Abstract
High frequency oscillations (HFO) are known as markers of epileptic areas in intracranial EEG and possibly scalp EEG. We compared distributions of HFO in the ripple band (80–250 Hz) in intracranial and scalp EEG with either a conventional 10–20-montage (10–20-EEG) or a high density recording using 128 electrodes (HD-EEG). HFO were visually identified, in all intracranial EEG channels (80–500 Hz) and all channels of the 10–20-EEG (scalp EEG 80–250 Hz). For the HD-EEG, HFO were analyzed in regions of interest using areas with HFO as seen on the 10–20-EEG as well as areas in the clinically-defined seizure onset zone (SOZ). 13 patients were included in the study, of whom 12 showed HFO in the ripple band. In 8 patients HD-EEG revealed additional regions of ripples compared to the 10–20-EEG. With HD-EEG, areas of highest ripple rates were corresponding between scalp and intracranial EEG in 7 patients (58%) and 8 (67%) patients showed highest ripple rates over the SOZ. In contrast, with 10–20-EEG only 2 patients (17%) had corresponding areas of highest ripple rates and only 3 patients (23%) showed highest ripple rates over the SOZ. HD-EEG proved to be better to identify scalp HFO in the ripple band compared to standard 10–20-EEG. Moreover, ripples in 10–20-EEG seem to lead to false localization of epileptic areas. In contrast ripples detected with HD-EEG were located over the seizure onset zone and maybe a promising tool to localize epileptic tissue in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epilepsy is one of the most common neurological diseases. Affecting approximately 50 million people of all ages worldwide, it is characterized by a great variability in etiology, clinical representation, response to treatment and outcome.
However, up to today there are no biomarkers that permit a reliable prediction of individual activity of disease, precise localization of epileptic tissue and prognosis. E. g., interictal spikes are commonly used diagnostically (Wirrell 2010) but lack specificity as they can infrequently be detected in healthy volunteers as well as more frequently in patients without a history of seizures (So 2010) and do not consistently reflect disease activity, as their occurrence changes in relation to seizures or medication level (Staba et al. 2014).
High frequency oscillations (HFO, 80–500 Hz) have become widely recognized as EEG markers for epileptic activity. In epileptic patients, irrespective of the type of epilepsy, HFO occurring during spikes and HFO outside of epileptic spikes have been shown to better delineate the seizure onset zone (SOZ) than epileptic spikes in general, which up to now are believed to be the most valid interictal clinical markers for epileptic tissue. (Wang et al. 2013; Pail et al. 2013; Jacobs et al. 2016) Moreover, the removal of HFO-generating brain tissue correlates with better postsurgical seizure outcome (Jacobs et al. 2010; Wu et al. 2010; Akiyama et al. 2011). HFO (ripple 80–250 Hz and fast ripple, FR 250–500 Hz) have first been recorded with intracranial microwires (Bragin et al. 1999, 2002; Staba et al. 2002) and then with depth and subdural electrodes (Ochi et al. 2007; Jacobs et al. 2008). However, several more recent studies have shown that HFO in the ripple band (80–250 Hz) are, although less frequently, also detectable in scalp EEG (Andrade-Valenca et al. 2011; van Klink et al. 2016). In infants with West syndrome, HFO have been found to correlate to the severity of hypsarrhythmia and to successful drug treatment (Kobayashi et al. 2015).
Comparing simultaneous scalp and intracranial EEG recordings of 11 patients, Zelmann et al. (2013) could prove that HFOs recorded from scalp have a distinct intracranial HFO correlate and thus are not just fast artifacts on the scalp. The same study however found evidence that events with a small extent such as HFOs are spatially undersampled with standard scalp and grid inter-electrode distances. Hence, high-density scalp electrode distributions seem necessary to obtain a solid sampling of HFO on the scalp.
Aim of This Study
In this study, we compared the occurrence of HFO in the ripple band (80–250 Hz, further referred to as ripple) in conventional and high-resolution scalp EEG as well as in intracranial EEG acquired from patients with epilepsy refractory to pharmacological treatment, to investigate the following hypotheses:
HD-EEG improves the identification of scalp ripples.
Correlation between ripples in scalp and intracranial EEG is higher when analyzed with HD-EEG than 10–20-EEG.
The area of highest ripple rates on scalp-EEG correlates with the SOZ.
Materials and Methods
Patients
All patients who underwent intracranial EEG recording as well as HD-EEG (128 electrodes) with a sampling rate of 2000 Hz at the Epilepsy Centre Freiburg between February 2011 and June 2012 were included. Seizure frequency had to allow for at least 4 h of interictal EEG uninterrupted by seizure. EEGs with prolonged periods of artifacts were excluded from analysis. All patients gave written informed consent for this study, which was approved of by the Freiburg Ethics Committee. Clinical data was drawn from the patients’ clinical charts at the time of the recording and during the postsurgical follow-up (3 months after surgery).
EEG Recording and Segment Selection
Scalp EEG
HD-EEG was obtained from all patients one night prior to placement of intracranial electrodes using 128 sintered Ag/AgCl elctrodes mounted on a cap (ANT Neuro, Enschede, Netherlands) according to the 10-5-System described by Oostenveld (Oostenveld and Praamstra 2001). EEG was recorded using Profusion EEG (Compumedics Limited, Abbotsford, Victoria, Australia) using a low pass filter of 800 Hz and a 2000 Hz sampling rate. A reference electrode was placed between the electrodes Fpz and AFz.
Intracranial EEG
Implantation of electrodes for intracranial EEG was done in Freiburg solely on clinical necessity. For intracranial EEG recording subdural strip electrodes [4, 6, 8 or 12 Contacts (AD-TECH, Medical Instrument Cooperation, Racine, Wi, USA)] or subdural grids [32, 40 or 64 Contacts, (AD-TECH)] were used as well as intracranial depth electrodes (5–18 Contacts, Dixi Medical, Besancon, France). A reference electrode for intracranial and scalp EEG was placed frontally.
Segment Selection
As ripples and Spikes occur most frequently during slow wave sleep (Staba et al. 2004; Bagshaw et al. 2009), a 1-h section of the EEG during slow wave sleep (according to the criteria of Rechtschaffen and Kahles 1968) with a time gap of at least 2 h after the precedent and before the following seizure was selected. Electrodes with continuous artifacts were excluded. To enable analysis using the Stellate-Harmonie Software (Stellate Systems, Inc., Montreal, Canada), EEG data were converted into a binary floating point format, permitting high pass filtering using the software ASA (ANT Neuro HQ, Enschede, the Netherlands, Butterworth filter second order, cutoff frequency 0.5 Hz) and then exported as edf-files.
Definition of SOZ
The seizure onset zone (SOZ) was determined independently from this study by the treating neurologists in the Epilepsy Centre and consisted of those contacts of the intracranial EEG and scalp EEG that showed initial seizure activity. As ripples can only be detected during an extremely time consuming procedure using filtered and time-axis-extended EEG, analysis of ripples was not part of the clinical procedure and was performed retrospectively for this study.
Event Identification
Scalp EEG
10–20-EEG
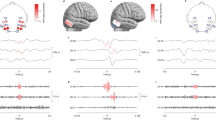
Using data from the recorded HD-EEG, for every patient a bipolar 10–20-Montage consisting of 20 electrodes (see Fig. 1a) was generated in the style of the “double banana”.
Distribution of HFO in scalp (a, b) and intracranial EEG (c) in patient no. 1. Panel a: 10–20-EEG. Panel b: distribution of ripples in the region of interest (ROI). Analyzed channels without occurrence of ripples are represented in grey; channels that had to be excluded due to artifacts are crossed out. Channels with occurrence of ripples are represented in color, intensity of color rises with ripple rates. Panel c: position of electrodes in intracranial EEG; Red dots represent contacts in the SOZ, black dots represent contacts outside the SOZ; MRI Scans depict electrode position and resection area
Epileptic spikes were marked throughout the selected 1-h section of the EEG, using a time scale of 10 s. per monitor width and were defined as interictal sharp sloping events with a duration of up to 70 ms.
Using the first 5 min of the selected EEG, ripples (80–200 Hz) and epileptic spikes were visually marked independently by JS and JJ as stated in Jacobs et al. (2016). Only events identified by both reviewers were used for further analysis. To ensure high specificity when detecting ripples in scalp-EEG, events likely due to artifact filtering were excluded (Andrade-Valenca et al. 2011). Unfiltered EEGs were carefully examined to exclude any visible co-occurring artifacts (see Fig. 2a for an example of a ripple).
a Exemplary ripple (underlined in grey) in scalp EEG of pat. Nr. 11. (A) unfiltered EEG, time scale 10 s/page (B) unfiltered EEG, timescale 0.8 s/page (C) high pass filtered EEG (80 Hz), time scale 0.8 s/page. b Patients with interictal spikes showed significantly higher ripple rates (Group A; 0.94 events/min) than patients without interictal spikes (Group B; 0.45 events/min); Bar chart shows mean and standard deviation; p < 0.05
HD-EEG (128 Channels)
For every patient, at least one region of interest (ROI) was defined, using information (ripples and seizure onset zone) gathered from the bipolar montage.
The ROI included
-
contacts of all ripple-showing channels of the bipolar 10–20-Montage
-
all contacts of the HD-EEG directly neighboring a contact showing ripples in 10–20-EEG
-
contacts that were comprised in the clinically-defined seizure onset zone (SOZ).
In patients whose SOZ was located in another brain region or the other hemisphere than the ripple generating area, an additional ROI for the SOZ was defined. All contacts comprised in the ROI where then represented against common average (128 channels), and marking of ripples and spikes as well as calculation of their rates for every channel was performed as stated above. For each channel, rates of spikes and ripples were calculated using MATLAB (The Mathworks Inc., Natick, Massachusetts, USA).
Intracranial EEG
Interictal HFOs (ripples and fast Ripples, FR) were marked in intracranial EEG for every channel visually as stated above using Harmonie Monitoring System. Using a vertically split display, the EEG was displayed with the time resolution of 0.3 s. per half monitor width, using a high pass filter of 80 Hz to display ripples (R, 80–250 Hz) on the left side and a high pass filter of 250 to display fast ripples (FR, 250–500 Hz). Ripples and FR were then identified as stated above throughout a 1 min section of the EEG, and their rates were calculated for every channel, as previously described (Jacobs et al. 2016).
Data Analysis
Using the MATLAB software (The Mathworks Inc., Natick, MA, USA), rates of epileptic spikes, ripples and FR were calculated for every channel of the scalp EEG, as well as rates of overlap of marked Spikes and ripples, and displayed in a color-coded schema, allowing for visual comparison. Ripple and spike rates ipsi- and contralateral to the clinically defined SOZ were compared. For every patient the region with the highest rate of ripples and FR in the intracranial EEG was localized as belonging to a certain brain region and compared with the localization of the area of highest ripple rate in the scalp EEG (for details see Table 1).
Results
Patient Inclusion
Of 17 patients, in whom a HD-EEG as well as an intracranial EEG was obtained, four had to be excluded due to massive artifacts in the scalp EEG. The 13 remaining patients were 8–52 years of age (mean 30.6 years), 7 were female, 6 were male. 11 patients showed at least one lesion in cranial MRI, 9 patients received epileptic surgery of whom 8 showed favorable post-operative outcome [Class I or II of Engel’s Classification (Engel et al. 1993)].
Table 1 shows the clinical data of each patient.
General EEG Events
Scalp EEG
Across all patients, a total of 237 channels were analyzed in a 10–20-EEG bipolar montage (“double-banana”). Using a common average montage, regions of interest (ROI) were defined (one in 3 patients, two ROIs in 7 patients and three ROIs in two patients). In total, 25 ROIs consisting of 351 channels were selected, of which 26 channels had to be excluded due to continuous artifacts.
Ripples could be detected in 12 patients, with a mean rate of 0.82 ± 1.3 events per minute per channel across all patients, totals ranging from 1 to 32 in 5 min. In 82% of the patients, spikes occurred (temporally and spatially) independently from ripples. In two patients (18%) spikes partially co-occurred with ripples, these patients (patients no. 6 and no. 11) showed considerably larger spike rates (both > 2.2 events/min) compared to the remaining patients.
Six percent of ripples co-occurred with spikes, and statistical analysis revealed a significantly higher mean rate of ripples in patients which also showed interictal spikes in comparison to patients without interictal spikes (p < 0.05; Fig. 2b).
Intracranial EEG
For the recording of intracranial EEG 7 grids, 29 strips and 72 depth electrodes (DE) were implanted, resulting in a total of 1177 channels to be analyzed. All patients showed HFO, with a mean rate of 28.8 ± 14.9 ripples per channel per min and a mean rate of 3.2 ± 2.2 FR per channel per minute. Statistical analysis showed significantly higher ripple rates in the SOZ (23.8 ± 32.4 events per channel per minute) than outside the SOZ (19.6 ± 34.2 events per channel per minute; p < 0.001; Kruskall–Wallis test) as well as higher rates for FR in the SOZ (1.6 ± 4.6 events per channel per minute) compared to outside the SOZ (1.0 ± 2.7 events per channel per minute, p < 0.05, Kruskall–Wallis test).
Comparison of Event Rates Between HD and 10–20 EEG
Ripple rates in HD-EEG were almost twice as high (mean rate 1.0 ± 1.56/min; in 56 channels; in 12 patients) as in the 10–20-EEG (mean rate 0.54 ± 0.73/min; in 36 channels, in 11 patients, Wilcoxon signed-rank test; p < 0.05).
Analysis of ripple local distribution in HD-EEG revealed corresponding areas of highest ripple rates in HD-EEG and 10–20-EEG in 7 patients (for details see Table 1). Additionally, analysis of HD-EEG allowed for detection of ripple generating areas undetected by the 10–20-EEG in 8 patients. In these patients, the additional contacts showed the highest ripple rates with decreasing ripple rates in neighboring contacts [see Fig. 1 for a typical example (patient no. 1)].
Epileptic spikes could be detected in 11 patients either in the 10–20-EEG or in the HD-EEG, or in both, with a mean rate of 1.12 ± 1.53 events/min. In the 10–20-EEG spikes occurred in 8 patients (in a total of 48 channels) with a mean rate of 1.08 ± 1.64 events/min, in the high density scalp EEG spikes occurred in 9 patients (in a total of 68 channels) with a mean rate of 1.14 ± 1.46 events/min (Wilcoxon signed-rank test; p < 0.05) .
Comparison of Events Rates Between Intracranial and Scalp EEG
In 7 of 12 (58%) patients, the area of highest ripple rate in the HD-EEG corresponded well to the area in which the highest ripple rates were detected in intracranial EEG, whereas if the 10–20-EEG was used for comparison only two patients (17%) showed highest ripple rates in the same area in the 10–20-EEG as in intracranial EEG. 4 of 12 patients (33%) displayed highest ripple rates in HD-EEG contralateral to the area of highest ripple rate in intracranial EEG. Of these patients two had only unilateral electrodes for intracranial EEG (patient no. 7—only right HS, patient no. 8 only left HS) and one (patient no. 9) had less DE on the left side (2 DE vs. 10 on the right side). One patient showed highest ripple rates in HD-EEG and intracranial EEG in two different areas belonging to the same hemisphere. This comparison is shown in more detail in Table 1.
Events Rate Correlation with the SOZ, Resection Area and Postoperative Outcome
Highest spike rates were found in the SOZ in 25% of the patients in the 10–20-EEG whereas in the HD-EEG 89% of the patients showed highest spike rates in the SOZ. In 5 patients, recording and analysis of HD-EEG revealed additional spikes that where localized in the ROI directly in the SOZ.
In the 10–20 EEG 3 (27%) of the patients showed highest ripple rates in the seizure onset zone (SOZ) as seen on the longterm EEG in the same clinical investigation. Of 8 (73%) patients who showed highest ripple rates outside the SOZ, 7 (64%) showed highest ripple rates contralateral to the SOZ and one patient showed equal ripple rates over the SOZ as over other areas of the brain. In the HD-EEG, 8 patients (67%) showed highest ripple rates in the SOZ, 4 patients in other brain areas, 3 of them contralateral to the SOZ (Fig. 3).
Of 9 patients who received epileptic surgery 6 patients showed a good correlation between the area of highest ripple rates in the HD-EEG and the resection area, as well as a favorable postoperative outcome [Class I or II of Engel’s Classification (Engel et al. 1993)]. Of two more patients with favorable postoperative outcome, one showed highest ripple rates in the same hemisphere as the SOZ and the resection area and one showed no correspondence between the area of highest ripple rates and the SOZ/resection area. One patient with good correspondence between the area of highest ripple rates and the SOZ chose not to undergo surgery (patient 11) and one patient (patient 16) who showed unfavorable postoperative outcome showed no correspondence between the area of highest ripple rates and the SOZ/resection area.
Discussion
In this study, the main findings were:
-
a.
Ripples are detectable in scalp EEG with both HD-EEG and 10–20-EEG.
-
b.
Higher ripple rates are seen with HD-EEG.
-
c.
Scalp ripples found in HD-EEG localize over the same brain regions as those found in intracranial EEG.
-
d.
HD-EEG allows for improved identification of the SOZ with ripples.
-
e.
Ripples seen in the 10–20-EEG might falsely localize the SOZ.
High Frequency Oscillations can be Detected in Scalp EEG
Studies concerning HFO in intracranial EEG have reached the conclusion, that HFO generating brain areas are relatively small, approximating 1 mm3. Thus, HFO research concentrated on intracranial EEG. However, more recent studies revealed that oscillations in the gamma, ripple or fast ripple band can be detected in scalp EEG of epileptic patients analyzing these frequency bands (Kobayashi et al. 2004, 2010, 2015) as well as by visual detection (Andrade-Valenca et al. 2011; Melani et al. 2013; Zelmann et al. 2013; Pizzo et al. 2016). In our study, ripples could be detected in 92% of our patients. This is in accordance with studies confirming that ripples can be detected in scalp EEG even though their generators are small (Zelmann et al. 2013) and their amplitude is much smaller than the amplitude of spikes (von Ellenrieder et al. 2014).
Mean ripple rate in scalp EEG in our study population was 0.82 ± 1.31 events/min. This is higher than previously reported [0.49 events/min (Andrade-Valenca et al. 2011), 0.12 events/min (Melani et al. 2013) and 0.21 events/min (Zelmann et al. 2013)] but in accordance with a more recent study by Pizzo and colleagues (Pizzo et al. 2016) who found a mean ripple rate of 0.85 ± 2.41 events/min. However, in our study, patient no. 6 showed much higher ripple rates than the remaining patients, accounting for 63% of total detected ripples. In our view this is mainly due to type of epileptic lesion (focal cortical dysplasia) and its relatively superficial localization (right frontal medial gyrus). Kerber and colleagues found that intracranial HFO rates in a homogenous patient population with FCD were higher than in a patient population with heterogonous brain lesions (Kerber et al. 2013). In our study, three more patients had FCD, but mean ripple rates comparable to the entire study population. This might be explained by localization of the FCD in deeper brain areas (temporal pole in patient no 8, subcallosal gyrus in patient no 13, insula in patient no 16). This suggests that superficial FCD cause more ripples that are detectable in scalp EEG. Another reason for higher ripple rates in patient no 6 is the co-occurrence of interictal spikes (58% of total spikes were detected in EEG of patient no 6), as a high rate of interictal spikes correlates to higher rates of ripples (Melani et al. 2013). Patient 6 not taken into account, the mean ripple rate in the remaining patients was 0.39 ± 0.28 events/min, which is in good accordance with the studies cited above.
Comparison of Event Rates Between HD-EEG and 10–20 EEG
The advantage of HD-EEG for the localization of epileptogenic brain tissue has been discussed for some time. Yamazaki and colleagues showed that analysis of HD-EEG (256 vs. 19 electrodes) lead to a better correspondence between iEEG and surface EEG regarding interictal spikes in patients with temporal lobe epilepsy: In HD-EEG, 45% of the spikes recorded by iEEG from temporal lobe proved to be visible, while this applied only to 22% of the interictal spikes when surface EEG was down sampled to 10–20-EEG (Yamazaki et al. 2012). In a second step, the authors confirmed - using electrical source imaging - that the interictal spikes recorded from the surface were generated close to the area within the temporal lobe where they were picked up by iEEG. Several other studies in patients with pharmakorefractory epilepsy showed that acquisition of HD-EEG with 76–256 electrodes and analysis using electrical source imaging allowed for more precise localization of epileptogenic zone or brain regions, which resection correlated with positive post-surgical outcome, than conventional EEG (Lu et al. 2012) or imaging techniques such as MRI, PET and SPECT (Brodbeck et al. 2011). All the while, the distribution of ripples in scalp EEG has not yet been investigated. However, Zelmann and colleagues conclude from their study cited above that ripples (in contrast to epileptic spikes)—being generated in small brain areas—can be detected as distinct events using a subdurally implanted grid of electrodes but will appear as one summed event in conventional EEG. As this would lead to a systematic undersampling of ripples in conventional EEG, in conclusion the authors propose the analysis of ripples using HD-EEG (Zelmann et al. 2013; von Ellenrieder et al. 2014).
In our study, analysis of HD-EEG allowed for increased detection of ripples as well as interictal spikes.
Highest spike rates were found in the SOZ in 25% of the patients in the 10–20-EEG. In 5 patients, analysis of HD-EEG revealed additional spikes that where localized in the SOZ, so that 89% of the patients showed highest spike rates in the SOZ proving interictal spikes to be quite specific for the SOZ in our cohort provided that analysis was based on HD-EEG.
Regarding ripples, analysis of HD-EEG allowed for more precise delineation of spatial distribution of ripples in 67% of patients, depicting a gradual decrease in ripple rates in neighboring contacts (e.g. patient no 1). In addition, ripples in HD-EEG occurred with a mean rate almost twice as high as in 10–20-EEG (1.0 ± 1.56 vs. 0.54 ± 0.73 events/min in conventional EEG). Both observations support the proposition of systematic undersampling of ripples in 10–20-EEG. On the one hand, those additional ripples could be detected in channels already showing ripples in the 10–20-Montage. On the other hand, additional ripples occurred in brain regions that are not well covered in the 10–20-Montage (e.g. patient 2, in whose high HD scalp EEG ripples could be detected directly above the seizure onset zone in the frontotemporal region, while the conventional EEG showed no ripple). Interestingly, analysis of HD-EEG led to the detection of additional ripples over the SOZ thus leading to the fact that the highest ripple rates were found above the SOZ. In this respect it is important to bear in mind that analysis of HD-EEG was performed after defining ROIs, which included the SOZ. Despite this potential contortion, our data shows that analysis of HD-EEG in clinically defined ROIs allows for detection of ripples that would have gone undetected in the same region using 10–20-EEG.
HFO and Spikes
HFO have been shown to occur in combination with spikes as well as independent of spikes (Urrestarazu et al. 2007). In intracranial EEG, approximately two-thirds of HFO occur with spikes (Jacobs et al. 2008), and it has been proved, that HFO co-occurring with spikes are more specific for the SOZ (Wang et al. 2013). Accordingly, spikes co-occurring with HFO have been shown to be more specific for the SOZ (Jacobs et al. 2008, 2011). Studies using scalp EEG revealed HFO to be more specific for the SOZ than spikes (Andrade-Valenca et al. 2011), a finding we cannot corroborate with our patient cohort as in our data, spikes proved to be more specific for the SOZ than ripples. As all studies on scalp HFO have been performed on limited numbers of patients and during a short period of time, the present difference in findings between our and previous studies might be in part a result of the selected patient groups and time periods for each study. Previous studies showed that mean ripple rates in patients with high rates of interictal spikes were higher (Melani et al. 2013), thus suggesting an interrelation between those two patterns. It has been reported that an increased number of electrodes (HD-EEG) leads to increased detection of spikes (Yamazaki et al. 2012), a finding we can confirm with our cohort. In addition, 3% of spikes co-occurred with ripples, a rate that is comparable to those reported previously (Andrade-Valenca et al. 2011; Melani et al. 2013). On the other hand 6% of ripples co-occurred with spikes. This rate is lower than previously reported [63% in (Andrade-Valenca et al. 2011), 44% in (Melani et al. 2013)]. We found a higher ripple rate in EEGs showing interictal spikes. As spikes are widely accepted as markers of epileptogenicity, this finding supports the idea that ripples in scalp EEG can be used as markers of epileptogenicity as well. All the while, the relatively low rate of immediate co-occurrence of ripples with spikes in our cohort supports the idea that patterns we defined as HFO do not occur due to filtering artifacts of spikes but represent distinct events generated by epileptic brain tissue.
Comparison of Event Rates Between Intracranial and Scalp EEG
HFO are known to be found in intracranial EEG as well as in scalp EEG of epileptic children and adults and are more specific for the SOZ than epileptic spikes (Jacobs et al. 2008, 2011; Andrade-Valenca et al. 2011; Melani et al. 2013). However, HFO-rates are much higher in intracranial EEG (Andrade-Valenca et al. 2011; Zelmann et al. 2013). This is mainly due to the fact that HFO are generated by relatively small areas of brain tissue beyond the scalp and are of a much smaller amplitude than epileptic spikes (Zelmann et al. 2013; von Ellenrieder et al. 2014), in consequence, the majority of these events is not detectable on the scalp. Another problem is the reduced spacial resolution of 10–20-EEG. However, this obstacle may be overcome by application of HD-EEG. As reported in previous studies cited above, we found lower ripple rates in scalp EEG (0.8 events/min) compared to intracranial EEG (28.8 events/min), corroborating the problem of under sampling ripples in scalp EEG.
One challenge of intracranial EEG being its spatial limitation as subdural or depth electrodes can never sample information from all brain regions, it is very important to have a good hypothesis when planning the electrode implantation. In our cohort, 58% of patients showed highest ripple rates in HD-EEG in brain regions that had shown highest HFO rates in intracranial EEG as well. This leads to the assumption that analysis of ripples in HD-EEG can help to define brain regions where intracranial electrodes should be implanted. However, because visual detection of HFO is very time consuming, we did not analyze all channels of the HD-EEG but only those belonging to the predefined ROI. However, for 71% of the patients a minimum of two ROI was defined, belonging to spatially distinct brain regions. The fact that highest ripple rates were found in the ROI corresponding to the area of highest HFO rates in intracranial EEG supports the hypothesis that analysis of ripple rates in HD-EEG yields valuable information for the planning of electrode implantation. However, to further investigate this hypothesis, a prospective study with a larger cohort is needed.
Event Rate Correlation with the SOZ, Resection Area and Postoperative Outcome
Previous studies have shown HFO to be found with significantly higher rates in the SOZ than outside the SOZ and to be more specific predictors for the SOZ than interictal spikes (Jacobs et al. 2008; Cho et al. 2012). Furthermore, surgical removal of HFO-generating brain tissue correlated with positive post-surgical outcome (Jacobs et al. 2010; Wu et al. 2010; Kerber et al. 2013), leading to HFO—recorded in intracranial EEG—being widely accepted as novel markers for epileptogenicity. Recent studies confirmed those findings for HFO in scalp EEG (Andrade-Valenca et al. 2011; Melani et al. 2013; Kobayashi et al. 2015). In this study, we analyzed the distribution of surface-recorded ripples in relation to the SOZ, identified in intracranial EEG as those contacts depicting initial seizure activity. 67% of our patients showed highest ripple rates in HD-EEG in the brain region corresponding to the intracranially defined seizure onset zone. Of 8 patients who underwent epileptic surgery with favorable outcome, only one showed no correspondence between the area of highest ripple rates in the HD-EEG and the clinical SOZ and resection area, thus further corroborating the clinical value of ripple analysis in HD-EEG. Interestingly, this correlation could only be observed in 27% of the patients when scalp EEG was applied according to the 10–20-EEG, leading to the observation that HD-EEG allows for better localization of the seizure onset zone than 10–20-EEG. Zelmann et al. already addressed the problem of undersampling of HFO when using conventional 10–20-EEG (Zelmann et al. 2013). Furthermore, due to this problem of undersampling analysis of 10–20-EEG can lead to false localization of the SOZ. We conclude that ripples in scalp EEG can be used as markers of epileptogenicity but correct localization of the SOZ using ripples requires analysis of HD-EEG.
Methodical Considerations
For this study, from all patients scalp EEG and intracranial EEG was recorded.
Having first been recorded using microelectrodes, it was then concluded that HFOs known to be generated by very small areas of brain tissue could not be correctly sampled using macroelectrodes that were in use for clinical purposes (Worrell et al. 2008). However, a more recent study found no significant difference between HFO rates whether micro or macro electrodes were used (Châtillon et al. 2013) and since that time, many studies could demonstrate that HFO recorded with macroelectrodes are appropriate as markers of epileptic tissue (Jacobs et al. 2008; Kerber et al. 2013) as they occur significantly more often in the SOZ.
Due to the assumed small generators of intracranial HFO it was long considered impossible to see HFO on the scalp. Recently some studies however demonstrated that HFO can be recorded on the scalp and moreover that there is a significant correlation between scalp HFO and the SOZ (Andrade-Valenca et al. 2011; Melani et al. 2013). Furthermore our group has previously shown with simultaneous scalp and intracranial EEG that scalp HFO have intracranial HFO correlates (Zelmann et al. 2013). This however does not mean that the scalp ripples recorded in this study represent the same neurophysiological phenomenon as those HFO recorded using microelectrodes (Bragin et al. 1999; Staba et al. 2002). Therefore conclusions about the mechanisms of HFO generation and about the clinical relevance of HFO investigated in intracranial EEG cannot be directly transferred to the scalp ripples which were identified in the current study.
In contrast to intracranial EEG, there are no automatic detectors that allow for reliable HFO detection and artifact rejection in scalp EEG. Therefore, analysis of HFO was performed visually to allow for reliable artifact detection and all events suspect to be artifacts were rejected. However, visual analysis requires some experience and is a very time consuming task (Zelmann et al. 2009). For this reason, our study is based on the analysis of relatively short EEG sections: 1 min for the intracranial EEG and 5 min for the scalp EEG, whereas in previous studies longer sections of 20–30 min of scalp EEG were analyzed (Melani et al. 2013; Zelmann et al. 2013; von Ellenrieder et al. 2014; Pizzo et al. 2016). As has been proposed before, we visually analyzed every single event on every contact separately (Andrade-Valenca et al. 2011). With this method no conclusion on synchronicity between events on neighboring channels can be drawn. It therefore might be that volume conduction leads to events detectable synchronously on several EEG channels especially on HD-EEG. This however cannot explain the higher ripple rates and new ripple generating regions which were identified on HD-EEG compared to 10–20-EEG and we therefore believe that it did not influence the main finding of the present study that HD-EEG might increase the possibility of identifying HFO active brain regions.
The mean ripple rate in our study is comparable to those previously reported; we therefore conclude that although ripples are recorded in scalp EEG much less frequently than in intracranial EEG (so that longer EEG sections need to be analyzed) our approach generated reliable information. However, to allow analysis of ripples to become part of the clinical routine in epilepsy diagnostics, a faster method of ripple detection is needed. Over the past few years, the development of automated HFO detectors has gained importance. Those devices have the advantage to work fast and objectively and of being able to process large data volumes. Automated HFO detection works well for intracranial EEG and is based on frequency analysis: local energy of a potential was put into reference to the energy of the whole of the EEG section due to be analyzed (Staba et al. 2002; Zijlmans et al. 2012; Crépon et al. 2010; Worrell et al. 2008). In more recent studies, instead of the entire EEG section, a baseline was defined (as those parts of the EEG without HFO) and used as a reference (Zelmann et al. 2010, 2012; Dümpelmann et al. 2012). In a recent study, the use of an automated detection device allowed for recording and analysis of several hours of intracranial EEG and detection of HFO over the entire period of time (Dümpelmann et al. 2012). While automatic detectors became more elaborate in the intracranial EEG, reliable scalp HFO detectors are still lacking. Currently published detectors suffer from low sensitivity and specificity (von Ellenrieder et al. 2012). The main challenges for scalp detectors are the large amount of artifacts in the high frequency range and the comparably low amplitude of scalp HFOs.
Scalp HFO are of smaller amplitude, as signals have to pass e.g. meninges, liquor, cranial bones and scalp tissue, (Zschocke and Hansen 2011). Another problem is that scalp EEG is much more vulnerable to artifacts, e.g. muscle activity. This, together with the lower amplitude of the signal leads to diminished signal-to-noise-ratio (Zelmann et al. 2012; von Ellenrieder et al. 2014), thus making scalp HFO more difficult to detect. At this point visual identification for these reasons is the only way to identify scalp HFO reliably even of one has to accept the above mentioned limitations of this technique.
Another challenge is the lower rate of HFO in scalp EEG, so that in order to yield reliable information, longer EEG sections and thus grater data volume needs to be processed. In our study, in order to test for artifacts, the filtered EEG that displayed ripples was always analyzed in parallel with the unfiltered EEG. Oscillations potentially due to artifact filtering were excluded from the analysis. For the recording of HD-EEG with 128 channels, we used an electrode hood as was already used in a previous study (Michel et al. 2004). As HFO are more frequent during slow wave sleep and easier to detect during the relative immobility of the patient (Staba et al. 2004; Bagshaw et al. 2009) we chose EEG segments accordingly. However, when using hoods, generally the impedance deteriorated and the cap may shift or slip during the night and we therefore had to exclude 23% of the patients of our original cohort and 7% of the channels in the remaining patients. Despite these challenges, we successfully detected ripples in scalp EEG and confirmed the relationship between high ripple rates and the localization of the SOZ, especially in HD-EEG.
For practical reasons and because of the high number of contacts, we restricted the analysis of predefined ROI that were located over brain areas where HFO occurred in the 10–20-EEG as well as those contacts belonging to the clinically defined SOZ. By this approach we accepted a potential distortion of our data towards brain regions which showed ripple activity on the 10–20-EEG. We can therefore not exclude that some regions on HD-EEG might even show higher ripple rates than we found in the selected ROI regions. Independently of this local restriction it was possible to confirm in the areas investigated that use of HD-EEG leads to the detection of additional ripples and to a more accurate delineation of the ripple distribution by gradual decrease of the ripple rate in neighboring contacts. In the future, the development of reliable automatic detection might allow are more complete assessment of HFO distribution in scalp EEG by analysis of all contacts and add more ROIs - also including clinical information. Especially in temporal lobe epilepsies the addition of lower temporal electrodes might be important.
The problem that analysis is limited to certain regions applies to the intracranial EEG as well, as in our study electrodes were implanted according to the clinical purpose of pre-surgical diagnostics to define the resection area. This limitation results in some uncertainties for the allocation of intracranially recorded HFO to areas of the scalp. In this area, more research is needed: it would be wishful to be able to analyze all channels of HD-EEG in a larger, more homogenous patient cohort prospectively in order to investigate whether analysis of HFO distribution can help to plan electrode implantation, as well as to define the area of brain that needs to be resected in order to allow for a favorable postoperative outcome.
The occurrence of HFO in HD-EEG has not been evaluated extensively in the past. In the present study we limited the analysis to ripples as this has been the mean frequency described in scalp HFO analysis previously (Andrade-Valenca et al. 2011b; van Klink et al. 2016). It certainly would be very interesting to analyze fast ripples (FR, 250–500 Hz) as well, which as we know from intracranial EEG studies might be even more closely linked to epileptic areas. However, the high content of high frequency artifacts greatly impedes the analysis of FR in scalp EEG. A solution for the diminished signal-to-noise-ratio might be the use of subdermal electrodes as suggested by Pizzo and coworkers (Pizzo et al. 2016), allowing for analysis of HFO both in the ripple as well in the fast ripple band.
Some studies also look at HFO in Magnetoencephalography (MEG), which is another non-invasive technique that covers the entire brain with high sensor density (Zijlmans et al. 2017). It might have the advantage to be less affected by skull conductivity and less susceptible to contamination from muscular activity (Tamilia et al. 2017). However, as with HD-EEG, HFO analysis is extremely time-consuming on all MEG channels (von Ellenrieder et al. 2016). More importantly, the availability of this promising technique is currently limited to a relatively low number of clinical centers which impedes the transition into clinical practice, whereas EEG techniques such as HD-EEG are available or could easily be implemented in almost every epilepsy unit. Depending on the localization of the generator of HFOs within the cortex, it is likely that some oscillations are better seen with MEG and others with HD-EEG, but this hypothesis calls for confirmation in future studies comparing HD-EEG and MEG directly. Furthermore, once reliable automatic detectors allow for processing of large data volumes, it would be most interesting to be able to extract more HFO features (e.g. frequency, duration) to increase the specificity of scalp HFO and to use source localization to identify their origin. It is however quite promising that this study found scalp HFO in HD-EEG and their distribution is promising for the clinical yield of the method.
Conclusion
HFO can be recorded in scalp EEG. However, as HFO occurr much less frequently in scalp EEG than in intracranial EEG and have small generators there is risk of undersampling in conventional scalp EEG using the 10–20-Montage. Our data suggests that undersampling can lead to a false localization of epileptic areas. This challenge can be overcome by using HD-EEG, which then allows for a more accurate localization of the seizure onset zone. Measuring HFO in HD-EEG might be a powerful non-invasive tool to identify epileptic areas and plan intracranial electrode placements accordingly.
Abbreviations
- DE:
-
Depth electrode
- EEG:
-
Electro-encephalography
- FR:
-
Fast ripple
- HD:
-
High density
- HFO:
-
High frequency oscillation
- HS:
-
Hemisphere
- MRT:
-
Magnet resonance tomography
- ROI:
-
Region of interest
- SOZ:
-
Seizure onset zone
References
Akiyama T, McCoy B, Go CY et al (2011) Focal resection of fast ripples on extraoperative intracranial EEG improves seizure outcome in pediatric epilepsy. Epilepsia 52:1802–1811. https://doi.org/10.1111/j.1528-1167.2011.03199.x
Andrade-Valenca LP, Dubeau F, Mari F et al (2011) Interictal scalp fast oscillations as a marker of the seizure onset zone. Neurology 77:524–531. https://doi.org/10.1212/WNL.0b013e318228bee2
Bagshaw AP, Jacobs J, LeVan P et al (2009) Effect of sleep stage on interictal high-frequency oscillations recorded from depth macroelectrodes in patients with focal epilepsy. Epilepsia 50:617–628. https://doi.org/10.1111/j.1528-1167.2008.01784.x
Bragin A, Engel J, Wilson CL, et al (1999) High-frequency oscillations in human brain. Hippocampus 9:137–142.
Bragin A, Wilson CL, Staba RJ et al (2002) Interictal high-frequency oscillations (80–500 Hz) in the human epileptic brain: entorhinal cortex. Ann Neurol 52:407–415. https://doi.org/10.1002/ana.10291
Brodbeck V, Spinelli L, Lascano AM et al (2011) Electroencephalographic source imaging: a prospective study of 152 operated epileptic patients. Brain J Neurol 134:2887–2897. https://doi.org/10.1093/brain/awr243
Châtillon CE, Zelmann R, Hall JA et al (2013) Influence of contact size on the detection of HFOs in human intracerebral EEG recordings. Clin Neurophysiol 124(8):1541–1546. https://doi.org/10.1016/j.clinph.2013.02.113
Cho JR, Joo EY, Koo DL et al (2012) Clinical utility of interictal high-frequency oscillations recorded with subdural macroelectrodes in partial epilepsy. J Clin Neurol Seoul Korea 8:22–34. https://doi.org/10.3988/jcn.2012.8.1.22
Crépon B, Navarro V, Hasboun D et al (2010) Mapping interictal oscillations greater than 200 Hz recorded with intracranial macroelectrodes in human epilepsy. Brain 133:33–45. https://doi.org/10.1093/brain/awp277
Dümpelmann M, Jacobs J, Kerber K, Schulze-Bonhage A (2012) Automatic 80-250Hz “ripple” high frequency oscillation detection in invasive subdural grid and strip recordings in epilepsy by a radial basis function neural network. Clin Neurophysiol Off J Int Fed Clin Neurophysiol 123:1721–1731. https://doi.org/10.1016/j.clinph.2012.02.072
Engel JJ, Van Ness PC, Rasmussen TB, Ojemann LM (1993) Outcome with respect to epileptic seizures. In: Engel J Jr (ed) Surgical treatment of the epilepsies, 2nd edn. Raven Press, New York, pp 609–621
Jacobs J, LeVan P, Chander R et al (2008) Interictal high-frequency oscillations (80–500 Hz) are an indicator of seizure onset areas independent of spikes in the human epileptic brain. Epilepsia 49:1893–1907. https://doi.org/10.1111/j.1528-1167.2008.01656.x
Jacobs J, Zijlmans M, Zelmann R et al (2010) High-frequency electroencephalographic oscillations correlate with outcome of epilepsy surgery. Ann Neurol 67:209–220. https://doi.org/10.1002/ana.21847
Jacobs J, Kobayashi K, Gotman J (2011) High-frequency changes during interictal spikes detected by time-frequency analysis. Clin Neurophysiol Off J Int Fed Clin Neurophysiol 122:32–42. https://doi.org/10.1016/j.clinph.2010.05.033
Jacobs J, Vogt C, LeVan P et al (2016) The identification of distinct high-frequency oscillations during spikes delineates the seizure onset zone better than high-frequency spectral power changes. Clin Neurophysiol 127:129–142. https://doi.org/10.1016/j.clinph.2015.04.053
Kerber K, LeVan P, Dümpelmann M et al (2013) High frequency oscillations mirror disease activity in patients with focal cortical dysplasia. Epilepsia 54:1428–1436. https://doi.org/10.1111/epi.12262
Kobayashi K, Oka M, Akiyama T et al (2004) Very fast rhythmic activity on scalp EEG associated with epileptic spasms. Epilepsia 45:488–496. https://doi.org/10.1111/j.0013-9580.2004.45703.x
Kobayashi K, Watanabe Y, Inoue T et al (2010) Scalp-recorded high-frequency oscillations in childhood sleep-induced electrical status epilepticus. Epilepsia 51:2190–2194. https://doi.org/10.1111/j.1528-1167.2010.02565.x
Kobayashi K, Akiyama T, Oka M et al (2015) A storm of fast (40–150 Hz) oscillations during hypsarrhythmia in West syndrome. Ann Neurol 77:58–67. https://doi.org/10.1002/ana.24299
Lu Y, Yang L, Worrell GA, He B (2012) Seizure source imaging by means of FINE spatio-temporal dipole localization and directed transfer function in partial epilepsy patients. Clin Neurophysiol Off J Int Fed Clin Neurophysiol 123:1275–1283. https://doi.org/10.1016/j.clinph.2011.11.007
Melani F, Zelmann R, Dubeau F, Gotman J (2013) Occurrence of scalp-fast oscillations among patients with different spiking rate and their role as epileptogenicity marker. Epilepsy Res 106:345–356. https://doi.org/10.1016/j.eplepsyres.2013.06.003
Michel CM, Lantz G, Spinelli L et al (2004) 128-channel EEG source imaging in epilepsy: clinical yield and localization precision. J Clin Neurophysiol Off Publ Am Electroencephalogr Soc 21:71–83
Ochi A, Otsubo H, Donner EJ et al (2007) Dynamic changes of Ictal high-frequency oscillations in neocortical epilepsy: using multiple band frequency analysis. Epilepsia 48:286–296. https://doi.org/10.1111/j.1528-1167.2007.00923.x
Oostenveld R, Praamstra P (2001) The five percent electrode system for high-resolution EEG and ERP measurements. Clin Neurophysiol 112:713–719. https://doi.org/10.1016/S1388-2457(00)00527-7
Pail M, Halámek J, Daniel P et al (2013) Intracerebrally recorded high frequency oscillations: simple visual assessment versus automated detection. Clin Neurophysiol Off J Int Fed Clin Neurophysiol 124:1935–1942. https://doi.org/10.1016/j.clinph.2013.03.032
Pizzo F, Ferrari-Marinho T, Amiri M et al (2016) When spikes are symmetric, ripples are not: bilateral spike and wave above 80 Hz in focal and generalized epilepsy. Clin Neurophysiol Off J Int Fed Clin Neurophysiol 127:1794–1802. https://doi.org/10.1016/j.clinph.2015.11.451
Rechtschaffen A, Kahles A (1968) A manual of standardized terminology, techniques and scoring system of sleep stages in human subjects. Brain Information Service/Brain Research Institute, University of California, Los Angeles
So EL (2010) Interictal epileptiform discharges in persons without a history of seizures: what do they mean? J Clin Neurophysiol Off Publ Am Electroencephalogr Soc 27:229–238. https://doi.org/10.1097/WNP.0b013e3181ea42a4
Staba RJ, Wilson CL, Bragin A et al (2002) Quantitative analysis of high-frequency oscillations (80–500 Hz) recorded in human epileptic hippocampus and entorhinal cortex. J Neurophysiol 88:1743–1752
Staba RJ, Wilson CL, Bragin A et al (2004) High-frequency oscillations recorded in human medial temporal lobe during sleep. Ann Neurol 56:108–115. https://doi.org/10.1002/ana.20164
Staba RJ, Stead M, Worrell GA (2014) Electrophysiological biomarkers of epilepsy. Neurotherapeutics 11:334–346. https://doi.org/10.1007/s13311-014-0259-0
Tamilia E, Madsen JR, Grant PE et al (2017) Current and emerging potential of magnetoencephalography in the detection and localization of high-frequency oscillations in epilepsy. Front Neurol. https://doi.org/10.3389/fneur.2017.00014
Urrestarazu E, Chander R, Dubeau F, Gotman J (2007) Interictal high-frequency oscillations (100–500 Hz) in the intracerebral EEG of epileptic patients. Brain J Neurol 130:2354–2366. https://doi.org/10.1093/brain/awm149
van Klink NEC, van’t Klooster MA, Leijten FSS et al (2016) Ripples on rolandic spikes: a marker of epilepsy severity. Epilepsia 57:1179–1189. https://doi.org/10.1111/epi.13423
von Ellenrieder N, Andrade-Valença LP, Dubeau F, Gotman J (2012) Automatic detection of fast oscillations (40–200 Hz) in scalp EEG recordings. Clin Neurophysiol 123:670–680. https://doi.org/10.1016/j.clinph.2011.07.050
von Ellenrieder N, Beltrachini L, Perucca P, Gotman J (2014) Size of cortical generators of epileptic interictal events and visibility on scalp EEG. NeuroImage 94:47–54. https://doi.org/10.1016/j.neuroimage.2014.02.032
von Ellenrieder N, Pellegrino G, Hedrich T et al (2016) Detection and magnetic source imaging of fast oscillations (40–160 Hz) recorded with magnetoencephalography in focal epilepsy patients. Brain Topogr 29:218–231. https://doi.org/10.1007/s10548-016-0471-9
Wang S, Wang IZ, Bulacio JC et al (2013) Ripple classification helps to localize the seizure-onset zone in neocortical epilepsy. Epilepsia 54:370–376. https://doi.org/10.1111/j.1528-1167.2012.03721.x
Wirrell EC (2010) Prognostic significance of interictal epileptiform discharges in newly diagnosed seizure disorders. J Clin Neurophysiol 27:239–248. https://doi.org/10.1097/WNP.0b013e3181ea4288
Worrell GA, Gardner AB, Stead SM et al (2008) High-frequency oscillations in human temporal lobe: simultaneous microwire and clinical macroelectrode recordings. Brain J Neurol 131:928–937. https://doi.org/10.1093/brain/awn006
Wu JY, Sankar R, Lerner JT et al (2010) Removing interictal fast ripples on electrocorticography linked with seizure freedom in children. Neurology 75:1686–1694. https://doi.org/10.1212/WNL.0b013e3181fc27d0
Yamazaki M, Tucker DM, Fujimoto A et al (2012) Comparison of dense array EEG with simultaneous intracranial EEG for Interictal spike detection and localization. Epilepsy Res 98:166–173. https://doi.org/10.1016/j.eplepsyres.2011.09.007
Zelmann R, Zijlmans M, Jacobs J et al (2009) Improving the identification of High Frequency Oscillations. Clin Neurophysiol 120(8):1457–1464. https://doi.org/10.1016/j.clinph.2009.05.029
Zelmann R, Mari F, Jacobs J et al (2010) Automatic detector of high frequency oscillations for human recordings with macroelectrodes. Conf Proc Annu Int Conf IEEE Eng Med Biol Soc 2010:2329–2333. https://doi.org/10.1109/IEMBS.2010.5627464
Zelmann R, Mari F, Jacobs J et al (2012) A comparison between detectors of high frequency oscillations. Clin Neurophysiol Off J Int Fed Clin Neurophysiol 123:106–116. https://doi.org/10.1016/j.clinph.2011.06.006
Zelmann R, Lina JM, Schulze-Bonhage A et al (2013) Scalp EEG is not a blur: it can see high frequency oscillations although their generators are small. Brain Topogr 27:683–704. https://doi.org/10.1007/s10548-013-0321-y
Zijlmans M, Jiruska P, Zelmann R et al (2012) High-frequency oscillations as a new biomarker in epilepsy. Ann Neurol 71:169–178. https://doi.org/10.1002/ana.22548
Zijlmans M, Worrell GA, Dümpelmann M et al (2017) How to record high-frequency oscillations in epilepsy: a practical guideline. Epilepsia 58:1305–1315. https://doi.org/10.1111/epi.13814
Zschocke S, Hansen H-C (eds) (2011) Klinische Elektroenzephalographie, 3rd edn. Springer, New York
Funding
JJ was supported by a Grant of the German Research Foundation (JA1725/2-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have potential conflict of interest to be disclosed.
Additional information
Handling Editor: Christoph M. Michel.
Rights and permissions
About this article
Cite this article
Kuhnke, N., Schwind, J., Dümpelmann, M. et al. High Frequency Oscillations in the Ripple Band (80–250 Hz) in Scalp EEG: Higher Density of Electrodes Allows for Better Localization of the Seizure Onset Zone. Brain Topogr 31, 1059–1072 (2018). https://doi.org/10.1007/s10548-018-0658-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10548-018-0658-3