Abstract
Microorganisms and specifically motile bacteria have been recently added to the list of micro-actuators typically considered for the implementation of microsystems and microrobots. Such trend has been motivated by the fact these microorganisms are self-powered actuators with overall sizes at the lower end of the micrometer range and which have proven to be extremely effective in low Reynolds number hydrodynamic regime of usually less than 10−2. Furthermore, the various sensors or taxes in bacteria influencing their movements can also be exploited to perform tasks that were previously considered only for futuristic artificial microrobots. Bacterial implementations and related issues are not only reviewed, but this paper also proposes many techniques and approaches that can be considered as building blocks for the implementations of more sophisticated microsystems and microrobots.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The conception of efficient, compact and self-powered microsystems and microrobots faces several technical challenges. One of the first challenges being investigated was certainly the actuation aspect of microrobots (Trimmer and Jebens 1989; Dario et al. 1992; Abbott et al. 2007; Sharma and Mittal 2008). Indeed, for microrobots designed to operate in a liquid medium, the main concern for the actuators lies in the fact that they must operate in low Reynolds number hydrodynamics (Happel and Brenner 1973; Purcell 1976) where self-propelled microorganisms (Hancock 1953) and in particular, bacterial flagellated actuation were known to be among the most efficient mechanisms in such a regime (Shoesmith 1960; Holwill and Burge 1963; Berg 1975; Keller and Rubinow 1976; Lighthill 1976; Greenberg and Canale-Parola 1977a; Berg and Turner 1979; Higdon 1979; Purcell 1997; Berg 2004; Chattopadhyay et al. 2006). Such observations stimulated research efforts to model flagellar bacterial motions (Chwang and Wu 1971; Winet and Keller 1976; de la Torre and Bloomfield 1977; Johnson and Brokaw 1979; Phan-Thien et al. 1987; Ramia 1991; Sleigh 1991; Goto et al. 2000; Lowe 2001; Trachtenberg et al. 2003; Earl et al. 2007; Hirofumi and Netz 2007; Lobaton and Bayen 2007). Although other modes of bacterial propulsion were studied (e.g. Spiroplasma, helical bacteria propelled by the propagation of kink pairs down the length of the cell body (Shaevitz et al. 2005; Yang et al. 2009)), the best understood and studied method based on flagellar propulsion was considered in assessing new biomimetic designs for microrobots (Rathore and Sharma 2010). These designs were inspired by the rotation of bacterial flagella (Berg and Anderson 1973; Lowe et al. 1987) while being validated and tested on macroscale implementations and prototypes (Honda et al. 1996; Edd et al. 2003; Ishiyama et al. 2003; Behkam and Sitti 2004; 2005, 2006a).
Then artificial micro-swimmers were developed starting with the controlled swimming motion of an artificial flexible flagellum made from a linear chain of colloidal magnetic particles linked by DNA and attached to a red blood cell (Dreyfus et al. 2005). The latter led to magnetically controlled artificial nano-structured propellers (Ghosh and Fisher 2009), helically symmetric flexible polymer structures (Garstecki et al. 2009), and micrometer-scale artificial bacterial flagella (ABF) (Zhang et al. 2009a; Tottori et al. 2012). ABF consists of a micro- or nano-scale helical propeller attached to a ferromagnetic bead acting as the head which is actuated using a torque from a rotational magnetic field. Interesting enough is the fact that ABF can have a size comparable to a natural flagellated bacterium.
Although accurate control of ABF has been demonstrated through micromanipulation tasks (Zhang et al. 2009b; 2010) and the fact that optimization of flagellar shape based on previous studies (Nasseri and Phan-Thien 1997; Fijita and Kawai 2001) and within nano- micro-fabrication constraints could potentially increase further the displacement velocity of such artificial swimmers, the approach still relies on an external source of power for propulsion. Indeed, since magnetism was and still the preferred choice for the actuation of such untethered micro-swimmers, the use of an external rotating magnetic field to induce a rotational torque on ABF for displacement purpose requires much less power compared to the use of a magnetic pulling force. This fact allows such artificial micro-swimmers mimicking flagellar propulsion to be miniaturized further while offering the possibility to increase the distance between the external source and the micro-swimmers for remote operations including potential medical interventions in the human body. But although improved significantly compared to other magnetically-actuated microdevices operating in low Reynolds number regime, the remote distance and the level of miniaturization achievable with an external source of power for propulsion still represent constraints that can limit its applicability for specific cases.
Toward the elimination of the need for an external source of power for actuation, the development of synthetic (artificial) molecular motors were investigated (Davis 1999; Balzani et al. 2000). But in a more practical point-of-view, considering the challenge of reproducing artificially the complexity of natural molecular machines, efforts were put forward to use natural entities for actuation purpose instead of replicating them artificially.
An initial example was a hybrid implementation consisting of an F1-adenosine triphosphate synthase (F1-ATPase) biomolecular motor actuating a fabricated inorganic nanopropeller (Soong et al. 2000). The F1-ATPase molecular motor is not only known to be the rotary motor found in flagellated bacteria (Berg 2003) but its conception relying on a rotor turning 360° stepwise inside a stator has design similarities with its counterpart macroscale electrical motor designed by engineers with the exception of the material and the energy source. Indeed, flagellated bacteria as self-powered actuators avoid the technical constraints related to electrical energy storage and generation that make miniaturization of self-powered untethered artificial microsystems and microrobots so difficult.
Bacteria use the free energy stored in transmembrane ion gradients to manufacture ATP to provide a proton flux (Meister et al. 1987) that generates a torque (Berg 1995; Samuel and Berg 1996; Ryu et al. 2000) for rotating the flagella and hence, achieving a corresponding swimming velocity (Chen and Berg 2000; Inoue et al. 2008). But more interesting is the fact that F1-ATPase was reported to convert chemical into mechanical energy with near 100 % efficiency (Yasuda et al. 1998). It is then not surprising that not only the ATPase molecular motors but the whole bacteria containing such efficient energy conversion unit were also considered as self-powered micro-actuators in microsystems.
2 Bacterial microsystems
Initial bacterial microsystems took the form of a monolayer consisting of flagellated bacteria attached to a solid surface and referred to as a bacterial carpet that was used to pump liquid in a microfluidic channel (Darnton et al. 2004; Kim and Breuer 2008) or to enhance mixing in microfluidic systems (Kim and Breuer 2007a). The chemotactic swimming characteristics of flagellated bacteria were also exploited to enhance controlled mixing in microfluidic systems (Kim and Breuer 2007b).
The static substrate used in microfluidic applications then evolved to integrate moving micro-parts. For instance, gliding bacteria were used to actuate a micro-rotary motor (Hiratsuka et al. 2006). Asymmetric sub-millimeter gears were also actuated by random movement of bacteria (Sokolov et al. 2010). Indeed, instead of carefully aligning and binding flagellated bacteria on the synthetic surface of a microsystem in order to have them work cooperatively, one strategy is to obtain a spontaneous and unidirectional motion or rotation of fabricated objects by immersing them in an active bacterial bath within an asymmetric environment (Angelani et al. 2009; Di Leonardo et al. 2010).
A microelectronic bacterial microsystem was also proposed (Martel 2006a; Lu et al. 2007a; b) and designed for the detection of live pathogenic bacteria using controlled flagellated magnetotactic bacteria (MTB) (Blakemore 1975; Blakemore 1982). Instead of relying on random motion of free-swimming bacteria as in some previous bacterial microsystems, another strategy was used where magnetotaxis (Frankel and Blakemore 1980; Frankel 1984; Debarros et al. 1990) inherent in MTB was exploited to magnetically guide them in a deterministic manner between pairs of microelectrodes in microfluidic channels drilled through a microelectronic integrated circuit. To accelerate the detection process, MTB coated with phages or antibodies with binding specificity to the targeted pathogens could be directed to sweep through the sample medium and redirected through the narrowed microfluidic channels with a potential pathogen attached to the MTB cell. In such a case, detection could be done through specific impedance signatures recorded by each pair of electrodes prior to be processed by the microelectronic circuit.
Magnetotactic bacteria as integral parts of microsystems were also considered for other purposes such as directional controlled microcarriers to transport samples in microfluidic microsystems (Lu and Martel 2006a; 2006b; 2007) which expanded for transport on microelectronic circuits and other microsystems (Martel 2007), and in micromanipulation. For instance, the orientation and location of the MTB can be controlled with minimum electrical power due to the proximity of micro-electromagnets arrays capable of inducing a directional electro-magnetic torque on the chain of aligned membrane magnetic nanoparticles known as magnetosomes synthesized inside each cell (Bazylinski and Frankel 2004) during cultivation. For example, micro-electromagnets consisting of multiple layers of lithographically patterned conductors were used to trap and control the orientation of a single or groups of MTB prior to remove their cellular membranes by cell lysis for leaving the magnetic nanoparticles on a substrate (Lee et al. 2004). Another system using micro-electromagnets arrays where micro- or nano-objects to be manipulated could be attached to the surface of the cell of the MTB has also been developed (André et al. 2007).
3 Bacterial microrobots
A motile bacterium can be seen as an actuator-sensor-processing unit, i.e., with the fundamental embedded functionalities found in autonomous robots. Sensory-based reactions or signal transduction mechanisms shown by motile microorganisms are known as “taxes” (positive and negative taxes indicate taxes towards the source and away from the source respectively) and the behavioral response of bacteria seeking optimal metabolic activity, a process referred to as energy taxis (Taylor and Zhulin 1998; Taylor et al. 1999; Alexandre and Zhulin 2001; Schweinitzer and Jesenhans 2010) or bacterial taxis (Glagolev 1980; Krell et al. 2011) when referring specifically to bacteria can be exploited to control these motile microorganisms to execute specific tasks.
The Paramecium, a microorganism propelled by cilia and much larger than a single bacterium, was initially used as a prototype biomicrorobot (also referred to as microbiorobot) to demonstrate this concept (Fearing 1991). This was done by controlling its motion along a planned trajectory using an approach based on previous observations of the galvanic response (Roberts 1970), the bioelectric control of ciliary activity (Eckert 1972), and the cilia frequency and orientation response (Machemer and Eckert 1975) of this microorganism.
In its simplest form, a single bacterium can be seen as a bacterial microrobot with an overall dimension in the extreme lower end of the micrometer (μm) scale. The same bacterium can also take the form of a bio-hybrid microrobot or microcarrier with one or more nanoscale synthetic or artificial objects to a few micrometers in size single object attached to its cell. A larger bio-hybrid microrobot can also be made of several motile bacteria acting as micro-actuators attached to a larger artificial microstructure.
For instance, several bacteria attached to a fragment of polydimethylsiloxane without motion control (steering) has been initially reported in (Darnton et al. 2004) and later with a polystyrene bead (Behkam and Sitti 2006b) with the aim to potentially applying it for patterned PDMS micro-cylinders (Behkman and Sitti 2008a), again without steering. Steering was done on a flagellated algae (slightly larger than a bacterium) using phototaxis to carry a few micrometer in diameter polystyrene bead attached to the cell using surface chemistry prior to be released using photochemistry (Weibel et al. 2005).
A method based on magnetotaxis for the directional control along a predetermined path or trajectory of one or more bacteria and a single bacterium carrying an attached object and acting like a microrobot has been initially filed in (Martel 2004; 2006b). Experimental results of a 3-micrometer bead attached to a single flagellated MC-1 bacterium being accurately controlled with magnetotaxis along a preprogrammed trajectory were initially published in (Martel 2006c) and described in more details in (Martel et al. 2006). One of the main motivations for using magnetotaxis directional control instead of another taxis-based directional control method was to assess the possibility of targeted delivery of therapeutic agents using such controlled bacterial microcarriers in the human blood vessels (Martel 2006d). Indeed, magnetism provides a suitable and noninvasive communication link between an external computerized control unit and the magnetotactic bacteria at any depths inside the human body. Furthermore, since the chain of magnetosomes in the cell of MTB responds to directional magnetic field as low as the geomagnetic field, the power required by a magnetic source for directional control purpose is therefore much lowered and hence easier to implement compared to the ones used to induce a directional displacement force on artificial entities including micro-swimmers. Unlike the Paramecium or the algae used as a directionally controlled biomicrorobot, the rounded cell of the MC-1 magnetotactic bacterium has a sufficiently small diameter (1–2 μm) to allow it to transit through the tiniest capillaries found in humans to reach targets such as tumors while offering the maximum surface on the cell to attach therapeutic payloads (Martel et al. 2009).
3.1 Directional control methods
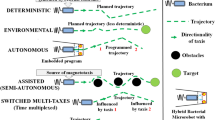
Directional control of bacteria is for sure a fundamental requirement for the implementation of bacterial microrobots. In this paper, we categorize the main directional control methods for bacteria as taxis-based or structural-based. In turn, taxis directional control as depicted in Fig. 1 is divided as deterministic, environmental, autonomous, assisted, and multi-taxes control.
3.1.1 Deterministic directional control
Deterministic directional or steering control allows a bacterium or a bacterial microrobot to follow precisely a predefined path or trajectory, often being under the control of an external computer. Although deterministic magnetotaxis directional control of motile bacteria (magnetotactic bacterial microrobots) has shown accurate results while offering significant advantages for many applications, other deterministic taxis-based control methods are also possible.
Besides magnetotactic bacterial microrobots, other deterministic taxis-based directional control methods offer the possibilities for the implementation of galvanotactic, phototactic, and electrophoretic bacterial microrobots, to name but only three examples. For instance, ultraviolet light was used to control the flagellar rotational motion and hence affecting the motility of the bacteria (phototactic stop-resume control), a phenomenum previously reported in (Taylor and Koshland 1975). In this example, direct current electric fields were used to achieve electrophoretic directional control of a two-dimensional movement of a micro-fabricated structure coated with a monolayer of motile bacteria (Steager et al. 2011). Unlike in galvanotaxis which is caused by a difference in electrophoretic mobility between the bacterial cell and the flagella (Shi et al. 1996), electrophoretic directional control is caused by the inherent charge of the bacterial cells. Although electrophoretic directional control could also be achieved by a charge in the bacterial artificial microstructure itself, it would impose specific requirements in the microstructure. Furthermore, having such taxis embedded in the bacterium itself, allows for the use of a single bacterium to act as a microrobot. The same holds true for other deterministic taxis modes including directional magnetic control using a torque on a magnetized bacterial artificial microstructure.
3.1.2 Environmental and autonomous directional control
Environmental and autonomous directional control methods for bacteria exploits a bacterial taxis acting as onboard sensor to seek and move towards (or away) a specific target without the help of a source of control external to the bacterial microrobot for autonomous directional control; and outside in the environmental liquid medium for environmental directional control. Although in some particular cases, environmental directional control could be done with a taxis mentioned in deterministic directional control to find the source inside a liquid medium influencing the same respective taxis, practically, another taxis including but not limited to aerotaxis (Baracchini and Sherris 1959; Stanbridge and Preston 1969; Barak et al. 1982; Shioi et al. 1988; Wong et al. 1995; Zhulin et al. 1996; Hou et al. 2000) and chemotaxis (Adler 1966; Berg and Brown 1972; Adler and Tso 1974; Lovely and Dahlquist 1975; Greenberg and Canale-Parola 1977b; Noble and Levine 1986; Adler 1988; Schnitzer et al. 1990; Crenshaw 1993; Zhulin and Armitage 1993; Hillesdon et al. 1995; Armitage and Schmitt 1997) is usually considered.
While aerotaxis is a general term that defines the movement of a microorganism toward (positive aerotaxis) or away (negative aerotaxis) from air or oxygen, the term oxytaxis is also used to indicate the movement or orientation towards a supply of oxygen. An example of environmental directional control is in aerotaxis that was exploited in clinical medicine where anaerobic motile bacteria were used to seek oxygen depleted areas in heterogeneous tumor tissue (Coley 1891; Nauts et al. 1946; Willis 1960) as discussed later in this paper.
The general principle of motion control of bacterial microrobots using oxygen gradients has been described in (Shechter and Martel 2010). This principle led to the possibility of programming bacterial microrobots to achieve environmental directional control using oxygen micro-bubbles in the surrounding fluidic environment or inside a bacterial artificial microstructure to implement autonomous directional control. Such concept referred to as oxygen programming, has been proposed and described in (Martel 2010) and it is represented schematically in Fig. 1 for autonomous directional control. With oxygen programming, not only the miniaturization constraints of powering an onboard electronic computer could be avoided since the approach does not rely on electrical energy, but the desired motion behavior of a single bacterium, a single aggregate or several aggregates of bacteria could also be defined from a computer graphical interface or a special computer language. Such commands or instructions could be compiled and written as a pattern of oxygen micro-bubbles of various sizes and locations inside the microstructure (autonomous) of a bacterial robot or in the surrounding fluidic environment (environmental). Sequential execution and the timing from one sequence to the next is then achieved by the consumption of oxygen by the bacteria being diffused from micro-bubbles of predetermined locations and sizes.
Bacterial chemotaxis on the other hand as first described by T.W. Engelmann and W.F. Pfeffer in 1881 and 1884 respectively, is known as the phenomenon in which bacteria or bacteria propelled microstructures such as microbeads (Kim et al. 2011) direct their movements according to certain chemicals in the fluid environment. As such, chemotaxis can be used in environmental directional control to seek particular sources of chemicals without external control. Negative chemotaxis can also be used to indicate by observation of the movement of the motile bacteria, the presence of other chemicals that are repulsive to the bacteria, e.g. phenol. Although never attempted yet, chemical programming as opposed to oxygen programming for autonomous directional control of bacterial microrobots could potentially be achieved for positive chemotaxis using a nutrient that can be consumed by the bacteria (e.g. glucose programming).
But besides the limitation in the types of chemicals that can be considered in positive chemotactic directional control for instance, chemotactic motion is not as straight and predictable (Berg 1983; Nossal 1983) as in pure deterministic directional control such as magnetotactic motion. As such and confirmed later with bacteria attached to a bead (Kim et al. 2011), chemotactic directional control may not be suitable for applications where directional control accuracy is critical. Indeed, chemotactic movement is the result of alternating random tumbles that re-orient the bacterium, and straight swimming phases. Temporal sensing in motile bacteria can help achieving longer straight swimming patterns only in the presence of a higher concentration of an attractant.
From observations, models of chemotactic motions that could potentially be used in applications involving chemotactic bacteria or chemotactic bacterial microrobots have also been developed (De Robertis and Peluffo 1951; Keller and Segel 1971; Brown and Berg 1974; Alt 1980; Koshland 1980; Alt et al. 1985; Tranquillo and Lauffenburger 1987; Rivero et al. 1989; Frymier et al. 1993; Spiro et al. 1997; Bearon and Pedley 2000; Muller et al. 2002; Othmer and Hillen 2002; Bearon 2003; Mello and Tu 2003; Arabagi et al. 2011). Such models could be used to predict the motion paths of bacteria and chemotactic bacterial microrobots in well known fluid environment or inversely, although not yet published, the chemotactic motion of the bacteria could be used against chemotactic behavioral models to estimate the locations and assessing the concentration of certain chemicals in the fluid environment.
An autonomous directional control microelectronic magnetotactic bacterial microrobot has also been proposed (Martel and André 2006; André and Martel 2007). The microrobot had an onboard control computer that was designed to generate from embedded software, the onboard directional magnetic fields. Such directional fields were intended for directional control of free-swimming MTB acting as micro-propulsion systems inside special embedded reservoirs. Since the onboard electronic circuit was powered by photovoltaic cells (André and Martel 2006), such type of hybrid implementation has the disadvantage of requiring an external power source. On the other hand, it could also deal with the addition of proper sensors, with attractants that are not compatible with known motile chemotaxis bacteria.
Other strategies relying on chemical properties instead of artificial technologies as the previous example can be used in some cases to influence the chemotactic responses. For instance, it was shown that change in intracellular pH of Escherichia coli mediates the chemotactic response to certain attractants and repellants (Repaske and Adler 1981). Again, this example emphasis the fact that the development of bacterial microrobots should be initiated from an interdisciplinary assessment of the methods best suited for a given task having specific requirements. Other environmental conditions can also influence the swimming direction of bacteria. For instance, porous media has an influence on the random walk for bacterial migration (Duffy et al. 1995).
3.1.3 Assisted directional control
Assisted bacterial directional control has been first proposed and described for tumor targeting (Martel 2006a; Martel et al. 2009). Indeed, the dimensions of capillaries in angiogenesis network providing the only routes to deliver therapeutic cargos carried by flagellated magnetotactic bacteria to a tumor are well below the spatial resolution of any medical imaging modalities. As such, no information of a path to be followed can be gathered and therefore, closed-loop servo control cannot be used in this particular application. Instead, an artificial magnetic pole generated at a targeted location in the tumor is used. Through magnetotaxis, the bacteria swim toward such artificial pole following the line of magnetic field oriented toward such pole. But like futuristic sophisticated artificial microrobots, they do so by avoiding obstacles and seeking a path along the direction of the tumor in this complex and chaotic blood network instead of remaining immobile when encountering a relatively large vessel wall. This type of autonomy referred to also as path finding (PF) capability, is critical to enhance targeting and hence, the therapeutic outcome. Various time-multiplexed magnetic field directional methods with frequencies adapted to the motion behavior of the bacteria are used to create such pole in a 3D space. The whole process is also assisted by various magnetic field modulation modes taking into account the characteristics of the angiogenesis network as well as the motion behaviors of the bacteria when encountering various types of obstacles.
3.1.4 Multi-taxes directional control
Although dual external stimuli-based deterministic directional control consisting of galvanotactic (change in swimming direction toward the cathode) with phototactic (high intensity broadband light causing a rotational motion of the cells) directional control of ciliate protozoa has been demonstrated (Kim et al. 2009), applying such approach with the use of more than one type of bacterial taxes for directional control is also possible.
For instance, the AMB-1 MTB shows phototaxis response (Chen et al. 2011) independent (i.e. with equal migration) of the wavelength (tested from 400 to 750 nm). This suggests that phototaxis could temporarily replace magnetotaxis for directional control by lowering the magnitude of the magnetic field. Such type of scenario of switching directional taxis has been proposed in (Martel et al. 2010) for switching between magnetotaxis and aerotaxis for the MC-1 MTB in this particular case. In (Martel 2010), magneto-aerotaxis (simultaneous multi-taxes instead of switched or time-multiplexed multi-taxes) as previously observed for marine coccoid bacteria (Frankel et al. 1997) has been proposed to achieve accurate directional control and maximum propelling force by aligning all bacteria while achieving full autonomy through on-board oxygen programming. Light-induced tumbling response in chemotaxis motility (chemo-phototaxis) has also been recorded (Macnab and Koshland 1974). Previous observations on the chemotactic, magnetotactic and tactile behaviours in magnetic spirilum (Spormann and Wolfe 1984) suggest that more than dual taxes in directional control are also possible. Although little is known so far about multi-taxes directional control used simultaneously or one at a time (switched multi-taxes), it is obvious that combining taxes offers many opportunities and flexibility in the development of bacterial microrobots characterized by a higher level of sophistication.
For instance, considering aerotaxis and magnetotaxis only, switched or simultaneous multi-taxes directional control could offer a significant advantage depending upon the application. Switched multi-taxes directional control could be used to seek a distant source of oxygen in an aqueous medium where the oxygen gradient would be too weak to be initially detectable by the bacteria. In such a case, magnetotaxis could be used to direct the bacteria closer to such a source prior to switch to aerotaxis. The same idea could be applied to other taxes including but not limited to phototaxis and chemotaxis. For simultaneous multi-taxes, magneto-aerotaxis in this particular case could be advantageous if aerotaxis is used to influence the movement of the bacteria whereas magnetotaxis would be used to force the alignment of the bacteria towards a suitable direction in order to optimize the flow generated by the flagella. In this particular case, the absence of magnetotaxis would lead to random orientations of the bacteria and hence, a far from optimal flow that could be used for moving micro-objects, to mention but only one example.
3.1.5 Structural directional control
As mentioned earlier, an asymmetric structure can result in a spontaneous and unidirectional motion or rotation of fabricated objects immersed in an active bacterial bath (Angelani et al. 2009; Di Leonardo et al. 2010). But other forms of structural directional control are possible. For instance, high throughput and dense structural directional controlled bacterial transport was achieved using geometrically-selected fluidic micro-channels and/or 3D microfiber structures (Martel and Mohammadi 2007) as depicted in Fig. 2.
Capillary force being exploited to implement high density fluidic channel networks for bacterial transport in square channel (top-left) and in 3D fiber networks; combining taxis and structural directional mode such as increasing the magnitude of the magnetic field within a specific range results in continuous back-and-forth movements of magnetotactic bacteria along a specific channel. (NanoRobotics Laboratory, École Polytechnique de Montréal)
For example a single square fluidic micro-channel replaced four channels for the bacteria by exploiting capillary force at each corners. The same approach was also applied between two or more microfibers and on a single fiber where a thin layer of fluid on the surface of the microfiber could be retained. In the latter case, it was demonstrated that a fluidic layer thickness of only a few micrometers was sufficient for the MTB to swim along providing efficient yet very dense transport channels where bacterial transport was done in two reciprocal directions simultaneously. By adding a higher magnitude magnetic field of just approximately 120 Gauss using stronger permanent magnets or placing the magnets closer to the fluidic microstructure, the bacteria automatically reversed their swimming directions when reaching the end of a swimming path which was not the case when the field intensity was lower. Hence, by integrating magnetic fields of various intensities within fluidic microstructures and constraining the swimming paths of the bacteria with thin layers of water, directional controlled micro-transport systems requiring no electrical power could be implemented. The same idea using MTB swimming in a thin fluidic layer has also been applied on electrical wires in microelectronic systems (Saeidlou et al. 2009). In (Shechter and Martel 2009), magnetic field and structural geometries have been exploited to change the directional preferences of MTB and hence, increasing the level of directional control in more complex structures without the use of an electrical controller.
Restricted geometries (Biodi et al. 1998) and solid planar surfaces can also be exploited to influence the motion behavior of flagellated bacteria (Harkes et al. 1992; Frymier et al. 1995; DiLuzio et al. 2005). For instance, the implementation of a solid surface can be used to force the bacteria to swim in circles as recorded in (Lauga et al. 2006) while constrained areas such as glass-capillaries have shown to have an influence on the chemotactic motions of bacteria (Berg and Turner 1990).
3.2 Swimming velocity and stop/resume control methods
Although directional control is critical for bacterial microrobots, swimming velocity control including stop and resume (also known as on/off) control may be a requirement for some specific tasks. Indeed, although the trend in many applications is to achieve the fastest execution of a given task including maximum velocities in transport or delivery where there will be no motivation for reducing the swimming velocities, in particular cases, especially when the bacteria are attached to an artificial microstructure, swimming velocity and/or stop/resume control can be desirable or essential.
Such bacterial velocity control can be achieved in different ways. For instance, it is known that the pH level can regulate genes for flagellar motility (Maurer et al. 2005) and as such, on/off control can be done by modifying the property of the liquid medium by adding the proper chemicals (Gannon et al. 1991; Gross and Logan 1995; Behkam and Sitti 2007). As mentioned earlier, phototactic stop/resume is also possible (Taylor and Koshland 1975). It was also known awhile ago that the temperature influence motility (Preston and Maitland 1952) and that the motility and the chemotaxis behavior of the Escherichia coli are also influenced by the temperature (Maeda et al. 1976). Such effect of temperature has been recorded at body temperature to assess the motility of the MC-1 magnetotactic bacteria as therapeutic microcarriers for cancer therapy in humans (Martel et al. 2009) indicating a continuous decrease before no motility is observed after ~40 min. of exposure. The increase in the pitch angle of the MTB helical motion at higher magnetic fields has also been observed (Yongxin et al. 2009). Such increase in amplitude of the helical motion of MTB translates onto an apparent decrease of the velocity along the longitudinal axis. Other environmental approaches are also possible such as the addition of oxygen and arginine that can also play a role on bacterial motility (Sheeris et al. 1957).
Structural approaches such as the proximity of a solid–liquid interface can also influence bacterial swimming speed (Frymier and Ford 1997). The same holds true for environmental approaches (Adler and Templeton 1967) such as a modification or an exploitation of the viscosity of the environmental liquid medium (Schneider and Doetsch 1974; Ramia et al. 1993). For instance, it is known that viscosity in linear-polymer solutions increases bacterial swimming speeds (Magariyama and Kudo 2002).
Although other methods are likely to be developed, these approaches provide an idea of the possibilities in bacterial velocity control. In turn, among several possible avenues, one could exploit the knowledge about the causes of the variations in swimming velocity of bacteria to use them to implement novel motile biosensors or measurement/characterization devices.
4 Attached vs. free-swimming bacteria
It is clear that when a single bacterium must carry nanometer-scale objects or a single object with dimensions of no more than a few micrometers, such load as depicted in Fig. 3 is typically attached to the cell. But when such artificial object to be moved (transported or manipulated), or when the application requires more than a single bacterium, then two approaches are possible: attaching or not attaching the bacteria to the artificial microstructure.
4.1 Bacterial adhesion and patterning
For the first approach, microbial adhesion (Busscher and Weerkamp 1987; Fletcher 1996; Hermansson 1999) and patterning are two fundamental requirements. Bacterial adhesion can be done in various ways including physicochemical approaches (van Loosdrecht et al. 1989), ionic attachments (Zita and Hermansson 1987), hydrogen bonds (Jucker et al. 1997), the use of antigen (e.g. Palomar et al. 1995), the exploitation of hydrophobic properties (van Loosdrecht et al., 1987; Van der Mei et al. 1998; Salerno et al. 2004) and the surface properties of the bacteria (Van der Mei et al. 1992), as well as electrostatic parameters (van Loosdrecht et al. 1990). Successful attachments have been done on many types of surface including but not limited to cells on cells (Van Oss 1989), polymeric surfaces (Tsuneda et al. 2003) including polydimethylsiloxane (Darnton et al. 2004) and polysterene (Rosenberg 1981; McEldowney and Fletcher 1986; Martel et al. 2006; Behkam and Sitti 2006b) as well as SU-8 microstructures (Steager et al. 2007), and glass (McClaine and Ford 2002a; McClaine and Ford 2002b). Efforts are also underway to attach using polyclonal and monoclonal antibodies other types of nanostructures such as drug-encapsulated liposomes being attached to the cell of the MC-1 bacteria to build new therapeutic microcarriers to treat colorectal cancer in humans (Martel et al. 2011). Reversible bacterial adhesion (Rijnaarts et al. 1995) including cells on cells (Hara et al. 2001) or with polysterene surface using photochemistry (Weibel et al. 2005) is also possible.
But blotting bacteria on the surface of a microstructure often leads to random displacement patterns or far from effective or optimal directional propelling force. Although random motion has been eliminated on such bacterial blotting on a microstructure by making a specific blotting area inactive after exposing ultraviolet rays (Steager et al. 2007), such approach results in less than optimal directional propelling force. As such, patterning oriented bacteria to a predetermined area is required for achieving maximum directional propelling force (Behkman and Sitti 2008a; 2008b; 2009). Such patterning can be done in various ways including but not limited to the use of a polymer film template (Rowan et al. 2002), via optical trapping (Haruff et al. 2002), using soft lithography (Cerf et al. 2008), or other oriented adhesion techniques (Jones et al. 2003).
4.2 Free-swimming bacterial implementations
Because the lifespan of bacteria is limited, attaching them to an artificial microstructure limits the useful operating life of the whole bio-hybrid microsystem or microrobot. For instance, an aggregate of free-swimming flagellated bacteria can be used to propel bio-hybrid microrobots (Martel and Mohammadi 2010; Khoshbakht Marvi et al. 2010). With MTB, magnetotaxis is used to construct and move such bacterial aggregation while aligning the bacteria to provide a cumulative directional force towards the artificial microstructure of the microrobot. As such, not only the same bacteria can be dispatched between several microrobots but can also be easily replaced at the end of their lifespan without compromising the operating life of the artificial microstructures.
5 Swarming and bioconvection
5.1 Swarming
Bacterial swarming can be defined as a collective behavior exhibited by bacteria aggregated together or a collective motion of a large number of self-propelled entities being the bacteria in this specific case. Swarming is critical in targeted cancer therapies (Martel et al. 2009; Martel et al. 2011) not only to achieve enhanced targeting efficacy but also to deliver a known therapeutic dose using a specific number of bacteria within an aggregate where each cell is carrying a specific amount of drug.
In the context of microsystems and microrobots, the swarming motility (Berg 2005) and more specifically its collective fluid dynamics (Kessler 1985; Pedley and Kessler 1992a; Hopkins and Fauci 2002; Dombrowski et al. 2004; Cisneros et al. 2007; Sokolov et al. 2007) is of special interest especially in non-contact microrobot propulsion and micromanipulation or microassembly tasks based on free-swimming bacteria such as in (Martel and Mohammadi 2010). In the latter example, magnetotaxis was used to not only aggregate the bacteria but also to obtain the maximum collective force acting on the artificial microstructures.
5.2 Bioconvection
Instead of creating a continuous directional flow as in the previous example, another strategy is to accumulate energy in the form of a swarm and to release such energy in one stroke to actuate a microstructure. Bioconvection ((Pedley and Kessler 1992b; Hill and Pedley 2005) is one possible method to achieve such mode of actuation. Bioconvection is defined as the process of spontaneous pattern formation in suspensions of up-swimming microorganisms. For bioconvection to occur such microorganisms must first be denser than the fluid medium which is the case for bacteria in water and second, they must up-swim. Such up-swim can be motivated by various taxes including but not limited to oxytaxis, magnetotaxis, chemotaxis, or phototaxis. Then in a fluid of finite depth, bacteria accumulate near the top surface forming an aggregation. The density of such aggregation or cell concentration then increases as more bacteria join and then becomes so high that it will sink with a falling concentrated plume being formed creating a dynamic force that can be exploited for actuation purpose.
6 Main applications
6.1 Microfluidic systems and microassembly
As mentioned previously, bacteria have been used in microfluidic systems to pump fluid (Darnton et al. 2004; Kim and Breuer 2008), to enhance mixing (Kim and Breuer 2007a; 2007b), for transports (Lu and Martel 2006a; 2006b; 2007) or to detect pathogens (Martel 2006a; Lu et al. 2007a; b), to name but some examples.
The first large-scale coordinated microassembly task using bacteria was published in (Martel and Mohammadi 2010) where micro-bricks were moved one at a time to build a small pyramid as previously recorded in a video (Martel and Mohammadi 2009). Such approach could lead to free-swimming mass-scale micro-assembly systems (Martel 2011) and towards the concept of bacterial micro-factories (André et al. 2006).
6.2 Medical applications
The main application of bacteria in the medical field has been in cancer therapy. Anaerobic bacteria have been investigated as tumor-targeting vectors (Pawelek et al. 2003) and for gene therapy (Lemmon et al. 1997; Chwanrow et al. 2010) based on initial reported observations (Coley 1891; Nauts et al. 1946; Willis 1960). Indeed, anaerobic bacteria were and still of special interest for such type of treatments since they are attracted to the oxygen starved areas of tumors which is precisely these areas which are the most difficult to reach with current cancer therapies such as chemotherapy, gene therapy, and radiotherapy (Kimura et al. 1980; Dang et al. 2001; Yazawa et al. 2001; Liu et al. 2002; Toso et al. 2002; Bettegowda et al. 2003; Forbes et al. 2003; Kasinskas and Forbes 2006).
To enhance tumor targeting, a robotic component in the form of assisted directional control using directional magnetic fields capable of guiding anaerobic flagellated magnetotactic bacteria acting as computer-controlled therapeutic microrobots effectively within a volume well beyond the range of attraction of the previous anaerobic bacteria towards oxygen depleted areas of tumor has been proposed and is under investigation (Martel et al. 2008; Martel et al. 2009; Felfoul et al. 2011). It was demonstrated experimentally that MTB were able to penetrate multicellular tumor spheroids (Mokrani et al. 2010) and the synthesis of a MTB-tagged agent for colorectal tumors in humans has already been initiated (Martel et al. 2011).
7 Discussion and conclusion
Table 1 summarizes in a chronological order some of the main milestones in the field of bacterial microsystems and microrobots. Although not exhaustive, it provides an idea of the progress made in recent years and the pace at which such field is progressing.
In an engineering point of view, motile bacteria are sophisticated highly efficient self-powered sensory-based micro-actuators that can be exploited for the development of new microsystems including microrobots and biosensors. Bacteria directional control and velocities can be achieved by exploiting taxes, as well as environmental and/or structural conditions. Such exploitation leads to applications ranging from micromanipulations, microassemblies to medical targeted interventions, including other bacterial applications under investigation. Such investigated applications include but are not limited to the fabrication of nanostructures such as tunable microbial conducting nanowire networks (Nikhil et al. 2011; Malvankar and Lovely 2012), and power generation such as in microbial fuel cells (Logan 2009).
Quorum sensing (Wolfe et al. 1987; Fuqua et al. 1994; Weiss et al. 2008) involving bacterial interactions and communications or signaling in a swarm is another aspect that could be exploited. Mutants (Armstrong et al. 1967; Parkinson 1978, 1981; Fisher 1997; Firtel and Chung 2000; Charon and Goldstein 2002; Voigt 2006; Snyder and Champness 2007; Schüler 2008; Mokrani et al. 2009) created through methods such as changes in cultivation parameters, addition or subtraction of components, or through genetics, to create bacteria better suited for particular microsystems, microrobots, applications or environmental conditions, is also a promising avenue.
Nonetheless, although it is difficult to provide an exhaustive and complete review and assessment on this relatively new field of research, one thing is sure, the use of motile bacteria in microsystems and in the field of microrobotics although in its infancy, is growing at a very fast pace. Furthermore, this particular field is likely to do so for awhile since it offers advantages and research opportunities beyond what is possible with entirely artificial microsystems and microrobots.
References
J.J. Abbott, Z. Nagy, F. Beyeller and B.J. Nelson, IEEE Robot. Autom. Mag., 92–103 (2007)
J. Adler, Science 153, 708–716 (1966)
J. Adler, Bot. Acta. 101, 93–100 (1988)
J. Adler, B. Templeton, J. Gen. Microbiol. 46, 175–184 (1967)
J. Adler, W.-W. Tso, Science 184(4143), 1292–1294 (1974)
G. Alexandre, I.B. Zhulin, J. Bacteriol. 183, 4681–4686 (2001)
W. Alt, J. Math. Biol. 9, 147–177 (1980)
W. Alt, T. Eisele, R. Schaaf, IMA J. Math. Appl. Med. Biol. 2, 109–129 (1985)
W. André and S. Martel, IEEE/RSJ Int. Conf. on Intelligent Robots and Systems (IROS), 1335–1340, Beijing, China (2006)
W. André, B. Moufarrej and S. Martel, 5th Int. Workshop on Microfactories (IWMF), Besançon, France (2006)
W. André and S. Martel, IEEE/ASME Int. Conf. on Advanced Intelligent Mechatronics (AIM), Zürich, Switzerland, (2007)
W. André, B. Moufarrej and S. Martel, Int. Symp. on Optomechatronic Technologies (ISOT), Lausanne, Switzerland (2007)
L. Angelani, R. Di Leonardo, G. Ruocco, Phys. Rev. Lett. 102, 048104 (2009)
V. Arabagi, B. Behkam, E. Cheung, M. Sitti, J. Appl. Phys. 109, 114702 (2011)
J.P. Armitage, R. Schmitt, Microbiol. 143, 3671–3682 (1997)
J.B. Armstrong, J. Adler and M.M. Dahl, 93, 390–398 (1967)
V. Balzani, A. Credi, F.M. Raymo, J. Fraser, Angew. Chem. Int. Ed. 39, 3348–3391 (2000)
O. Baracchini, J.C. Sherris, J. Path. Bact. 77, 565 (1959)
R. Barak, I. Nur, Y. Okon, Y. Henis, J. Bacteriol. 152, 643–649 (1982)
D.A. Bazylinski, R.B. Frankel, Nat. Rev. 2, 217–230 (2004)
R.N. Bearon, Phys. Fluids 15, 1552–1563 (2003)
R.N. Bearon, T.J. Pedley, Bull. Math. Biol. 62, 775–791 (2000)
B. Behkam, M. Sitti, ASME J. Dyn. Syst. Meas. Control. 128(1), 36–43 (2006)
B. Behkam, M. Sitti, Appl. Phys. Lett. 90, 023902 (2007)
B. Behkam, M. Sitti, Appl. Phys. Lett. 93, 223901 (2008)
B. Behkam and M. Sitti, Proc. of the IEEE Int. Conf. on Robotics and Automation (ICRA), 1022–1027 (2009)
B. Behkam and M. Sitti, Proc. of the ASME Int. Mech. Eng. Congr., Anaheim, CA, USA, 59621–59626 (2004)
B. Behkam and M. Sitti, IEEE/ASME Int. Conf. on Advanced Mechatronics (AIM), Monterey, CA, USA, 37–42 (2005)
B. Behkam and M. Sitti, Proc. of the IEEE Eng. in Medicine and Biology Society, (2006b)
B. Behkman and M. Sitti, (2008a) Proc. of the IEEE RAS-EMBC Int. Conf. on Biomedical Robotics and Biomechatronics, 753–757 (2008a)
H. Berg, Sci. Am. 233, 36–44 (1975)
H.C. Berg, Biophys. J. 68, 163s–167s (1995)
H.C. Berg, Annu. Rev. Biochem. 72, 19–54 (2003)
H. Berg, Curr. Biol. 15, R599–R600 (2005)
H.C. Berg, R.A. Anderson, Nature 245, 380–382 (1973)
H.C. Berg, D.A. Brown, Nature 239, 500–504 (1972)
H. Berg, E. coli in Motion, (Springer-Verlag 2004)
H.C. Berg, L. Turner, Nature 278, 349–351 (1979)
H.C. Berg, L. Turner, Biophys. J. 58, 919–930 (1990)
H.C. Berg, Random Walks in Biology, (Princeton University Press, 1983)
C. Bettegowda, L.H. Dang, R. Abrams et al., Proc. Natl. Acad. Sci. U. S. A. 100(25), 15083–15088 (2003)
S.A. Biodi, J.A. Quinn, H. Goldfire, AICHE J. 44, 1923–1929 (1998)
R.P. Blakemore, Science 190, 377–379 (1975)
R.P. Blakemore, Annu. Rev. Microbiol. 36, 217–238 (1982)
D.A. Brown, H.C. Berg, Proc. Natl. Acad. Sci. U. S. A. 71, 1388–1392 (1974)
H.J. Busscher, A.H. Weerkamp, FEMS Microbiol. Rev. 46, 165–173 (1987)
A. Cerf, J.-C. Cau, C. Vieu, Colloids Surf. B: Biointerfaces 65, 285–291 (2008)
N.W. Charon, S.T. Goldstein, Annu. Rev. Genet. 36, 47–73 (2002)
S. Chattopadhyay, R. Moldovan, C. Yeung, X.L. Wu, Proc. Natl. Acad. Sci. U. S. A. 103(37), 13712–13717 (2006)
X. Chen, H. Berg, Biophys. J. 78, 1036–1041 (2000)
C. Chen, Q. Ma, W. Jiang, T. Song, Appl. Microbiol. Biotechnol. 90(1), 269–275 (2011)
A.T. Chwang, T.Y. Wu, Proc. R. Soc. Lond. Ser. B 178, 327–346 (1971)
K.B. Chwanrow, M. Cronin, D. O’Hanlon, G.C. O’Sullivan, M. Tangney, Bioeng. Bugs 1, 385–394 (2010)
L.H. Cisneros, R. Cortez, C. Dombrowski, R.E. Goldstein, J.O. Kessler, Exp. Fluids 43, 737–753 (2007)
B. Coley, Ann. Surg. 14, 199–220 (1891)
H.C. Crenshaw, Bull. Math. Biol. 55, 231–255 (1993)
L.H. Dang, C. Bettegowda, D.L. Huso, K.W. Kinzler, B. Vogelstein, Proc. Natl. Acad. Sci. U. S. A. 98, 15155–15160 (2001)
P. Dario, R. Valleggi, M.C. Carroza, M.C. Montesi, M. Cocco, J. Micromech, Microeng. 2, 141–157 (1992)
N. Darnton, L. Turner, K. Breuer, H.C. Berg, Biophys. J. 86, 1863–1870 (2004)
A.P. Davis, Nature (London) 401, 120–121 (1999)
J.G. de la Torre, V.A. Bloomfield, Biophys. J. 20, 49–67 (1977)
E. De Robertis, C.A. Peluffo, Proc. Soc. Exp. Biol. Med. 78, 584 (1951)
H. Debarros, D.M.S. Esquivel, M. Farina, Sci. Prog. 74, 347–359 (1990)
R. Di Leonardo, L. Angelani, D. Dell’Arciprete, G. Ruocco, V. Iebba, S. Schippa, M.P. Conte, F. Mecarini, F. De Angelis, E. Di Fabrizio, Proc. Natl. Acad. Sci. U. S. A. 107, 9541–9545 (2010)
W.R. DiLuzio, L. Turner, M. Mayer, P. Garstecki, D.B. Weibel, H.C. Berg, G.M. Whitesides, Nature 435, 1271–1274 (2005)
C. Dombrowski, L. Cisneros, S. Chatkaew, R.E. Goldstein, J.O. Kessler, Phys. Rev. Lett. 93, 0981031–0981034 (2004)
R. Dreyfus, J. Baudry, M.L. Roper, M. Fermigier, H.A. Stone, J. Bibette, Nature 437(6), 862–865 (2005)
K.J. Duffy, P.T. Cummins, R.M. Ford, Biophys. J. 68, 800–806 (1995)
D.J. Earl, C.M. Pooley, J.F. Ryder, I. Bredberg, J.M. Yeomans, J. Chem. Phys. 126, 064703 (2007)
R. Eckert, Science 176, 473–481 (1972)
J. Edd, S. Payen, B. Rubinski, M. Stoller and M. Sitti, Proc. of the IEEE/RSJ Int. Conf. on Intelligent Robots and Systsems, 2583–2588 (2003)
R. Fearing, 2nd Int. Symp. On Micromachines and Human Sciences, 1–15 (1991)
O. Felfoul, M. Mohammadi, L. Gaboury and S. Martel, Proc. IEEE/RSJ Int. Conf. on Intelligent Robots and Systems (IROS), San Francisco, USA (2011)
T. Fijita, T. Kawai, JSME Int. J. Ser. C 44, 952–957 (2001)
R.A. Firtel, C.Y. Chung, BioEssays 22, 603–615 (2000)
P.R. Fisher, BioEssays 19(5), 397–407 (1997)
M. Fletcher, Bacterial adhesion: Molecular and Ecological Diversity. Volume Bacterial Attachment in Aquatic Environments: A Diversity of Surfaces and Ahesion Strategies, (Wiley, 1996)
N.S. Forbes, L.L. Munn, D. Fukumura, R.K. Jain, Cancer Res. 63, 5188–5193 (2003)
R.B. Frankel, Annu. Rev. Biophys. Bioeng. 13, 85–103 (1984)
R.B. Frankel, R.P. Blakemore, J. Magn. Magn. Mater. 15–18(3), 1562–1564 (1980)
R.B. Frankel, D.A. Bazylinski, M.S. Johnson, B.L. Taylor, Biophys. J. 73, 994–1000 (1997)
P.D. Frymier, R.M. Ford, AICHE J. 43, 1341–1347 (1997)
P.D. Frymier, R.M. Ford, P.T. Cummins, Chem. Eng. Sci. 48, 687–699 (1993)
P.D. Frymier, R.M. Ford, H.C. Berg, P.T. Cummings, Proc. Natl. Acad. Sci. U. S. A. 92, 6195–6199 (1995)
W.C. Fuqua, S.C. Winans, E.P. Greenberg, J. Bacteriol. 176, 269–275 (1994)
J.Y. Gannon, Y. Tan, P. Baveye, M. Alexander, Appl. Environ. Microbiol. 57, 2497–2501 (1991)
P. Garstecki, P. Tierno, D.B. Weibel, F. Sagues, J. Phys, J. Phys. Condens. Matter 21, 204110 (2009)
A. Ghosh, P. Fisher, Nano Letters 9(6), 2243–2246 (2009)
A.N. Glagolev, J. Theor. Biol. 82, 171–185 (1980)
T. Goto, R. Inaoka, Y. Tokano, JSME Int. J. Ser. C 43(4), 875–881 (2000)
E.P. Greenberg, E. Canale-Parola, J. Bacteriol. 132, 356–358 (1977a)
E. Greenberg, E. Canale-Parola, J. Bacteriol. 130, 485–494 (1977b)
M.J. Gross, B.E. Logan, Appl. Environ. Microbiol. 61, 1750–1756 (1995)
G.J. Hancock, Proc. R. Soc. Lond. A 217, 96–121 (1953)
J. Happel, H. Brenner, Low Reynolds Number Hydrodynamics (Kluwer Academic Publishers, Dordrecht, 1973)
M. Hara, A. Yamaki, J. Miyake, Mater. Sci. Eng. C. 17, 107–112 (2001)
G. Harkes, J. Dankert, J. Feijen, Appl. Environ. Microbiol. 58, 1500–1505 (1992)
H.M. Haruff, J. Munakata-Marr, D.W.M. Marr, Colloids Surf. B: Biointerfaces 27, 189–195 (2002)
M. Hermansson, Colloids Surf. B: Biointerfaces 14, 105–119 (1999)
J.L.L. Higdon, J. Fluid Mech. 90(4), 685–711 (1979)
N.A. Hill, T.J. Pedley, Fluid. Dynam. Res. 37, 1–20 (2005)
A.J.T. Hillesdon, T.J. Pedley, J.O. Kessler, Bull. Math. Biol. 57, 299–344 (1995)
Y. Hiratsuka, M. Miyata, T. Tada, T.Q.P. Uyeda, Proc. Natl. Acad. Sci. U. S. A. 103, 13618 (2006)
W. Hirofumi, R.R. Netz, Phys. Rev. Lett. 99, 108102 (2007)
M.E.J. Holwill, R.E. Burge, Arch. Biochem. Biophys. 101, 249–260 (1963)
T. Honda, K.I. Arai, K. Ishiyama, IEEE Trans. Magn. 32, 5085–5087 (1996)
M.M. Hopkins, L.J. Fauci, J. Fluid Mech. 455, 149–174 (2002)
S. Hou, R.W. Larsen, D. Boudko, C.W. Riley, E. Karatan, M. Zimmer, G.W. Ordal, M. Alam, Nature 403, 540–544 (2000)
Y. Inoue, C. Lo, H. Fukuoka, H. Takahashi, Y. Sowa et al., J. Mol. Biol. 376, 1251–1259 (2008)
K. Ishiyama, K.I. Arai, M. Sendoh, A. Yamazaki, J. Micromechatronics 2, 77–86 (2003)
R.E. Johnson, C.J. Brokaw, Biophys. J. 25(1), 113–127 (1979)
J. Jones, J. Feick, D. Imoudu, N. Chukwumah, M. Vigeant, O. Velegol, Appl. Environ. Microbiol. 69, 6515–6519 (2003)
B. Jucker, H. Harms, S. Hug, A. Zehnder, Colloids Surf. B: Biointerfaces 9, 331–343 (1997)
R.W. Kasinskas, N.S. Forbes, Biotech. Bioeng. 94(4), 710–721 (2006)
J.B. Keller, S.I. Rubinow, Biophys. J. 16, 151–170 (1976)
E. Keller, L. Segel, J. Theor. Biol. 30, 225–234 (1971)
J.O. Kessler, Contemp. Phys. 10, 202–210 (1985)
E. Khoshbakht Marvi, M. Mohammadi and S. Martel, 12th Int. Conf. on New Actuators (ACTUATOR), Bremen, Germany (2010)
M. Kim, K. Breuer, J. Fluids Eng. 129, 319–324 (2007a)
M. Kim, K. Breuer, Anal. Chem. 79, 955–959 (2007b)
M.J. Kim, K.S. Breuer, Small 4, 111–118 (2008)
D.H. Kim, D. Casale, L. Köhidai, M.J. Kim, Appl. Phys. Lett. 94, 163901 (2009)
D. Kim, A. Liu and M. Sitti, IEEE/RSJ Int. Conf. in Intelligent Robots and Systems, San Francisco, USA (2011)
N.T. Kimura, S. Taniguchi, K. Aoki, T. Baba, Cancer Res. 40, 2061–2068 (1980)
D. Koshland, Bacterial chemotaxis as a model behavioral system (Raven, New York, 1980)
T. Krell, J. Lacal, F. Munoz-Martinez, J.A. Reyes-Darias, B.H. Cadirci, C. Garcia-Fontana, J.L. Ramos, Environ. Microbiol. 13(5), 1115–1124 (2011)
E. Lauga, W.R. DiLuzio, G.M. Whitesides, H.A. Stone, Biophys. J. 90(2), 400–412 (2006)
H. Lee, A.M. Purdon, V. Chu, R.M. Westervelt, Nano Letters 4(5), 995–998 (2004)
M.J. Lemmon, P. Van Zijl, M.E. Fox, M.L. Mauchline, A.J. Garcia, N.P. Minton, J.M. Brown, Gene Ther. 4(8), 791–796 (1997)
J. Lighthill, SIAM Rev. 18, 161–173 (1976)
S.-C. Liu, N.P. Minton, A.J. Giaccia, J.M. Brown, Gene Ther. 9, 291–296 (2002)
E. Lobaton and A. Bayen, Proc. of the Americam Control Conf., New York City, USA (2007)
B.E. Logan, Nat. Rev. Microbiol. 7, 375–381 (2009)
P.S. Lovely, F.W. Dahlquist, J. Theor. Biol. 50, 477–496 (1975)
C.P. Lowe, Future Generat. Comput. Syst. 17, 853–862 (2001)
G. Lowe, M. Meister, H.C. Berg, Nature 325, 637–640 (1987)
Z. Lu and S. Martel, The Nanotechnology Conf. and Trade Show (NSTI) Nanotech.., Boston, MA, USA (2006a)
Z. Lu and S. Martel, 28th IEEE-EMBS Annual Int. Conf. of the Eng. in Med. and Biol. Soc., New York, USA, 3415–3418 (2006b)
Z. Lu and S. Martel, The 14th Int. Conf. on Solid-state Sensors and Actuators, Lyon, France (2007)
Z. Lu, R. Denomme and S. Martel, The 11th Int. Conf. on Miniaturized Systems for Chemistry and Life Sciences (μTAS), Paris, France, (2007a)
Z. Lu, J. El-Fouladi, Y. Savaria and S. Martel, The 11th Int. Conf. on Miniaturized Systems for Chemistry and Life Sciences (μTAS), Paris, France (2007b)
H. Machemer, R. Eckert, J. Comp. Physiol. 104, 247–260 (1975)
R. Macnab, D.E. Koshland, J. Mol. Biol. 85, 399–406 (1974)
K. Maeda, Y. Imae, J.I. Shioi, F. Oosawa, J. Bacteriol. 127, 1039–1046 (1976)
Y. Magariyama, S. Kudo, Biophys. J. 83, 733–739 (2002)
N.S. Malvankar, D.R. Lovley, Microbial Nanowires: A New Paradigm for Biological Electron Transfer and Bioelectronics, ChemSusChem. 5(6), 1039–1046 (2012)
S. Martel, Method of controlling magnetotactic bacteria, US Provisonal Patent Application No. 60/576,609 (2004)
S. Martel, The First IEEE/RAS-EMBS Int. Conf. on Biomed. Robotics and Biomechatronics, 829–834 (2006a)
S. Martel, Method and system for controlling micro-objects or micro-particles, Patent Application Publication, US 2006/0073540 A (2006b)
S. Martel, Proc. of the Int. Conf. on Microtechnologies in Medicine and Biology, Okinawa, Japan, 89–92 (2006c)
S. Martel, 2nd ASM/IEEE EMBS Conf. on Bio, Micro and Nanosystems, San Francisco, CA (2006d)
S. Martel and W. André, Int. Advanced Robotics Programme (IARP), Paris, France (2006)
S. Martel, C. Tremblay, S. Ngakeng, G. Langlois, Appl. Phys. Lett. 89, 233804–233806 (2006)
S. Martel, 16th European Microelectronics and Packaging Conference (EMPC), Oulu, Finland (2007)
S. Martel and M. Mohammadi, The 11th Int. Conf. on Miniaturized Systems for Chemistry and Life Sciences (μTAS), Paris, France (2007)
S. Martel, O. Felfoul and M. Mohammadi, The 2nd IEEE RAS/EMBS Int. Conf. on Biomedical Robotics and Biomechatronics (BioRob), Scottsdale, AZ, USA (2008)
S. Martel and M. Mohammadi, Swarm of bacteria builds tiny pyramid, http://www.youtube.com/watch?v=fCSOdQK5PIY (2009)
S. Martel, M. Mohammadi, O. Felfoul, Z. Lu, P. Pouponneau, Int. J. Robot. Res. 28(4), 571–582 (2009)
S. Martel, Int. Symp. on Experimental Robotics (ISER), New Delhi, India (2010)
S. Martel and M. Mohammadi, Proc. of the IEEE Int. Conf. on Robotics and Automation (ICRA), Anchorage, Alaska, USA (2010)
S. Martel, M. Mohammadi and N. Mokrani, ASME First Global Congress on NanoEngineering for Medicine and Biology (NEMB), Houston, TX, USA (2010)
S. Martel, ASME Int. Manufacturing Science and Eng. Conf., Corvalis, OR, USA (2011)
S. Martel et al., SN-38 (or 5-FU) drug encapsulation in liposomes transported by magnetotactic bacteria for localized colorectal cancer treatment, Quebec Consortium for Drug Discovery (CQDM) (2011)
L. Maurer, E. Yohannes, S. Bondurant, M. Radmacher, J. Slonczewski, J. Bacteriol. 187, 304–319 (2005)
J.W. McClaine, R.M. Ford, Appl. Environ. Microbiol. 68, 1280–1289 (2002a)
J.W. McClaine, R.M. Ford, Biotech. Bioeng. 78, 179–189 (2002b)
S. McEldowney, M. Fletcher, Appl. Environ. Microbiol. 52, 460–465 (1986)
M. Meister, G. Lowe, H.C. Berg, Cell 49, 643–650 (1987)
B.A. Mello, Y. Tu, Biophys. J. 84, 2943–2956 (2003)
N. Mokrani, M. Mohammadi and S. Martel, The 5th Int. Conf. on Microtech. in Med. and Biol. (MMB) Conf., Quebec City, Canada (2009)
N. Mokrani, O. Felfoul, F. Afkhami Zarreh, M. Mohammadi, R. Aloyz, G. Batist and S. Martel, 32nd Annual Int. Conf. of the IEEE Eng. in Medicine and Biology Society, Buenos Airos, Argentina (2010)
S.D. Muller, J. Marchetto, S. Airaghi and P. Koumoutsakos, IEEETrans. on IEEE Trans. Evol. Comput. 6(1), (2002)
S. Nasseri, N. Phan-Thien, Comput. Mech. 20, 267–271 (1997)
H.C. Nauts, W.E. Swift, B.L. Coley, Cancer Res. 6, 205–216 (1946)
S. Nikhil et al. Nature Nanotechnology. 6, 573–579 (2011)
P.B. Noble, M. Levine, Computer-Assisted Analyses of Cell Locomotion and Chemotaxis (CRC Press, Boca Raton, 1986)
R. Nossal, J. Stat. Phys. 30, 391–399 (1983)
H.G. Othmer, T. Hillen, SIAM J. Appl. Math. 62, 1222–1250 (2002)
J. Palomar, A. Leranov, M. Vinas, Microbios 81, 107–113 (1995)
J.S. Parkinson, J. Bacteriol. 135, 45–53 (1978)
J.S. Parkinson, Symp. Soc. Gen. Microbiol., Cambridge (1981)
J.M. Pawelek, K.B. Low, D. Bermudes, Lancet Oncol. 4, 548–556 (2003)
T.J. Pedley, J.O. Kessler, Annu. Rev. Fluid Mech. 24, 313–358 (1992a)
T.J. Pedley, J.O. Kessler, Sci. Prog. 76, 105–123 (1992b)
N. Phan-Thien, T. Tran-Cong, M. Ramia, J. Fluid Mech. 184, 533–549 (1987)
N.W. Preston, H.B. Maitland, J. Gen. Microbiol. 7, 117 (1952)
E.M. Purcell, Am. J. Phys. 45, 3–11 (1976)
E.M. Purcell, Proc. Natl. Acad. Sci. 94, 11307–11311 (1997)
M. Ramia, Biophys. J. 60, 1057–1078 (1991)
M. Ramia, D.L. Tullock, N. Phan-Thien, Biophys. J. 65, 755–778 (1993)
J.S. Rathore, N.N. Sharma, ASME J. Nanotech. Eng. Med. 1, 031001 (2010)
D.R. Repaske, J. Adler, J. Bacteriol. 145, 1196–1208 (1981)
H. Rijnaarts, W. Norde, E. Bouwer, J. Lyklema, A. Zehnder, Colloids Surf. B: Biointerfaces 4, 5–22 (1995)
M.A. Rivero, R.T. Tranquillo, H.M. Buettner, D.A. Lauffenburger, Chem. Eng. Sci. 44, 2881–2897 (1989)
A.M. Roberts, J. Theor. Biol. 27, 97–106 (1970)
M. Rosenberg, Appl. Environ. Microbiol. 42, 375–377 (1981)
B. Rowan, M. Wheeler, R. Crooks, Langmuir 18, 9914–9917 (2002)
W.S. Ryu, R.M. Berry, H.C. Berg, Nature 403, 444–447 (2000)
S. Saeidlou, G. Bringout, C. Dubois, and S. Martel, Fourth Int. ICST Conf. on Nano-Networks (Nano-Net), Luzern, Switzerland (2009)
M. Salerno, B. Logan, D. Velegol, Langmuir 20, 10625–10629 (2004)
A.D. Samuel, H.C. Berg, Biophys. J. 71, 918–923 (1996)
W.R. Schneider, R.N. Doetsch, J. Bacteriol. 117, 696–701 (1974)
M.J. Schnitzer, S.M. Block, H.C. Berg, E.M. Purcell, Symp. Soc. Gen. Microbiol. 46, 15–34 (1990)
D. Schüler, FEMS Microbiol. Rev. 32(4), 654–672 (2008)
T. Schweinitzer, C. Jesenhans, Arch. Microbiol. 192(7), 507–520 (2010)
J.W. Shaevitz, J.Y. Lee, D.A. Fletcher, Cell 122(6), 941–945 (2005)
N.N. Sharma, R.K. Mittal, Int. J. Smart Sens. Intell. Syst. 1(1), 87–109 (2008)
E. Shechter and S. Martel, The 5th Int. Conf. on Microtech. in Med. and Biol. (MMB) Conf., Quebec City, Canada (2009)
E. Shechter and S. Martel, IEEE/ASME Int. Conf. on Advanced Intelligent Mechatronics (AIM), Montréal, Canada (2010)
J.C. Sheeris, N.W. Preston, J.G. Shoesmith, J. Gen. Microbiol. 16, 86 (1957)
W. Shi, B.A.D. Stocker, J. Adler, J. Bacteriol. 178, 1113–1119 (1996)
J. Shioi, R.C. Tribhuwan, S.T. Berg, B.L. Taylor, J. Bacteriol. 170, 5507–5511 (1988)
J.G. Shoesmith, J. Gen. Microbiol. 22, 528–535 (1960)
M.A. Sleigh, Protoplasma 164, 45–53 (1991)
L. Snyder, W. Champness, Molecular Genetics of Bacteria, 3rd edn. (ASM Press, Washington, DC, 2007)
A. Sokolov, I. Aranson, J. Kessler, R. Goldstein, Phys. Rev. Lett. 98, 1591021–1591024 (2007)
A. Sokolov, M.M. Apodaca, B.A. Grzybowski, I.S. Aranson, Proc. Natl. Acad. Sci. U. S. A. 107(3), 969–974 (2010)
R. Soong, D. Bachand, H.P. Neves, A.G. Olkhovets, H.G. Craighead, C.D. Montemagno, Science 290, 1555–1558 (2000)
P.A. Spiro, J.S. Parkinson, H.G. Othmer, Proc. Natl. Acad. Sci. U. S. A. 94, 7263–7278 (1997)
A.M. Spormann, R.S. Wolfe, FEMS Microbiol. Lett. 22, 171–177 (1984)
T.N. Stanbridge, N.W. Preston, J. Gen. Microbiol. 55, 29–36 (1969)
E. Steager, C. Kim, J. Patel, S. Bith, C. Naik, L. Reber, M. Kim, Appl. Phys. Lett. 90, 263901–263903 (2007)
E.B. Steager, M.S. Sakar, D.H. Kim, V. Kumar, G.J. Pappas, M.J. Kim, J. Micromech. Microeng. 21, 035001 (2011)
B.L. Taylor, D.E. Koshland, J. Bacteriol. 123, 557–569 (1975)
B.L. Taylor, I.B. Zhulin, Mol. Microbiol. 28, 683–690 (1998)
B.L. Taylor, I.B. Zhulin, M.S. Johnson, Annu. Rev. Microbiol. 53, 103–128 (1999)
J.F. Toso, V.J. Gill, P. Hwu, F.M. Marincola, N.P. Restifo, D.J. Schwartzentruber, R.M. Sherry, S.L. Topalian, J.C. Yang, F. Stock, L.J. Freezer, K.E. Morton, C. Seipp, L. Haworth, S. Mavroukakis, D. White, S. MacDonald, J. Mao, M. Sznol, S.A. Rosenberg, J. Clin. Oncol. 20, 142–152 (2002)
S. Tottori, L. Zhang, F. Qiu, K.K. Krawczyk, A. Franco-Obregon, B.J. Nelson, Adv. Mater. 24(6), 816–816 (2012)
S. Trachtenberg, D. Fishelov, M. Ben-artzi, Biophys. J. 85, 1345–1357 (2003)
R. Tranquillo, D. Lauffenburger, J. Math. Biol. 25, 229–262 (1987)
W. Trimmer and R. Jebens, IEEE Int. Conf. on Robotics and Automation, Scottsdale, AZ, USA, 1547–1552 (1989)
S. Tsuneda, H. Aikawa, H. Ayashi, A. Yuasa, A. Hirata, FEMS Microbiol. Lett. 223, 287–292 (2003)
H. Van der Mei, M. Cown, M. Genet, P. Rouxhet, H. Busscher, Can. J. Microbiol. 38, 1033–1041 (1992)
H. Van der Mei, R. Bos, H. Busscher, Colloids Surf. B: Biointerfaces 11, 213–221 (1998)
M. van Loosdrecht, J. Lyklema, W. Norde, G. Schraa, A. Zehnder, Appl. Environ. Microbiol. 53, 1893–1897 (1987)
M.C. van Loosdrecht, M.C.M.J. Lyklema, A.J.B. Zehnder, Microb. Ecol. 17, 1–15 (1989)
M.C. van Loosdrecht, M.C.M.J. Lyklema, A.J.B. Zehnder, Aquat. Sci. 52, 103–114 (1990)
J. Van Oss, Cell Biophys. 14, 1–16 (1989)
C.A. Voigt, Curr. Opin. Biotechnol. 17, 548–557 (2006)
D. Weibel, P. Garstecki, D. Ryan, W.R. DiLuzio, M. Mayer, J.E. Seto, G.M. Whitesides, Proc. Natl. Acad. Sci. 102, 11963–11967 (2005)
L.E. Weiss, J.P. Badalamenti, L.J. Weaver, A.R. Tascone, P.S. Weiss, T.L. Richard, P.C. Cirino, Biotechnol. Bioeng. 100, 1251–1255 (2008)
A.T. Willis, Anaerobic Bacteriology in Clinical Medicine (Butterworth, London, 1960), p. 40
H. Winet, S.R. Keller, J. Exp. Biol. 65, 577–602 (1976)
A.J. Wolfe, M.P. Conley, T.J. Kramer, H.C. Berg, J. Bacteriol. 169, 1878–1885 (1987)
L.S. Wong, M.S. Johnson, I.B. Zhulin, B.L. Taylor, J. Bacteriol. 177, 3985–3991 (1995)
J. Yang, C.W. Wolgemuth, G. Huber, Phys. Rev. Lett. 102, 218102 (2009)
R. Yasuda, H. Noji, K. Kinosita, M. Yoshida, Cell 93(7), 1117–1124 (1998)
K. Yazawa, M. Fujimori, T. Nakamura et al., Breast Canc. Res. Treat. 66, 165–170 (2001)
P. Yongxin, W. Lin, L. Jinhua, T. Lanxiang, D. Chenglong, L. Qingsong, Z. Rixiang, M. Winklhofer, N. Petersen, Biophys. J. 97, 986–991 (2009)
L. Zhang, J.J. Abbott, L. Dong, B.E. Kratochvil, H. Zhang, K.E. Peyer and B.J. Nelson, IEEE/RSJ Int. Conf. on Intelligent Robots and Systems, St. Louis, USA, 1401–1401 (2009b)
L. Zhang, J.J. Abbott, L.X. Dong, B.E. Kratochvil, D.J. Bell, B.J. Nelson, Appl. Phys. Lett. 94, 064107 (2009)
L. Zhang, K.E. Peyer, B.J. Nelson, Lab Chip 10, 2203–2215 (2010)
I.B. Zhulin, J.P. Armitage, J. Bacteriol. 175, 952–958 (1993)
I.B. Zhulin, V.A. Bespalov, M.S. Johnson, B.L. Taylor, J. Bacteriol. 178, 5199–5204 (1996)
A. Zita, M. Hermansson, Appl. Environ. Microbiol. 60, 3041–3048 (1987)
Acknowledgments
Dr. Mohammadi from the NanoRobotics Laboratory at EPM is acknowledged for its help in the literature review process. During the writing of this paper, the author was supported by the Research Chair of École Polytechnique in Nanorobotics and a Discovery Grant from the National Research Council of Canada (NSERC).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martel, S. Bacterial microsystems and microrobots. Biomed Microdevices 14, 1033–1045 (2012). https://doi.org/10.1007/s10544-012-9696-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10544-012-9696-x