org/10.3390/molecules21010013
Ko KP, Kim SW, Ma SH, Park B, Ahn Y, Lee JW, Lee MH, Kang E, Kim LS, Jung Y, Cho YU, Lee B, Lin JH, Park SK (2013) Dietary intake and breast cancer among carriers and noncarriers of BRCA mutations in the Korean Hereditary Breast Cancer Study. Am J Clin Nutr 98(6):1493–1501. https://doi.org/10.3945/ajcn.112.057760
Han SA, Park SK, Ahn SH, Lee MH, Noh DY, Kim LS, Noh WC, Jung Y, Kim KS, Kim SW, Korean Breast Cancer Study Group (2011) The Korean hereditary breast cancer (KOHBRA) study: protocols and interim report. Clin Oncol 23(7):434–441. https://doi.org/10.1016/j.clon.2010.11.007
Ahn YJ, Lee JE, Paik HY, Lee HK, Jo IH, Kim KC (2003) Development of a semi-quantitative food frequency questionnaire based on dietary data from the Korea national health and nutrition examination survey. Nutr Sci 6(3):173–184
Ahn Y, Kwon E, Shim JE, Park MK, Joo Y, Kimm K, Park C, Kim DH (2007) Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur J Clin Nutr 61(12):1435–1441. https://doi.org/10.1038/sj.ejcn.1602657
The Korean Nutrition Society (2009) Food values. The Korean Nutrition Society, Seoul
Chen Y, Huang J, He X, Gao Y, Mahara G, Lin Z, Zhang J (2019) A novel approach to determine two optimal cut-points of a continuous predictor with a U-shaped relationship to hazard ratio in survival data: simulation and application. BMC Med Res Methodol 19(1):96. https://doi.org/10.1186/s12874-019-0738-4
Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ, Panel M (2011) Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St Gallen. International expert consensus on the primary therapy of early breast cancer 2011. Ann Oncol 22(8):1736–1747. https://doi.org/10.1093/annonc/mdr304
Jeong SH, An YS, Choi JY, Park B, Kang D, Lee MH, Han W, Noh DY, Yoo KY, Park SK (2017) Risk reduction of breast cancer by childbirth, breastfeeding, and their interaction in Korean women: heterogeneous effects across menopausal status, hormone receptor status, and pathological subtypes. J Prev Med Public Health 50(6):401–410. https://doi.org/10.3961/jpmph.17.152
Keeney MG, Couch FJ, Visscher DW, Lindor NM (2017) Non-BRCA familial breast cancer: review of reported pathology and molecular findings. Pathology 49(4):363–370. https://doi.org/10.1016/j.pathol.2017.03.002
Park B, Hopper JL, Win AK, Dowty JG, Sung HK, Ahn C, Kim SW, Lee MH, Lee J, Lee JW, Kang E, Yu JH, Kim KS, Moon BI, Han W, Noh DY, Park SK, KOHBRA Study Group (2017) Reproductive factors as risk modifiers of breast cancer in BRCA mutation carriers and high-risk non-carriers. Oncotarget 8(60):102110–102118. https://doi.org/10.18632/oncotarget.22193
Antoniou AC, Rookus M, Andrieu N, Brohet R, Chang-Claude J, Peock S, Cook M, Evans DG, Eeles R, EMBRACE, Nogues C, Faivre L, Gesta P, GENEPSO, van Leeuwen FE, Ausems MG, Osorio A, GEO-HEBON, Caldes T, Simard J, Lubinski J, Gerdes AM, Olah E, Furhauser C, Olsson H, Arver B, Radice P, Easton DF, Goldgar DE (2009) Reproductive and hormonal factors, and ovarian cancer risk for BRCA1 and BRCA2 mutation carriers: results from the International BRCA1/2 Carrier Cohort Study. Cancer Epidemiol Biomarkers Prev 18(2):601–610. https://doi.org/10.1158/1055-9965.EPI-08-0546
Schrijver LH, Olsson H, Phillips KA, Terry MB, Goldgar DE, Kast K, Engel C, Mooij TM, Adlard J, Barrowdale D, Davidson R, Eeles R, Ellis S, Evans DG, Frost D, Izatt L, Porteous ME, Side LE, Walker L, Berthet P, Bonadona V, Leroux D, Mouret-Fourme E, Venat-Bouvet L, Buys SS, Southey MC, John EM, Chung WK, Daly MB, Bane A, van Asperen CJ, Gomez Garcia EB, Mourits MJE, van Os TAM, Roos-Blom MJ, Friedlander ML, McLachlan SA, Singer CF, Tan YY, Foretova L, Navratilova M, Gerdes AM, Caldes T, Simard J, Olah E, Jakubowska A, Arver B, Osorio A, Nogues C, Andrieu N, Easton DF, van Leeuwen FE, Hopper JL, Milne RL, Antoniou AC, Rookus MA, EMBRACE, GENEPSO, BCFR, HEBON, kConFab, IBCCS (2018) Oral contraceptive use and breast cancer risk: retrospective and prospective analyses from a BRCA1 and BRCA2 mutation carrier cohort study. JNCI Cancer Spectr 2(2):pky023. https://doi.org/10.1093/jncics/pky023
Antoniou AC, Goldgar DE, Andrieu N, Chang-Claude J, Brohet R, Rookus MA, Easton DF (2005) A weighted cohort approach for analysing factors modifying disease risks in carriers of high-risk susceptibility genes. Genet Epidemiol 29(1):1–11. https://doi.org/10.1002/gepi.20074
Lin DY, Wei LJ (1989) The robust inference for the cox proportional hazards model. J Am Stat Assoc 84(408):1074–1078. https://doi.org/10.1080/01621459.1989.10478874
Piegorsch WW, Weinberg CR, Taylor JA (1994) Non-hierarchical logistic models and case-only designs for assessing susceptibility in population-based case-control studies. Stat Med 13(2):153–162
Wu AH, Yu MC, Tseng CC, Pike MC (2008) Epidemiology of soy exposures and breast cancer risk. Br J Cancer 98(1):9–14. https://doi.org/10.1038/sj.bjc.6604145
Dong JY, Qin LQ (2011) Soy isoflavones consumption and risk of breast cancer incidence or recurrence: a meta-analysis of prospective studies. Breast Cancer Res Treat 125(2):315–323. https://doi.org/10.1007/s10549-010-1270-8
Cho YA, Kim J, Park KS, Lim SY, Shin A, Sung MK, Ro J (2010) Effect of dietary soy intake on breast cancer risk according to menopause and hormone receptor status. Eur J Clin Nutr 64(9):924–932. https://doi.org/10.1038/ejcn.2010.95
Baglia ML, Zheng W, Li H, Yang G, Gao J, Gao YT, Shu XO (2016) The association of soy food consumption with the risk of subtype of breast cancers defined by hormone receptor and HER2 status. Int J Cancer 139(4):742–748. https://doi.org/10.1002/ijc.30117
Iwasaki M, Hamada GS, Nishimoto IN, Netto MM, Motola J Jr, Laginha FM, Kasuga Y, Yokoyama S, Onuma H, Nishimura H, Kusama R, Kobayashi M, Ishihara J, Yamamoto S, Hanaoka T, Tsugane S (2009) Dietary isoflavone intake and breast cancer risk in case-control studies in Japanese, Japanese Brazilians, and non-Japanese Brazilians. Breast Cancer Res Treat 116(2):401–411. https://doi.org/10.1007/s10549-008-0168-1
Tuli HS, Tuorkey MJ, Thakral F, Sak K, Kumar M, Sharma AK, Sharma U, Jain A, Aggarwal V, Bishayee A (2019) Molecular mechanisms of action of genistein in cancer: recent advances. Front Pharmacol 10:1336. https://doi.org/10.3389/fphar.2019.01336
Kim GY, Suh J, Jang JH, Kim DH, Park OJ, Park SK, Surh YJ (2019) Genistein inhibits proliferation of BRCA1 mutated breast cancer cells: the GPR30-Akt axis as a potential target. J Cancer Prev 24(4):197–207. https://doi.org/10.15430/JCP.2019.24.4.197
Bernard-Gallon DJ, Satih S, Chalabi N, Rabiau N, Bosviel R, Fontana L, Bignon YJ (2010) Phytoestrogens regulate the expression of genes involved in different biological processes in BRCA2 knocked down MCF-7, MDA-MB-231 and MCF-10a cell lines. Oncol Rep 23(3):647–653. https://doi.org/10.3892/or_00000680
Mavaddat N, Barrowdale D, Andrulis IL, Domchek SM, Eccles D, Nevanlinna H, Ramus SJ, Spurdle A, Robson M, Sherman M, Mulligan AM, Couch FJ, Engel C, McGuffog L, Healey S, Sinilnikova OM, Southey MC, Terry MB, Goldgar D, O’Malley F, John EM, Janavicius R, Tihomirova L, Hansen TV, Nielsen FC, Osorio A, Stavropoulou A, Benitez J, Manoukian S, Peissel B, Barile M, Volorio S, Pasini B, Dolcetti R, Putignano AL, Ottini L, Radice P, Hamann U, Rashid MU, Hogervorst FB, Kriege M, van der Luijt RB, HEBON, Peock S, Frost D, Evans DG, Brewer C, Walker L, Rogers MT, Side LE, Houghton C, EMBRACE, Weaver J, Godwin AK, Schmutzler RK, Wappenschmidt B, Meindl A, Kast K, Arnold N, Niederacher D, Sutter C, Deissler H, Gadzicki D, Preisler-Adams S, Varon-Mateeva R, Schonbuchner I, Gevensleben H, Stoppa-Lyonnet D, Belotti M, Barjhoux L, GEMO Study Collaborators, Isaacs C, Peshkin BN, Caldes T, de la Hoya M, Canadas C, Heikkinen T, Heikkila P, Aittomaki K, Blanco I, Lazaro C, Brunet J, Agnarsson BA, Arason A, Barkardottir RB, Dumont M, Simard J, Montagna M, Agata S, D’Andrea E, Yan M, Fox S, kConFab Investigators, Rebbeck TR, Rubinstein W, Tung N, Garber JE, Wang X, Fredericksen Z, Pankratz VS, Lindor NM, Szabo C, Offit K, Sakr R, Gaudet MM, Singer CF, Tea MK, Rappaport C, Mai PL, Greene MH, Sokolenko A, Imyanitov E, Toland AE, Senter L, Sweet K, Thomassen M, Gerdes AM, Kruse T, Caligo M, Aretini P, Rantala J, von Wachenfeld A, Henriksson K, SWE-BRCA Collaborators, Steele L, Neuhausen SL, Nussbaum R, Beattie M, Odunsi K, Sucheston L, Gayther SA, Nathanson K, Gross J, Walsh C, Karlan B, Chenevix-Trench G, Easton DF, Antoniou AC, Consortium of Investigators of Modifiers of BRCA1/2 (2012) Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol Biomarkers Prev 21(1):134–147. https://doi.org/10.1158/1055-9965.EPI-11-0775
Kim H, Choi DH (2013) Distribution of BRCA1 and BRCA2 mutations in Asian patients with breast cancer. J Breast Cancer 16(4):357–365. https://doi.org/10.4048/jbc.2013.16.4.357
Virk-Baker MK, Barnes S, Krontiras H, Nagy TR (2014) S-(-)equol producing status not associated with breast cancer risk among low isoflavone-consuming US postmenopausal women undergoing a physician-recommended breast biopsy. Nutr Res 34(2):116–125. https://doi.org/10.1016/j.nutres.2013.12.002
Acknowledgements
We thank all investigators of the KOHBRA study : Beom Seok Kwak, Byeong-Woo Park, Byung Ho Son, Byung-In Moon, Cha Kyong Yom, Chan Heun Park, Chan Seok Yoon, Chang Hyun Lee, Dae Sung Yoon, DongYoung Noh, Doo Ho Choi, Eundeok Chang, Eun-Kyu Kim, Eunyoung Kang, Hae Kyung Lee, Hai-Lin Park, Hyde Lee, Hyeong-Gon Moon, Hyun-Ah Kim, Il-Kyun Lee, Jeong Eon Lee, Jihyoun Lee, Jong Won Lee, Jong-Han Yu, Joon Jeong, Jung Han Yoon, Jung-Hyun Yang, Keumhee Kwak, Ki-Tae Hwang, Ku Sang Kim, Lee Su Kim, Min Hee Hur, Min Ho Park, Min-Hyuk Lee, Myung Chul Chang, Nam Sun Paik, Sang Ah Han, Sang Seol Jung, Sang Uk Woo, Se Jeong Oh, Sehwan Han, Sei Joong Kim, Sei-Hyun Ahn, Seok-Jin Nam, Seung Sang Ko, Sung Hoo Jung, Sung Soo Kang, Sung Yong Kim, Sung-Won Kim, Tae Hyun Kim, Tae Wan Won, Tae Woo Kang, Wonshik Han, Woo-Chul Noh, Yong Lai Park, Yongsik Jung, Young Jin Suh, Young Tae Bae, Young Up Cho, Young-Ik Hong, Sue K. Park, Yoon Joo Jung, Su Yun Choi, Young Bum Yoo, Soo-Jung Lee.
Funding
This work was supported by the Seoul National University Research Grant in 2016. This study was partially supported by a grant from the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (1020350).
Author information
Authors and Affiliations
Contributions
The authors’ responsibilities were as follows—Eun Ji Sim, Kwang-Pil Ko, Choonghyun Ahn, Sang Min Park, Young-Joon Surh, and Sue K. Park: designed research; Eun Ji Sim, Kwang-Pil Ko, Choonghyun Ahn, and Sue K. Park: conducted research; Sung-Won Kim, Min-Hyuk Lee, Jong Won Lee, Jeng Eon Lee, Ku Sang Kim, Cha Kyong Yom, Hyun-Ah Kim, and Sue K. Park: performed data collection; Eun Ji Sim, Kwang-Pil Ko, Choonghyun Ahn, Seokyung An and Sue K. Park: analyzed data or performed statistical analyses; Eun Ji Sim, Kwang-Pil Ko and Sue K. Park: wrote the draft of the manuscript paper; Sue K. Park: had primary responsibility for final content; all authors: read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedure performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was registered at clinicaltrials.gov as NCT00595348, and approved by the Institutional Review Boards of Seoul National University (IRB number: C-0709–050-219) and all ethics committees of each participating centers.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sim, E.J., Ko, KP., Ahn, C. et al. Isoflavone intake on the risk of overall breast cancer and molecular subtypes in women at high risk for hereditary breast cancer. Breast Cancer Res Treat 184, 615–626 (2020). https://doi.org/10.1007/s10549-020-05875-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-05875-0