Abstract
Physiological and morphological properties of the human intestine cannot be accurately mimicked in conventional culture devices such as well plates and petri dishes where intestinal epithelial cells form a monolayer with loose contacts among cells. Here, we report a novel microfluidic cell culture device (μFCCD) that can be used to culture cells as a human intestinal model. This device enables intestinal epithelial cells (Caco-2) to grow three-dimensionally on a porous membrane coated with fibronectin between two polydimethylsiloxane (PDMS) layers. Within 3 days, Caco-2 cells cultured in the μFCCD formed villi- and crypt-like structures with small intercellular spaces, while individual cells were tightly connected to one another through the expression of the tight junction protein occludin, and were covered with a secreted mucin, MUC-2. Caco-2 cells cultured in the μFCCD for 3 days were less susceptible to bacterial attack than those cultured in transwell plates for 21 days. μFCCD-cultured Caco-2 cells also displayed physiologically relevant absorption and paracellular transport properties. These results suggest that our intestinal model more accurately mimics the morphological and physiological properties of the intestine in vivo than the conventional transwell culture model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In vitro cell culture models are widely used evaluate the effect of xenobiotic compounds and study the mechanism of diseases. While the cell culture methods has long been established, in vitro cell culture models still suffer from several drawbacks, as these systems often do no recapitulate important aspects of biological systems (Meyvantsson and Beebe 2008). Consequently, this limitation of currentcell culture models has contributed to the rising cost of drug development (Dickson and Gagnon 2004). Through the combination of microtechnology and conventional cell cultrue techniques, novel microsystems have been created, with improvement in physiological relevance compared conventional models. Collectively termed organ-on-a-chip (OOC), this technology enables screening the effect of compounds and studying themechanism of diseases in a more phyiologically realistic context (Sung et al. 2013; Huh et al. 2012).
In vitro gut epithelium models are often used to study the absorption of drugs, study the mechanism of gut-related diseases, as well as bacterial or viral interaction (Artursson et al. 2001; Duffy 2000; Straub et al. 2013). Caco-2 cells, a human colon epithelial cancer cell line, exhibit some of the characteristics of the human small intestine, such as tight junctions between cells, drug transporter and efflux proteins, and phase II conjugation enzymes, and have been widely used as an in vitro model for drug absorption as well as bacterial infection. (van Breemen and Li 2005). However, Caco-2 cells are not able to replicate some of the important characteristics of the human intestine. For example, distinct properties of the human intestine include mucin production, microvilli formation, and tight junction formation. Cells grown in conventional culture systems such as transwell plates and petri dishes do not display these properties and therefore do not accurately reflect the biological properties of the intestine. Thus, tremendous effort has been focused on developing physiologically-relevant in vitro intestinal models, for example by using three-dimensional culture of epithelial cells (Li et al. 2013; Leonard et al. 2010) or providing mechanical stimuli (Kim et al. 2012).
Intestinal cells in vivo produce mucins, which play an important role in protecting, lubricating, and hydrating intestinal tissue surface (Andrianifahanana et al. 2006). Among these mucins, mucin 2 (MUC-2), which is a secreted mucin, covers the apical surface of the intestinal tissue, thereby preventing pathogenic bacteria from invading host cells. MUC-2 deficiency may cause chronic diseases, such as inflammatory bowel disease (IBS) (Byrd and Bresalier 2004). Caco-2, a human cell line derived from a colonic epithelial tumor, retains the epithelial function of the small intestine and thus has been frequently used as an in vitro model of the intestine (Biazik et al. 2010). However, these cells produce only a small amount of MUC-2 (Bu et al. 2011) compared to other intestinal cell lines such as the goblet cell line, and are therefore considered unsuitable for studying bacterial interactions with the intestinal epithelial layer.
The apical surface of intestinal cells is covered with thousands of microvilli. The main function of microvilli on the apical surface is absorption. Microvilli produce enzymes that breakdown complex compounds into simpler, smaller compounds that can be easily absorbed by intestinal cells. They also act as mechanosensors by sensing fluid flow in the lumen and converting this information via biochemical responses into enhanced absorption (Guo et al. 2000).
Intestinal cellsin vivo are tightly connected with one another by tight junctions. These tight junctions prevent proteins from moving freely across tissue boundaries (Gumbiner 1987, 1993; Schneeberger 1992; Staddon and Rubin 1996). Occludin is the main protein related to tight junction formation and is important for the maintenance of tissue environments. Disruption of occludin regulation often causes inflammation, leading to tissue edema and damage. Therefore, understanding tight junction regulation could establish a future treatment direction for intestinal diseases (Zanassi et al. 2001).
Microfluidic cell culture devices (μFCCDs) have been used to culture various types of cells, including intestinal cells. The intestine plays an important role in digestion and absorption of nutrients as well as immunity homeostasis. Cells cultured in a μFCCD with porous membranes were reported to form small villi structures and were used for adsorption research (Esch et al. 2012; Kim et al. 2012; Kim and Ingber 2013). Most recently, a μFCCD incorporating a hydrogel as a scaffold was used to induce cells to form 3D villi-like structures mimicking intestinal villi (Kim et al. 2013; Sung et al. 2011). Despite these achievements, μFCCDs that enable cells to reconstitute other structural features of the intestine, such as tight junctions and microvilli, have yet to be developed.
In this study, we modified a μFCCD to reconstitute the physiological and morphological functions of intestinal cells by culturing intestinal epithelial (Caco-2) cells on a porous membrane under microfluidic conditions. Morphological functions of the cells grown in the μFCCD were investigated by observing villi- and crypt-like structures, tight junctions, and mucin production, while the physiological functions of cells grown in the μFCCD were investigated by measuring paracellular transport of large molecules, TEER, and susceptibility to bacterial attack. These features were compared to those of cells cultured in transwell plates to verify the relevance of intestinal cells grown in our μFCCD as an intestinal model for absorption and host-bacteria interactions.
2 Materials and methods
2.1 Fabrication of the μFCCD
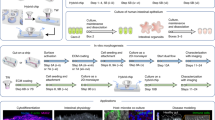
We used a μFCCD composed of five polydimethylsiloxane (PDMS) layers and a polyester membrane (7 mm × 7 mm) 10 μm-thick with pores (average diameter of 0.4 μm) (Corning Inc., Acton, MA, USA) (Fig. 1a). All PDMS layers had the same width (2 cm) and length (3 cm), but their thickness varied. Layers 1, 2, and 5 were 2 mm-thick, while layers 3 and 4 were 1 mm-thick. Diameters of the large and small holeswere5 mm and 300 μm, respectively.Flow channels (red) were 50 μm wide, 2 cm long, and 50 μm high. Layer 1 had five small holes. Layer 2 had four small holes, one large hole, and one flow channel. Layer 3 has one small hole and two large holes. Layer 4 had one small hole, two large holes, and one flow channel. Layer 5 had neither a hole nor a flow channel. The polyester membrane was prepared by cutting the membrane part from the well (Corning) with scissors. The polyester membrane was coated with 0.05 % fibronectin (Sigma-Aldrich) for 1 h and was placed between layers 3 and 4. Each PDMS layer was treated with O2 plasma and carefully aligned to each other to form a chip. The inlet of the flow channel had bubble trap chambers (5 mm diameter: 2 mm height) to prevent holes from being blocked with bubbles. Flexible plastic tubing (Tygon) was connected to the inlets and outlets (Ishikawa et al. 2011; Lentle and Janssen 2008; Olesen and Edvinsson 1988). Both layer 3 and 4 were 1 mm thick to provide sufficient amounts of nutrients to the cell in the channel (Imura et al. 2009). Layer 2 worked as a bubble trap and should be tall enough to inhibit transport of small air bubbles into the cell culture chamber. Layer 1 and 5 were thick enough to prevent tearing of the layers.
Schematic of the μFCCD. a Process used to assemble the μFCCD. μFCCD was composed of five PDMS layers and a polyester membrane (green)(7 mm wide and 8 mm long). All PDMS layers had the same width (2 cm) and length (3 cm) with different thickness: layers 1, 2, and 5 were 2 mm-thick, while layers 3 and 4were 1 mm-thick. The diameters of the large and small chambers were 5 mm and 300 μm, respectively.Flow channels (red) were 50 μm wide, 2 cm long, and 50 μm high. b Operation of the μFCCD. A syringe pump was used to perfuse medium through tubing to the upper and lower (red) microchannels. Caco-2cellswere seeded onto the membrane (green) coated with fibronectin
2.2 Cell culture
The human colon carcinoma cell line Caco-2 (KCLB #30037, passages31–40) was cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (Welgene, Daegu, Korea) containing 10 % fetal bovine serum (FBS; Welgene) and 1 % penicillin-streptomycin(Welgene). Cells were maintained in a humidified incubator with 5 % CO2 at 37 °C. Trypsinized cells were seeded at a density of 5 × 105 cells/cm2 ontothe μFCCDat a flow rate of 0.5 μl/minor the wells of a24-transwell plate (Corning, Cortland, NY, USA).
2.3 Immunostaining
Once cells formed a monolayer, cells in the μFCCD were taken out by cutting the membrane out using a laser sharp. Then, they were fixed with 10 % neutralized formalin at room temperature for 15 min, and permeabilized with 0.1 % Triton X-100 for 10 min. They were then stained with rhodamine phalloidin (RP) (Invitrogen) at a dilution ratioof1:200in Dulbecco’s phosphate buffered saline (DPBS) (Welgene) for 20 min on a rocker (FINEPCR, Gunpo-Si, Korea), followed by staining with 4,6-diamidino-2-phenylindole(DAPI)(Sigma-Aldrich) at a dilution ratio of 1:10,000 in DPBS for 5 min on the rocker. Cells were washed three times with DPBS between each step. Images of stained cells were taken using confocal laser scanning microscopy (CLSM, Zeiss LSM 510, Carl Zeiss).
Cells were stained for occludin, a tight junction marker, with mouse anti-occludin antibodies (Invitrogen). In detail, cells were first permeabilized with 0.1 % Triton X-100 for 10 min, then blocked with 4 % bovine serum albumin (BSA; Invitrogen) in DPBS and incubated with mouse anti-occludin antibody labelled with Alexa 594 (Invitrogen) at a dilution ratio of 1:200 for 2 h at RT. Then, cells were washed with DBPS to remove unbound antibody and stained again with 4′,6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich) at a dilution ratio of 1:10,000 in DPBS for 5 min. Images of stained cells were taken using fluorescence microscopy (TE2000U, Nikon).
Cells were stained for MUC-2 using rabbit anti-MUC-2 antibody (Sigma-Aldrich). In brief, cells were incubated with rabbit anti-MUC-2 antibody at a 1:200 dilution ratio in DPBS for 2 h at room temperature and then DPBS was used to wash away unbound antibody. Cells were then incubated with anti-rabbit FITC (fluorescein isothiocyanate)-conjugated antibody (Sigma-Aldrich) for 1 h and unbound antibody was washed away with DPBS.
2.4 Transmission electron microscopy (TEM) imaging
Cells in the μFCCD were taken out of the chip before the imaging. Then, cells were pre-fixed in 2.5 % glutaraldehyde in phosphate buffer (pH 7.2) for 4 h and then post-fixed in 1 % osmium tetroxide solution for 1 h (Kiernan 1999). Cell samples were then dehydrated in graded alcohol, and transited to propylene oxide solution. They were infiltrated with resin (Epon 812; Sigma-Aldrich) on a rotary shaker for 12 h. Samples were embedded in fresh resin overnight and the fresh resin was cured by placing the samples in a drying oven at 60 °C for 24 h. A sample section thickness of 100 nm was obtained by ultra-microtome cutting. Sections were stained with toluidine blue (1:200 in DPBS) and observed under a transmission electron microscope (JEOL 1400).
2.5 Scanning electron microscopy (SEM) imaging
Cells in the μFCCD were taken out as mentioned above and fixed with 3.7 % paraformaldehyde for 15 min, 2.5 % glutaraldehyde for 2 h, and 1 % osmium tetroxide for 1 h in a sequential manner. Samples were then washed using distilled water several times, then freeze-dried for 24 h and coated with 7 nm-thick platinum using a spin coater. Finally, images were taken with a scanning electron microscope (JSM-6700F; JEOL).
2.6 Aminopeptidase activity assay
Aminopeptidase activity of the brush border of the cell surface was measured using DMEM containing 1.5 mML-alanine-4-nitroanilide hydrochloride (A4N) (Sigma-Aldrich) as a substrate for the enzyme. Substrate solution was applied to cells grown in transwell plates or in the μ FCCD. For the transwell-cultured cells, 200 μl of solution was added to the top chamber of the plate and then the plate was incubated in a humidified incubator with 5 % CO2 at 37 °C for 2 h. After the incubation, a 100 μl aliquot were carefully taken from each well and its optical density was measured at 405 nm using a microplate reader (Molecular Devices, Sunnyvale, CA, USA). For the μFCCD, the same substrate solution was flowed into the microchamber through the top channel at a rate of 0.5 ml/min. Eluents were pooled for 1 h and the pooled sample was diluted two-fold and then transferred to a 96-well plate before measurement at 405 nm.
2.7 TEER and permeability assay
Tight junctional integrity of cells was evaluated through transepithelial electric resistance (TEER) measurement using an epithelial voltammeter (Millicell ERS-2) (Millipore, Bedford, MA, USA) (Kim et al. 2014) with a chopstick-type electrode set (4 mm wide and 1 mm thick). To measure TEER values in Caco-2 cells cultured in transwell plates, cells were cultured in transwell plates for 21 days and both sides of the insert chamber of each transwell were then filled with media.
Cell permeability was measured using FITC-labeled dextran (4 kDa; Sigma-Aldrich) (Kim et al. 2014). Cells in transwells were first washed with Hank’s Balanced Salt Solution (HBSS) (Sigma), and then 200 μl of FITC-dextran 4 kDa (1 mg/ml) in HBSS was added to the top chamber and 1 ml of HBSS was added to the lower chamber followed by incubation in a 5 % CO2 incubator at 37°C for several hours. Every hour, aliquots (100 μl) were removed from the lower chamber and transferred to 96-well plates (Costar, Corning) to measure fluorescence intensity using a microplate reader (Molecular Devices) at excitation and emission wavelengths of 492 and 518 nm, respectively. Cells in the μFCCD were washed by flowing HBSS into the cell culture chamber at a rate of 0.5 μl/min for 1 h. Then, FITC-labeled dextran (1 mg/ml) in HBSS was perfused through the upper channel at 0.5 μl/min and aliquots (30 μl) were collected every hour from the outlet of the lower channel. The fluorescent intensity of each aliquot was measured as described above.
2.8 Gene expression analyses
Total RNA was extracted from cells cultured in either transwell plates for 21 days or the μFCCD for 3 days using an RNeasy Micro Kit (Qiagen). cDNA fragments were then synthesized from the extracted RNA using an AccuPower® Cycle Script RT PreMix kit (Bioneer, Daejeon, Korea). Gene expression was determined byRT-PCR using a Lightcycler® Nano System (Roche, Germany).
2.9 Bacterial invasion assay
Bacterial invasion was assessed using Salmonella enterica serovar Typhimurium (χ3339). This strain was transformed with green fluorescent protein (GFP). In brief, the strain was cultured overnight in Luria-Bertani broth (LB) containing 0.1 % ampicillin (100 mg/ml; Sigma Aldrich) at 37°C and 220 rpm. Cultures were re-inoculated into fresh LB containing 0.1 % ampicillin at a dilution of 1:100 and cultured as described above until the optical density (OD) reached 1.0.
Then, S. Typhimurium cells were co-incubated with Caco-2 cells grown in transwell plates or the μFCCD at a multiplicity of infection (MOI) of 100 for 3 h to test the invasion ability of the bacterial cells (Izumikawa et al. 1998). After washing three times with DPBS, Caco-2 cells were treated with 0.1 μg/ml gentamycin in DMEM for 3 h to kill bacterial cells remaining on the surface of the host cells. Host cells were then lysed with trypsin/EDTA and bacterial cell number was counted using a standard colony counting method. For fluorescence imaging, host cells and bacterial cells were first fixed with formalin, then permeabilized with 0.1 % Triton-X 100, and finally stained with RP (1:200 in PBS) for 20 min to visualize invasion by fluorescence microscopy.
3 Results and discussion
3.1 Reconstitution of the mucus layer of the intestine on the chip
Intestinal epithelial cells need laminar flow to properly perform uptake and transport of nutrients (Basson 2003). Caco-2 cells were cultured on a porous membrane at various flow rates (0.1 to 10 μl/min) in the μFCCD (Fig. 1) for 3 days. Cells showed a decrease in proliferation rate when they were cultured for longer than 3 days (data not shown). Shear stressesat different flow rates were calculated by a reference formulation (Oh et al. 2012). Optimum flow rate was then determined by investigating the effect of flow rates on MUC-2 production by the cells grown in the μFCCD. As shown in Fig. 2a, the highest MUC-2 production was observed at the flow rate of 0.5 μl/min. Similarly, a recent study reported that fluid shear at 0.5 μl/min enhanced MUC-2 production in Caco-2 cells (Kim et al. 2012).When Caco-2 cells were cultured in transwell plates for 21 days, which requires them to form a monolayer, they produced a very low amount of MUC-2 compared to those cultured in the μFCCD for 3 days, which was the time required for them to fully fill the membrane surface of the chip (Fig. 2c). The production level of both cultures was further verified by qRT-PCR (Fig. 2d). Because the intestinal epithelium produces mucins in vivo that protect epithelial cells from bacterial and other biological attacks as well as toxins (Andrianifahanana et al. 2006; Ishikawa et al. 2011; Lentle and Janssen 2008; Olesen et al. 1988), a physiologically-relevant in vitro intestinal model requires mucin production. Among various mucins, MUC-2, which belongs to the secreted mucin family, covers the apical surface of intestinal tissue and plays an important role in protecting the intestinal epithelium from bacterial attack. Our results suggest that Caco-2 cells cultured in the μFCCD at 0.5 μl/minfor 3 days reconstituted the mucus layer of the endothelial epithelium.
Effect of flow rate on MUC-2 production by Caco-2 cells cultured in the μFCCD. a MUC-2 (green) production in cells (red: actin staining; blue: DAPI staining) cultured in the μFCCD for 3 days at different flow rates (0.1 to 10 μl/min). b CLSM images of cells stained with actin (red) and DAPI (blue). c CLSM images of cells cultured in either transwell plates for 21 days or the μFCCD for 3 days at a flow rate of 0.5 μl/min after staining with MUC-2 antibody (green), actin (red), and DAPI (blue). Details of each staining method are provided in the Materials and methods. d MUC-2 expression of cells cultured in either transwellplatesfor 21 days or the μFCCD at 0.5 μl/min for 3 days. Expression levels of MUC-2 in cells grown in the μFCCD and transwell plates were determined by qRT-PCR. * P < 0.01; Student’s t-test
As discussed above, the mucus layer protects the intestinal epithelium from microbial and chemical attacks. To evaluate the protective effect of MUC-2 production by Caco-2 cells, host cells grown in both transwells and the μFCCD were challenged with S. Typhimurium which can adhere to and invade host cells (Van Asten et al. 2000). As shown in Fig. 3a, b, and c, the numbers of both adherent and invaded bacterial cells per host cell were lower for μFCCD-cultured cells than transwell-cultured cells, indicating that the mucus layer that formed due to enhanced MUC-2 production acted as a physical barrier. These results suggest that Caco-2 cells cultured in the described μFCCD will be highly useful for studying host-bacterial interactions in the intestine.
S. Typhimurium adherence to and invasion of Caco-2 cells cultured in transwell plates for 21 days or μFCCD at 0.5 μl/min for 3 days. a Fluorescence images showing attachment of S. Typhimurium labeled with GFP to host cells stained with actin (red). Before imaging, S. Typhimurium was co-incubated with host cells cultured in transwell plates or the μFCCD at a MOI of 100 for 3 h. b Enumeration of S. Typhimurium cells that adhered to host cells. c Enumeration of S. Typhimurium cells that invaded host cells. S. Typhimurium cells that were not able to invade into the host cells and therefore remained on the surface of the cells were lysed with trypsin/EDTA before enumeration. * P < 0.01; Student’s t-test
3.2 Reconstitution of intestinal villi and cryptson the chip
Villi increase the surface area of the intestine for absorption, while crypts store stem cells and goblet cells, which have various functions. To investigate the effect of fluidic conditions on formation of villi and crypts, cells cultured in either transwell plates for 21 days or the μFCCD at 0.5 μl/min for 3 days were stained with DAPI and RP to visualize the nucleus and F-actin, respectively. Cells cultured in the μFCCD formed villi-like and crypt-like structures, while those grown in transwells failed to form any of these structures (Fig. 2b). Villi-like and crypt-like structures were previously reported when intestinal cells were grown under microfluidic conditions (Kim et al. 2012). Histology images (Fig. 4a) prepared by staining cells with toluidine blue, which stains nucleic acids blue and polysaccharides purple, revealed that the tissue structures formed by cells cultured in the μFCCD were taller than those that formed in cells grown in transwells. TEM images (Fig. 4b) showed that cells cultured in the μFCCD were tightly packed, as indicated by both the narrow intercellular space (IS) and small membranous vacuoles (Mb), compared to cells grown in transwells. Small vacuoles indicate that tissue is healthy (Panteris et al. 1993; Park et al. 2011; Schouten 1991). In addition, μFCCD-cultured cells had more microvilli than transwell-cultured cells (Fig. 4c). This result is further supported by the results shown in Fig. 4d. μFCCD-cultured cells had higher aminopeptidase activity than transwell-cultured cells. Aminopeptidase, which is an enzyme localized on the surface of the microvillus matrix, cleaves small peptides to amino acids and thus help intestinal epithelial cells to reabsorb amino acids (Hansen et al. 1992; Goodman 2010). These results suggested that cells grown in the μFCCD were more tightly connected to each other, more metabolically active, and had a larger surface area for absorption than those grown in transwells.
Villi and microvilli of Caco-2 cells cultured in either transwell plates for 21 days or μFCCD at 0.5 μl/min for 3 days. a Histology images of cells stained with toluidine blue. Cells grown in the μFCCD were taller than those grown in the transwell plates. b TEM images. The intercellular space (IS) is marked by the red and yellow lines while tight junctions are marked with arrowheads. I and II: Cells grown in a transwell plate for 21 days. III and IV: Cells grown in theμFCCDfor 3 days. Inset images show microvilli (Mv). N: nucleus. Mb: membranous vacuole. c SEM images of cells showing microvilli. d Aminopeptidase activity. *P < 0.01; Student’s t-test
3.3 Reconstitution of an intestinal barrier function on the chip
Conventional culture systems where cells grow as a monolayer with loose contacts between cells cannot reconstitute the human intestine where cells grow three-dimensionally and are tightly connected to each other. Figure 5a shows that cells cultured in the μFCCD stained more strongly for the tight junction protein occludin (Feldman et al. 2005; McCarthy et al. 1996) than cells cultured in transwell plates, which explains why the TEER value of cells grown in the μFCCD was higher than that of cells grown in transwells (Fig. 5b). Figure 5c shows that the Papp of cells grown in the μFCCD was lower than that of cells grown in transwells. This could be due to the increase in MUC-2 production by μFCCD-cultured cells. Diffusion of hydrophilic molecules is considerably lower in mucus than aqueous solutions (Lieleg et al. 2010), which may have retarded diffusion of dextran into the cell layer covered with MUC-2 in the μFCCD. Another conceivable reason for the lower apparent permeability in μFCCD is the stronger formation of tight junctions verified by occludin expression and TEM images of the cells in μFCCD. FITC-dextran, a hydrophilic molecule, mainly crosses intestinal epithelium via paracellular route through water-filled pores between the cells (Jin et al. 2009). The stronger formation of tight junctions in the Caco-2 cells in μFCCD may have inhibited transport of FITC-dextran across the epithelium. In a study where Caco-2 cells were co-cultured with goblet cells in three dimensional matrix, decrease in TEER and increase in permeability was observed, which was explained by decreased tight junction integrity by the authors (Li et al. 2013). Based on our observation on the tight junctions shown in Figs. 2 and 4, it is plausible that the fluidic environment of μFCCD caused the increase in the tight junction integrity.
Evaluation of the barrier function of Caco-2 cells cultured in either transwell plates for 21 days or the μFCCD at 0.5 μl/min for 3 days. a CLSM images of cells stained with DAPI (blue) and anti-occludin antibody (red). b TEER values of cells. c Paracellular transport coefficient of FITC-dextran (4 kDa) by Caco-2 cells. Papp: apparent permeability coefficient. d Expression of occludin by qRT-PCR* P < 0.01; Student’s t-test
In a recent study (Kim et al. 2012), a PDMS microfluidic device was used to subject Caco-2 cells to the cyclic strain and the shear stress that match in vivo conditions. In this study, 10 % strain with mean frequency of 0.15 Hz was used, and the volume flow rate of 0.5 μl/min exerted the shear stress of 0.02 dyne/cm2 on the cells. In our study, a similar flow rate was introduced, but the difference in the geometry of a cell culture chamber resulted in a lower shear stress of approximately 2 × 10−4 dyne/cm2, although the circular-shaped chamber creates non-uniform distribution of shear stress across the chamber. Our study suggests that Caco-2 cells can respond to lower shear stress than previously reported, and consequently change their physiological properties.
4 Conclusions
Both intrinsic and extrinsic barriers need to be reconstituted in in vitro intestinal models to accurately mimic the human intestine. The intrinsic barrier function is related to formation of tight junctions between intestinal epithelial cells, while the extrinsic barrier function is related to the production of mucin proteins. These two barriers are structural parts of the intestinal epithelium and are required for proper transport of molecules as well as immunity to microorganisms.
In this study, we demonstrated thatCaco-2 cells grown in a μFCCD formed villi- and crypt-like structures, had tight junctions between cells, and produced MUC-2, thereby mimicking the structure of the intestinal epithelium in vivo. This structural mimicry resulted in physiologically relevant absorption and strong immunity to a bacterial pathogen. Our intestinal model is therefore highly suitable for studying drug absorption as well as host-bacteria interactions.
References
M. Andrianifahanana, N. Moniaux, S.K. Batra, Regulation of mucin expression: mechanistic aspects and implications for cancer and inflammatory diseases. Biochim. Biophys. Acta 1765(2), 189–222 (2006)
P. Artursson, K. Palm, K. Luthman, Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv. Drug Deliv. Rev. 46(1–3), 27–43 (2001)
M.D. Basson, Paradigms for mechanical signal transduction in the intestinal epithelium. Category: molecular, cell, and developmental biology. Digestion 68(4), 217–225 (2003)
J.M. Biazik, K.A. Jahn, Y. Su, Y.N. Wu, F. Braet, Unlocking the ultrastructure of colorectal cancer cells in vitro using selective staining. World J. Gastroenterol. 16(22), 2743–2753 (2010)
X.D. Bu, N. Li, X.Q. Tian, P.L. Huang, Caco-2 and LS174T cell lines provide different models for studying mucin expression in colon cancer. Tissue Cell 43(201), 201–206 (2011)
J.C. Byrd, R.S. Bresalier, Mucins and mucin binding proteins in colorectal cancer. Cancer Metastasis Rev. 23(1–2), 77–99 (2004)
M. Dickson, J.P. Gagnon, Key factors in the rising cost of new drug discovery and development. Nat. Rev. Drug Discov. 3(5), 417–429 (2004)
L.C. Duffy, Interactions mediating bacterial translocation in the immature intestine. J. Nutr. 130(2S Suppl), 432S–436S (2000)
M.B. Esch, J.H. Sung, J. Yang, C. Yu, J. Yu, J.C. March, M.L. Shuler, On chip porous polymer membranes for integration of gastrointestinal tract epithelium with microfluidic ‘body-on-a-chip’ devices. Biomed. Microdevices 14(5), 895–906 (2012)
G.J. Feldman, J.M. Mullin, M.P. Ryan, Occludin: structure, function and regulation. Adv. Drug Deliv. Rev. 57(6), 883–917 (2005)
B.E. Goodman, Insights into digestion and absorption of major nutrients in humans. Adv. Physiol. Educ. 34(20), 44–53 (2010)
B.M. Gumbiner, Structure, biochemistry, and assembly of epithelial tight junctions. Am. J. Physiol. 253(6 Pt 1), C749–C758 (1987)
B.M. Gumbiner, Breaking through the tight junction barrier. J. Cell Biol. 123(6 Pt 2), 1631–1633 (1993)
P. Guo, A.M. Weinstein, S. Weinbaum, A hydrodynamic mechanosensory hypothesis for brush border microvilli. Am J Physiol Renal Physiol. 279(4), F698–F712 (2000)
J. Hansen, L. Andrew, R. Ruedy, M. Sato, Potential climate impact of mount pinatubo eruption. Geophys. Res. Lett. 19(2), 215–218 (1992)
D.H. Huh, Y.S. Torisawa, G.A. Hamilton, H.J. Kim, D.E. Ingber, Microengineered physiological biomimicry: organs-on-chips. Lab Chip 12(12), 2156–2164 (2012)
Y. Imura, Y. Asano, K. Sato, E. Yoshimura, A microfluidic system to evaluate intestinal absorption. Anal. Sci. 25, 1403–1407 (2009)
T. Ishikawa, T. Sato, G. Mohit, Y. Imai, T. Yamaguchi, Transport phenomena of microbial flora in the small intestine with peristalsis. J. Theor. Biol. 279(1), 63–73 (2011)
K. Izumikawa, Y. Hirakata, T. Yamaguchi, H. Takemura, S. Maesaki, K. Tomono, S. Igimi, M. Kaku, Y. Yamada, S. Kohno, S. Kamihira, Escherichia coli O157 interactions with human intestinal Caco-2 cells and the influence of fosfomycin. J. Antimicrob. Chemother. 42(3), 341–347 (1998)
Y. Jin, Y. Takegahara, Y. Sugawara, T. Matsumura, Y. Fujinaga, Disruption of the epithelial barrier by botulinum haemagglutinin (HA) proteins - differences in cell tropism and the mechanism of action between HA proteins of types A or B, and HA proteins of type C. Microbiology 155(Pt 1), 35–45 (2009)
J.A. Kiernan, Histological and histochemical methods: theory and practice (Butterworth Heinemann, Oxford, 1999). x, 502 p
H.J. Kim, D.E. Ingber, Gut-on-a-Chip microenvironment induces human intestinal cells to undergo villus differentiation. Integr. Biol. 5(9), 1130–1140 (2013)
H.J. Kim, D. Huh, G. Hamilton, D.E. Ingber, Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip 12(12), 2165–2174 (2012)
S.H. Kim, J.W. Lee, I. Choi, Y.C. Kim, J.B. Lee, J.H. Sung, A microfluidic device with 3-d hydrogel villi scaffold to simulate intestinal absorption. J. Nanosci. Nanotechnol. 13(11), 7220–7228 (2013)
S.H. Kim, M. Chi, B. Yi, S.H. Kim, S. Oh, Y. Kim, S. Park, J.H. Sung, Three-dimensional intestinal villi epithelium enhances protection of human intestinal cells from bacterial infection by inducing mucin expression. Integr. Biol. 6(12), 1122–1131 (2014)
R.G. Lentle, P.W.M. Janssen, Physical characteristics of digesta and their influence on flow and mixing in the mammalian intestine. J. Comp. Physiol. B. 178(6), 673–690 (2008)
F. Leonard, E.M. Collnot, C.M. Lehr, A three-dimensional coculture of enterocytes, monocytes and dendritic cells to model inflamed intestinal mucosa in vitro. Mol. Pharm. 7, 2103–2119 (2010)
N. Li, D. Wang, Z. Sui, X. Qi, L. Ji, X. Wang, L. Yang, Development of an improved three-dimensional in vitro intestinal mucosa model for drug absorption evaluation. Tissue Eng. Part C Methods 19(9), 708–719 (2013)
O. Lieleg, I. Vladescu, K. Ribbeck, Characterization of particle translocation through mucinhydrogels. Biophys. J. 98(9), 1782–1789 (2010)
K.M. McCarthy, I.B. Skare, M.C. Stankewich, M. Furuse, S. Tsukita, R.A. Rogers, R.D. Lynch, E.E. Schneeberger, Occludin is a functional component of the tight junction. J. Cell Sci. 109, 2287–2298 (1996)
I. Meyvantsson, D.J. Beebe, Cell culture models in microfluidic systems. Annu. Rev. Anal. Chem. 1, 423–449 (2008)
K.W. Oh, L. Lee, B. Ahn, E.P. Furlani, Design of pressure-driven microfluidic networks using electric circuit analogy. Lab Chip 12(3), 515–545 (2012)
J. Olesen, L. Edvinsson, Basic mechanisms of headache, Amsterdam; NY, USA: Elsevier; Sole distributors for the USA and Canada, Elsevier Science Pub. Co. xxvi, 492 p (1988)
S.P. Olesen, P.F. Davies, D.E. Clapham, Muscarinic-activated K+ current in bovine endothelial cells. Circ. Res. 62(2), 1059–1064 (1988b)
E. Panteris, P. Apostolakos, B. Galatis, Microtubule organization, mesophyll cell morphogenesis, and intercellular space formation in Adiantum capillus veneris leaflets. Protoplasma 172(2–4), 97–110 (1993)
S. Park, H.J. Chun, J.S. Jang, B. Keum, Y.S. Seo, Y.S. Kim, Y.T. Jeen, H.S. Lee, S.H. Um, C.D. Kim, H.S. Ryu, C.S. Uhm, S.J. Lee, Is intercellular space different among layers in normal esophageal mucosa? An electron microscopic study. Dig. Dis. Sci. 56(12), 3492–3497 (2011)
B.M.H. Schneeberger, Die Musikerfamilie Fürstenau: Untersuchungen zu Leben und Werk (Lit, Münster, 1992)
J.P. Schouten, Revolution of the mystics: on the social aspects of Vīraśaivism (Kok Pharos Pub. House, Kampen, 1991). xiii, 331 p
J.M. Staddon, L.L. Rubin, Cell adhesion, cell junctions and the blood-brain barrier. Curr. Opin. Neurobiol. 6(5), 622–627 (1996)
T.M. Straub, J.R. Hutchison, R.A. Bartholomew, C.O. Valdez, N.B. Valentine, A. Dohnalkova, R.M. Ozanich, C.J. Bruckner-Lea, Defining cell culture conditions to improve human norovirus infectivity assays. Water Sci. Technol. 67(4), 863–868 (2013)
J.H. Sung, J. Yu, D. Luo, M.L. Shuler, J.C. March, Microscale 3-D hydrogel scaffold for biomimetic gastrointestinal (GI) tract model. Lab Chip 11(3), 389–392 (2011)
J.H. Sung, M.B. Esch, J.M. Prot, C.J. Long, A. Smith, J.J. Hickman, M.L. Shuler, Microfabricated mammalian organ systems and their integration into models of whole animals and humans. Lab Chip 13(7), 1201–1212 (2013)
F.J. Van Asten, H.G. Hendriks, J.F. Koninkx, B.A. Van der Zeijst, W. Gaastra, Inactivation of the flagellin gene of Salmonella enterica serotype Enteritidis strongly reduces invasion into differentiated Caco-2 cells. FEMS Microbiol. Lett. 185(2), 175–179 (2000)
R.B. van Breemen, Y. Li, Caco-2 cell permeability assays to measure drug absorption. Expert Opin. Drug Metab. Toxicol. 1, 175–185 (2005)
P. Zanassi, M. Paolillo, A. Feliciello, E.V. Avvedimento, V. Gallo, S. Schinelli, cAMP-dependent protein kinase induces cAMP-response element-binding protein phosphorylation via an intracellular calcium release/ERK-dependent pathway in striatal neurons. J. Biol. Chem. 276(15), 11487–11495 (2001)
Acknowledgments
This work was supported by a grant from the Korea Science and Engineering Foundation (KOSEF) funded by the Korea government (MOST) (#2012-0001138), and by a grant from the Public Welfare & Safety Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (#2012R1A2A2A01012221 and #2012-0006522). JHS and BL acknowledges support from Hongik University Research Fund.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chi, M., Yi, B., Oh, S. et al. A microfluidic cell culture device (μFCCD) to culture epithelial cells with physiological and morphological properties that mimic those of the human intestine. Biomed Microdevices 17, 58 (2015). https://doi.org/10.1007/s10544-015-9966-5
Published:
DOI: https://doi.org/10.1007/s10544-015-9966-5