Abstract
Cadmium (Cd) is an ubiquitous environmental pollutant that has been associated with male reproductive toxicity in animal models. However, little is known about the reproductive toxicity of Cd in birds. To investigate the toxicity of Cd on male reproduction in birds and the protective effects of selenium (Se) against subchronic exposure to dietary Cd, 100-day-old cocks received either Se (as 10 mg Na2SeO3 per kg of diet), Cd (as 150 mg CdCl2 per kg of diet) or Cd + Se in their diets for 60 days. Histological and ultrastructural changes in the testis, the concentrations of Cd and Se, amount of lipid peroxidation (LPO), the activities of the antioxidants superoxide dismutase (SOD) and glutathione peroxidase (GPx), and apoptosis and serum testosterone levels were determined. Exposure to Cd significantly lowered SOD and GPx activity, Se content in the testicular tissue, and serum testosterone levels. It increased the amount of LPO, the numbers of apoptotic cells and Cd concentration and caused obvious histopathological changes in the testes. Concurrent treatment with Se reduced the Cd-induced histopathological changes in the testis, oxidative stress, endocrine disorder and apoptosis, suggesting that the toxic effects of cadmium on the testes is ameliorated by Se. Se supplementation also modified the distribution of Cd in the testis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is known to be both extremely toxic and ubiquitous in natural environments. Globally, Cd pollution has been increasing and has been identified as a potential health threat to wildlife. Moreover, Cd toxicity might be more common among natural populations of vertebrates than has been appreciated to date. The ingestion of even trace quantities of Cd can influence not only the physiology and health of individual organisms, but also the demographics and the distribution of species (Larison et al. 2000; Lucia et al. 2009). Accompanying population declines, there have been two shifts in lesser scaup (Aythya affinis) demographics: a decrease in the proportion of young birds and an increase in the male-to-female ratio (Pollock and Machin 2009). Cd contamination perhaps played an important role in this. Cd toxicity has also been linked to declines in body condition in birds (Wayland et al. 2002; Anteau et al. 2007). Although there are negative effects of Cd exposure on the male reproductive system in humans and rodents, little is known about the effects of Cd poisoning in birds.
Cd is a ubiquitous environmental pollutant that has been associated with male reproductive toxicity and cellular stress signaling in animal models (Yu et al. 2008). The testes are important targets of Cd, which cause both acute and chronic toxicity in laboratory animals (IARC 1993). Recent studies have shown that Cd exposure can cause changes in testicular histopathology, increased oxidative stress, endocrine disruption and increased apoptosis in rodents, rabbit, dog, calf stallion and humans (Waalkes 2000; Takiguchi and Yoshihara 2006; Siu et al. 2009). However, it is not clear how dietary Cd might affect the avian testis.
Considering the high sensitivity of the testicular tissue to Cd insult, prevention and/or therapeutic interventions are of major concern (Yadav and Khandelwal 2008). Several substances have been shown to have a protective effect against Cd-induced injury and of these selenium (Se) is considered one of the most efficient. Although numerous studies have shown that Se can protect against Cd toxicity in mammals in vitro and vivo (Yiin et al. 1999; Santos et al. 2005; El-Sharaky et al. 2007; Newairy et al. 2007; Ognjanović et al. 2008; Jihen et al. 2008, 2009; Messaoudi et al. 2009; Lazarus et al. 2009; Zhou et al. 2009), the mechanisms of this detoxification have not yet been entirely clarified. Several hypotheses for the antagonistic behavior have been proposed, such as the formation of a complex between Cd and Se (Santos et al. 2004, 2005; Lazarus et al. 2006), antagonism to Cd-induced DNA damage and apoptosis (Yu and Chen 2004; Zhou et al. 2009), amelioration of antioxidant systems (El-Sharaky et al. 2007; Newairy et al. 2007; Ognjanović et al. 2008; Jihen et al. 2009; Messaoudi et al. 2009) and influences on Cd absorption and organ redistribution (Lazarus et al. 2009). Some studies found a protective effect of Se against the testicular injury induced by Cd in rodents and humans. However, the effects of feeding Se on Cd-induced testicular injury in cocks has not been studied.
A recent study indicated that Cd is biomagnified by one particular genus of plants (Larison et al. 2000). Cd ingestion can cause potential health affects in birds through their food web. This led us to investigate Cd-induced testicular toxicity in cocks and the protective effects of Se. Therefore, we investigated the testicular injury in cocks after a sub-chronic exposure to Cd and the simultaneous administration of Cd and Se by a dietary route. We evaluated testicular histopathology, oxidative stress, endocrine disruptors and apoptosis in cock testes.
Materials and methods
Birds and experimental design
All procedures used in this experiment were approved by the Institutional Animal Care and Use Committee of Northeast Agricultural University. Twenty-four 100-day-old Isa Brown cocks were divided randomly into four groups (n = 6 per group). Group I (Control) were fed a basal diet; group II (Se-treated) were fed with the basic diet supplemented with 10 mg/kg Na2SeO3; group III (Cd/Se-treated group) were fed with the basic diet supplemented with 150 mg/kg CdCl2 + 10 mg/kg Na2SeO3 and group IV (Cd-treated group) were fed the basal diet supplemented with 150 mg/kg CdCl2. Birds were maintained in the Laboratory Animal Center, College of Veterinary Medicine, Northeast Agricultural University, China, kept under a 16/8 h light/dark cycle and given free access to standard food and water. On the 60th day of the experiment, all of the cocks were fasted overnight. Following euthanasia, the testes were immediately excised, blotted and then rinsed with ice-cold 0.9% NaCl solution. They were dried with filter paper and weighed. Fractions of testes (500 mg) were homogenized using glass Teflon homogenizer in cold 0.9% NaCl solution. The homogenates were centrifuged at 1000×g for 10 min at 4°C. The supernatant was collected and used for the experiments. The blood collected via cardiac puncture was allowed to clot and the serum was obtained by centrifugation at 1000×g for 10 min.
Estimation of the ratio of testis weight to body weight and testicular Cd and Se concentrations
After euthanasia, the testes from experimental cocks were collected and weighed. The ratio of testis weight to body weight was calculated for each cock.
The content of Cd in the testes was detected using flame atomic absorption spectrometry (FAAS). The optimal operating conditions were: wavelength λ 228.8 nm, slit 0.2 nm, burner height 5.0 mm, light current I 2.0 mA, acetylene discharge 1.5 l/min, air discharge 6.0 l/min. The wet tissue samples (1.0 g) were cut into small pieces with a stainless-steel knife and were transferred into beakers. For digestion, 25 ml of concentrated HNO3/HCl (4:1) was added to each beaker and warmed on a low temperature electric hot plate to solution transparence. The samples were metered volume to 10 ml by 0.5% HNO3 and measured using FAAS. The content of Cd was calculated from a standard curve.
The content of Se in the testes was estimated by the method described by Hasunuma et al. (1982). The assay is based on the principle that Se in samples following acid digestion is converted to selenous acid. The reaction between selenous acid and aromatic-o-diamines, such as 2,3-diamino-naphthalene (DAN), leads to the formation of 4,5-benzopiazselenol, which displays a brilliant lime-green fluorescence when excited at 366 nm in cyclohexane. Fluorescence emission in extracted cyclohexane was read using a spectrophotometer using 366 nm as the excitation wavelength and 520 nm as the emission wavelength. The content of Se was calculated from a standard curve.
Histological and ultrastructural observations
Testicular specimens were fixed in 10% buffered neutral formalin and were processed for paraffin wax sectioning. Sections of about 5 μm thickness were stained with hematoxylin and eosin for light microscopy.
For electron microscopy, testicular specimens were fixed with 2.5% glutaraldehyde in 0.1 M sodium phosphate buffer (pH 7.2) for 3 h at 4°C, washed in the same buffer for 1 h at 4°C and postfixed with 1% osmium tetroxide in sodium phosphate buffer for 1 h at 4°C. The tissues were then dehydrated in graded series of ethanol, starting at 50% each step for 10 min, after two changes in propylene oxide. The tissue specimens were embedded in Araldite. Ultrathin sections were stained with Mg-uranyl acetate and lead citrate for transmission electron microscope (TEM) evaluation.
In situ apoptosis detection
Cleavage of genomic DNA during apoptosis yields single strand breaks (nicks) in high molecular weight DNA. Apoptotic nuclei in tissue sections were identified using the in situ terminal deoxynucleotidyl transferase (TdT)-mediated deoxyuridine triphosphate (dUTP) biotin nick-end labeling (TUNEL) technique that identifies DNA strand breaks by labeling their free 3′-OH termini. We used in situ cell death detection kit (Roche Diagnostics GmbH, Mannheim, Germany). The method distinguishes apoptotic cells from those undergoing necrosis, because damaged DNA in the former leads to a different distribution of staining and nuclear morphology. Paraffin wax-embedded tissue sections were treated with proteinase K and the endogenous peroxidase activity was blocked with hydrogen peroxide. The sections were incubated at 37°C with the terminal TdT/nucleotide mixture for 1 h. Then, the reaction was stopped and the slides were rinsed with phosphate buffered saline. Nuclear labeling was developed with horseradish peroxidase and diaminobenzidine. Hematoxylin was used for counterstaining. Quantitative evaluation of the apoptotic index was performed by manual counting of positively stained nuclei at 400× magnification. Apoptosis was determined in five testes from each group of cocks by counting at least 1000 cells from 5 to 6 sections of each testis. The results are expressed as the percentage of TUNEL-positive cells among the total number of cells counted.
Determination of protein content
Protein determinations were made using the dye-binding method of Bradford (1976). Bovine serum albumin (BSA) was used to construct the standard curve.
Lipid peroxidation
Lipid peroxidation (LPO) was assessed by measuring malondialdehyde (MDA) formation, using the thiobarbituric acid assay (Esterbauer and Cheeseman 1990). The content of LPO in testes was carried out with the MDA detection kit (A003-1, Nanjing Jiancheng Bioengineering Institute, P. R. China) according to the manufacturer’s protocol.
Antioxidant assays
The activity levels of the antioxidants superoxide dismutase (SOD) and glutathione peroxidase (GPx) were measured in testicular homogenates of all experimental cocks. The GPx activity assay was performed with kits according to the manufacturer’s protocol (A005-1, Nanjing Jiancheng Bioengineering Institute, P. R. China). The SOD activity was measured using SOD detection kit (A001-1, Nanjing Jiancheng Bioengineering Institute, P. R. China) according to the manufacturer’s protocol.
Determination of the testosterone level in serum
Radioimmunoassay (RIA) for serum testosterone was carried out with a testosterone 125I RIA Kit (Beijing North Institute of Biological Technology, P. R. China) according to the manufacturer’s protocol. Radioactivity was determined using an automatic gamma counter. All samples were run in duplicate in a single assay to avoid interassay variation.
Statistical analysis
Statistical analysis of all data was performed using SPSS for Windows (version 13; SPSS Inc., Chicago, IL, USA). When a significant value (P < 0.05) was obtained by one-way analysis of variance, further analysis was carried out. All data showed a normal distribution and passed equal variance testing. Differences between means were assessed using Tukey’s honestly significant difference test for post hoc multiple comparisons. Data are expressed as the mean ± standard deviation.
Results
Testis weight/body weight ratios and Cd and Se concentrations
After 60 days exposure to Cd in the feed, there were no significant differences in the growth rates between groups. However, as expected, the final body weight was less in the Cd group than in the other groups. The relative testis weight was reduced by the sub-chronic Cd administration and became greater when cocks were co-treated with Cd and Se than when treated with Cd alone. This measure showed no change compared with the control group when cocks were treated with Se alone (Table 1). Table 1 also shows a significant (P < 0.01) accumulation of Cd in the testis compared with the values in the control cocks. Co-treatment with Cd and Se induced a significant (P < 0.05) change in the level of Cd accumulated in the testis compared with the level of the Cd group. Se in the diet reduced the level of Cd in the testicular tissue and significantly increased the Se content of the testicular tissue in the Cd + Se group (P < 0.01). Se content in the Se-treated group was significantly increased compared with the other groups (P < 0.05 or P < 0.01) and Cd concentration was decreased compared with the control group.
Histopathology and ultrastructure
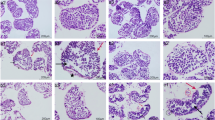
Histology of the testes in control and Se-treated cocks showed normal testicular architecture with regular seminiferous tubular morphology and normal spermatogenesis (Fig. 1a, b). Cocks treated with Cd only showed testicular lesions, with edematous testes, severe necrosis and degeneration of seminiferous tubules (Fig. 1d). In addition, defoliation of many spermatocytes into the lumen of seminiferous tubules was observed. Intense fibrosis was also observed in the central area of the testis. Spermatogenesis was almost absent in the seminiferous tubules. In the Cd + Se group, the interstitial edema and partial loss of spermatogenic cells remained similar to the Cd-exposed cocks (Fig. 1c). Mild restoration of spermatogenesis was observed in a very few seminiferous tubules.
Histology (Panel A, hematoxylin and eosin staining, ×400) and TUNEL staining (Panel B, counterstained with hematoxylin, ×1000) of the seminiferous tubules of cock testes in different groups. a, d Normal testis in the control group. a, b, f, e Normal testes in the control and Se groups. c Section of testis from a cock in the Cd/Se-treated group. d Section of testis from a cock in the Cd-treated group. Many seminiferous tubules were edematous with an intact germinal layer but are undergoing degeneration along with loss of spermatogenesis. g Seminiferous tubules of a cock testis from the Cd/Se-treated group showing a low level of apoptosis. h Seminiferous tubules of a cock testis from the Cd-treated group showing increased numbers of apoptotic cells (arrows)
Electron microscopy showed normal testicular ultrastructure in the control and Se groups (Fig. 2a). Cd treatment alone caused extensive testicular tissue damage. Dilated cisternae of the smooth endoplasmic reticulum (SER), markedly swollen mitochondria with degeneration or loss of cristae and blebbing of the membranes with cytoplasmic vacuolation were observed in spermatocytes and Sertoli cells (Fig. 2c1–3). Leydig cells and spermatocytes showed chromatin condensation and margination (Fig. 2c4–5). Spermatocytes displayed morphological characteristics of apoptosis, including chromatin condensation (Fig. 2c4), cell shrinkage and apoptotic body formation (Fig. 2c5). Chromatin condensation showed as crescentic bodies with a clear boundary of the nuclear membrane (Fig. 2c6). Se co-treatment restored the normal electron microscopic appearance of testicular tissue, similar to that observed in the control group. Slightly dilated cisternae of the SER and swollen mitochondria were observed in the spermatogenic (Fig. 2b1–3), Sertoli (Fig. 2b4) and Leydig cells (Fig. 2b5). Cytoplasmic vacuolation and abnormal chromatin distribution of Sertoli cells and spermatocytes were not evident.
Transmission electron microscopy of testicular tissues. The control and Se group showed normal spermatocytes (a, ×6000). Spermatocytes in the Cd/Se-treated group showed slightly dilated smooth endoplasmic reticulum (SER) cisternae and swollen mitochondria (b1 and 3, ×6000; b2, ×12000). Sertoli and Leydig cells in the Cd/Se-treated group also showed slightly dilated SER cisternae and swollen mitochondria (b4, ×8000; b5, ×12000). Spermatocytes in the Cd-treated group had markedly swollen mitochondria with degenerated or missing cristae, dilated SER cisternae and cytoplasmic vacuolation (c1, 2, ×6000). Leydig cells in the Cd-treated group had markedly swollen mitochondria with loss of cristae and nuclear chromatin condensation (c3, ×12000; c4, ×6000). Spermatocytes in the Cd-treated group displayed morphological characteristics of apoptosis, including cell shrinkage and apoptotic body formation (black arrows) (c5, ×10000). Spermatocytes in the Cd-treated group displayed morphological characteristics of apoptosis, including chromatin condensation showing as crescentic bodies (white arrows) with a clear boundary under the nuclear envelope (c6 ×10000). Key: SER smooth endoplasmic reticulum, MI mitochondria, NU nucleus
Apoptosis in the testes
The number of apoptotic cells in the testes shown by TUNEL assay was significantly increased in the Cd-treated group compared with the control and Se groups (Fig. 1e, f and h). Interestingly, most of the apoptotic cells in the testes were found in the spermiogenic region (Fig. 1g and h). Se supplementation significantly reduced the number of apoptotic cells in the Cd/Se-treated group compared with the Cd group (P < 0.01; Fig. 3). The numbers of apoptotic cells were similar in the control and Se groups (Figs 3 and 1e, f).
Effects of Se treatment on testicular apoptosis in Cd-treated cocks. Each value is the mean ± SD of at least six cocks. Statistically significant differences: a P < 0.05 compared with control group; b P < 0.01 compared with the control group; * P < 0.05 compared with the Cd + Se group; ** values differs significantly from the Cd + Se group (P < 0.01)
Lipid peroxidation
As shown in Table 2, the LPO levels of serum and testis tissue in the Cd group were significantly higher than in the control and Se group (P < 0.05 and P < 0.01, respectively), but the levels in serum were not significantly different between the control, Se and Cd + Se groups. Se supplementation significantly reduced the LPO levels in the Cd/Se-treated group compared with the Cd group (P < 0.01).
Antioxidant activity
The SOD and GPx activity levels in the serum and testicular tissue of the experimental cocks are shown in Table 2. Cd administration significantly reduced the activities compared with the control and Se groups. Thus, Se treatment was able to counter the inhibition of antioxidant activity caused by Cd.
Effects on testosterone levels in serum
As shown in Fig. 4, the testosterone level in the serum of the Cd-treated cocks was decreased significantly. However, Cd/Se co-treatment was able to reverse this. The testosterone levels showed no statistically significant differences between the control and Se groups (Fig. 4).
Effects of Se treatment on serum testosterone level in Cd-treated cocks. Each value is the mean ± SD of at least six cocks. Statistically significant differences: a P < 0.05 compared with the control group; b P < 0.01 compared with the control group; * P < 0.05 compared with the Cd + Se group; ** values differs significantly from the Cd + Se group (P < 0.01)
Discussion
Cd, a very harmful environmental pollutant, is widely dispersed in ecosystems worldwide and is transferred among various levels of the food chain. Elevated levels of Cd have been reported in birds and mammals caused by long-range transport of pollution (Kumar et al. 2007; Pan et al. 2008; Roodbergen et al. 2008). Testicular changes in response to Cd toxicity have been seen in a variety of animal models. Thus, dietary Cd induced moderate histopathological changes in young bank voles (Bonda et al. 2004). Similarly, adult male rats have been shown to develop gonadal damage following administration of Cd (El-Ashmawy and Youssef 1999). Histologically, the toxic effects of Cd in the rat testes showed as severely damaged seminiferous tubules with degeneration and disintegration of spermatogenic cells and Leydig cells (Burukoğlu and Baycu 2008; Manna et al. 2008; Fouad et al. 2009). In the testis of Cd-treated rats there were significant increases in lipid peroxidation and the Sertoli cell tight junction was damaged by Cd exposure leading to a breakdown of the blood–testis barrier (Kusakabe et al. 2008). The present study demonstrated that dietary Cd induced similar histopathological changes in the testes (edema, necrosis, degeneration of seminiferous tubules and apoptosis in the spermiogenic region of the seminiferous epithelium (Figs 1 and 2) as was observed following Cd administration in rodents (Liu et al. 2001; Xu et al. 2005; Burukoğlu and Baycu 2008; Fouad et al. 2009).
It is well known that testes are very sensitive to Cd toxicity, which causes biochemical and functional changes. Cd induces oxidative damage by increasing the production of reactive oxygen species (ROS; Chen et al. 2008; Liu et al. 2008) and decreasing the biological activities of some antioxidants, such as SOD and GPx (Kara et al. 2007; Fouad et al. 2009), which play an important role in antioxidant defense and in the elimination of free radicals. Our results are in agreement with previous studies, which clearly demonstrated that Cd exposure increases LPO and suppresses the antioxidant defense mechanisms in testicular tissue with significant reductions in testicular function and androgen secretion (Liu et al. 2001; Sen Gupta et al. 2004b; Kara et al. 2007; Acharya et al. 2008; Fouad et al. 2009).
Mitochondria are important targets of metal toxicity including Cd (Belyaeva et al. 2008). It has been proposed that Cd initially binds to protein thiols in the mitochondrial membrane, affects the mitochondrial permeability transition, inhibits the respiratory chain reaction and that this generates ROS (Dorta et al. 2003). These effects on mitochondrial electron transfer are the major source of Cd-generated ROS not only in mammalian cells but also in plants (Heyno et al. 2008). In this study, markedly swollen mitochondria with degenerated or missing cristae were observed in spermatocytes and Sertoli cells (Fig. 2c1–3) by electron microscopy. These results are in agreement with a previous study (Belyaeva et al. 2008).
In male rodents, it is well established that Cd significantly alters the circulating level of testosterone (Lafuente et al. 2001, 2004; Sen Gupta et al. 2004a). Cd treatment also impairs testosterone production in isolated Leydig cells (Yang et al. 2003), demonstrating that the disruption of steroidogenesis is likely to be an initial target of Cd toxicity as an endocrine modulator. Here we observed that Cd exposure decreased the relative testicular weight in cocks together with a reduction in the serum testosterone level. This can be explained by the obvious pathological changes and apoptosis in the testicular cell components (e.g., spermatogenic, Leydig and Sertoli cells).
Se has a paradoxical position in animal nutrition because it is well established both as a natural toxicant and as an essential micronutrient. Recent evidence indicates that Se has a positive influence on the male reproductive system (Boitani and Puglisi 2008). In this study, concurrent treatment with Se and Cd reduced the Cd-induced testicular histopathological changes, oxidative stress, endocrine disorder and apoptosis. Together, this evidence suggests that Se treatment counteracts the toxic effect of cadmium on the avian testis. This element is a well-established antioxidant and can prevent or decrease the harmful effects of oxidants and ROS in various tissues. The effect of Se effect on GPx activity may be attributed to an increase in the bioavailability of Se following co-treatment with sodium selenite, which is reflected in increased GPx activity (Jamba et al. 2000; El-Sharaky et al. 2007; Newairy et al. 2007; Ognjanović et al. 2008; Jihen et al. 2009; Messaoudi et al. 2009). This upregulation of GPx production induced by Se may explain why the GPx and SOD activity levels in the serum and testicular tissues of the Cd + Se group were higher than in the Cd group in our study. Dietary Se also decreased the MDA concentration in the Cd + Se group. These results can be explained by the important role of Se in preventing LPO and in protecting the integrity and functioning of testicular tissues. However, some have reported that Se exerts its protective effect by significantly decreasing Cd accumulation in organs or by inducing a redistribution of Cd in rodents (Yiin et al. 1999; Jamba et al. 2000; Ognjanović et al. 2008; Lazarus et al. 2009). We found here that Se supplementation reduced the distribution of Cd to the testicular tissues (Table 1) and also decreased Cd concentrations in the liver and kidney (unpublished results, JL Li). Cd forms an equimolar complex with Se ingested as selenite, in the form of selenide in plasma. This complex binds to selenoprotein P as a high molecular weight protein complex and changes the metabolism of Cd (Sasakura and Suzuki 1998). Selenoprotein P is the most common Se-binding protein and is important for the supply of SE to organs, especially the testis and kidney. It has been implicated in selenium transport, selenium detoxification and antioxidant defense systems (Schomburg et al. 2003). Furthermore, the activity of Se within animal systems is mainly elicited via selenoproteins (Stadtman 2000). Hence, we hypothesize that selenoproteins, especially selenoprotein P, might play an important role in the detoxification and interactions between Se and Cd.
In conclusion, our study demonstrated that dietary exposure to Cd caused histopathological changes, oxidative stress, endocrine disorder and apoptosis in cock testes. There were significant reductions in testicular function and androgen secretion. Moreover, dietary Se ameliorated these effects by enhancing antioxidant systems and by decreasing Cd accumulation in the testis. However, our experimental design could not give information about the relationship between Cd retention and selenoprotein synthesis, so further investigations are needed to clarify this.
References
Acharya UR, Mishra M, Patro J, Panda MK (2008) Effect of vitamins C and E on spermatogenesis in mice exposed to cadmium. Reprod Toxicol 25(1):84–88
Anteau MJ, Afton AD, Custer CM, Custer TW (2007) Relationships of cadmium, mercury, and selenium with nutrient reserves of female lesser scaup (Aythya affinis) during winter and spring migration. Environ Toxicol Chem 26(3):515–520
Belyaeva EA, Dymkowska D, Wieckowski MR, Wojtczak L (2008) Mitochondria as an important target in heavy metal toxicity in rat hepatoma AS-30D cells. Toxicol Appl Pharmacol 231(1):34–42
Boitani C, Puglisi R (2008) Selenium, a key element in spermatogenesis and male fertility. Adv Exp Med Biol 636:65–73
Bonda E, Wlostowski T, Krasowska A (2004) Testicular toxicity induced by dietary cadmium is associated with decreased testicular zinc and increased hepatic and renal metallothionein and zinc in the bank vole (Clethrionomys glareolus). Biometals 17(6):615–624
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Burukoğlu D, Baycu C (2008) Protective effects of zinc on testes of cadmium-treated rats. Bull Environ Contam Toxicol 81(6):521–524
Chen L, Liu L, Huang S (2008) Cadmium activates the mitogen-activated protein kinase (MAPK) pathway via induction of reactive oxygen species and inhibition of protein phosphatases 2A and 5. Free Radic Biol Med 45(7):1035–1044
Dorta DJ, Leite S, DeMarco KC, Prado IM, Rodrigues T, Mingatto FE et al (2003) A proposed sequence of events for cadmium-induced mitochondrial impairment. J Inorg Biochem 97(3):251–257
El-Ashmawy IM, Youssef SA (1999) The antagonistic effect of chlorpromazine on cadmium toxicity. Toxicol Appl Pharmacol 161(1):34–39
El-Sharaky AS, Newairy AA, Badreldeen MM, Eweda SM, Sheweita SA (2007) Protective role of selenium against renal toxicity induced by cadmium in rats. Toxicology 235(3):185–193
Esterbauer H, Cheeseman KH (1990) Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol 186:407–421
Fouad AA, Qureshi HA, Al-Sultan AI, Yacoubi MT, Ali AA (2009) Protective effect of hemin against cadmium-induced testicular damage in rats. Toxicology 257(3):153–160
Hasunuma R, Ogawa T, Kawanishi Y (1982) Fluorometric determination of selenium in nanogram amounts in biological materials using 2,3-diaminonaphthalene. Anal Biochem 126(2):242–245
Heyno E, Klose C, Krieger-Liszkay A (2008) Origin of cadmium-induced reactive oxygen species production: mitochondrial electron transfer versus plasma membrane NADPH oxidase. New Phytol 179(3):687–699
IARC (1993) Beryllium, cadmium, mercury, and exposures in the glass manufacturing industry. IARC Monograph 58, Lyon
Jamba L, Nehru B, Bansal MP (2000) Effect of selenium supplementation on the influence of cadmium on glutathione and glutathione peroxidase system in mouse liver. J Trace Elem Exp Med 13(3):299–304
Jihen el H, Imed M, Fatima H, Abdelhamid K (2008) Protective effects of selenium (Se) and zinc (Zn) on cadmium (Cd) toxicity in the liver and kidney of the rat: histology and Cd accumulation. Food Chem Toxicol 46(11):3522–3527
Jihen el H, Imed M, Fatima H, Abdelhamid K (2009) Protective effects of selenium (Se) and zinc (Zn) on cadmium (Cd) toxicity in the liver of the rat: effects on the oxidative stress. Ecotoxicol Environ Saf 72(5):1559–1564
Kara H, Cevik A, Konar V, Dayangac A, Yilmaz M (2007) Protective effects of antioxidants against cadmium-induced oxidative damage in rat testes. Biol Trace Elem Res 120(1–3):205–211
Kumar P, Prasad Y, Patra AK, Swarup D (2007) Levels of cadmium and lead in tissues of freshwater fish (Clarias batrachus L.) and chicken in Western UP (India). Bull Environ Contam Toxicol 79(4):396–400
Kusakabe T, Nakajima K, Suzuki K, Nakazato K, Takada H, Satoh T, Oikawa M, Kobayashi K, Koyama H, Arakawa K, Nagamine T (2008) The changes of heavy metal and metallothionein distribution in testis induced by cadmium exposure. Biometals 21(1):71–81
Lafuente A, Márquez N, Pérez-Lorenzo M, Pazo D, Esquifino AI (2001) Cadmium effects on hypothalamic-pituitary-testicular axis in male rats. Exp Biol Med (Maywood) 226(6):605–611
Lafuente A, Gonzalez-Carracedo A, Romero A, Cano P, Esquifino AI (2004) Cadmium exposure differentially modifies the circadian patterns of norepinephrine at the median eminence and plasma LH, FSH and testosterone levels. Toxicol Lett 146(2):175–182
Larison JR, Likens GE, Fitzpatrick JW, Crock JG (2000) Cadmium toxicity among wildlife in the Colorado Rocky Mountains. Nature 406(6792):181–183
Lazarus M, Orct T, Blanusa M, Kostial K, Pirsljin J, Beer-Ljubic B (2006) Effect of selenium pre-treatment on cadmium content and enzymatic antioxidants in tissues of suckling rat. Toxicol Lett 164(S1):S191. doi:10.1016/j.toxlet.2006.07.055
Lazarus M, Orct T, Jurasoviae J, Blanusa M (2009) The effect of dietary selenium supplementation on cadmium absorption and retention in suckling rats. Biometals 22:973–983. doi:10.1007/s10534-009-9249-9
Liu J, Corton C, Dix DJ, Liu Y, Waalkes MP, Klaassen CD (2001) Genetic background but not metallothionein phenotype dictates sensitivity to cadmium-induced testicular injury in mice. Toxicol Appl Pharmacol 176(1):1–9
Liu J, Qian SY, Guo Q, Jiang J, Waalkes MP, Mason RP, Kadiiska MB (2008) Cadmium generates reactive oxygen- and carbon-centered radical species in rats: insights from in vivo spin-trapping studies. Free Radic Biol Med 45(4):475–481
Lucia M, Andre JM, Gonzalez P, Baudrimont M, Gontier K, Maury-Brachet R, Davail S (2009) Impact of cadmium on aquatic bird Cairina moschata. Biometals (in press). doi:10.1007/s10534-009-9232-5
Manna P, Sinha M, Sil PC (2008) Cadmium induced testicular pathophysiology: prophylactic role of taurine. Reprod Toxicol 26(3–4):282–291
Messaoudi I, Hammouda F, El Heni J, Baati T, Said K, Kerkeni A (2009) Reversal of cadmium-induced oxidative stress in rat erythrocytes by selenium, zinc or their combination. Exp Toxicol Pathol (in press). doi:10.1016/j.etp.2009.04.004
Newairy AA, El-Sharaky AS, Badreldeen MM, Eweda SM, Sheweita SA (2007) The hepatoprotective effects of selenium against cadmium toxicity in rats. Toxicology 242(1–3):23–30
Ognjanović BI, Marković SD, Pavlović SZ, Zikić RV, Stajn AS, Saicić ZS (2008) Effect of chronic cadmium exposure on antioxidant defense system in some tissues of rats: protective effect of selenium. Physiol Res 57(3):403–411
Pan C, Zheng G, Zhang Y (2008) Concentrations of metals in liver, muscle and feathers of tree sparrow: age, inter-clutch variability, gender, and species differences. Bull Environ Contam Toxicol 81(6):558–560
Pollock B, Machin KL (2009) Corticosterone in relation to tissue cadmium, mercury and selenium concentrations and social status of male lesser scaup (Aythya affinis). Ecotoxicology 18(1):5–14
Roodbergen M, Klok C, van der Hout A (2008) Transfer of heavy metals in the food chain earthworm Black-tailed godwit (Limosa limosa): comparison of a polluted and a reference site in The Netherlands. Sci Total Environ 406(3):407–412
Santos FW, Oro T, Zeni G, Rocha JB, do Nascimento PC, Nogueira CW (2004) Cadmium induced testicular damage and its response to administration of succimer and diphenyl diselenide in mice. Toxicol Lett 152(3):255–263
Santos FW, Zeni G, Rocha JB, Weis SN, Fachinetto JM, Favero AM et al (2005) Diphenyl diselenide reverses cadmium-induced oxidative damage on mice tissues. Chem Biol Interact 151(3):159–165
Sasakura C, Suzuki KT (1998) Biological interaction between transition metals (Ag, Cd and Hg), selenide/sulfide and selenoprotein P. J Inorg Biochem 71(3–4):159–162
Schomburg L, Schweizer U, Holtmann B, Flohe L, Sendtner M, Kohrle J (2003) Gene disruption discloses role of selenoprotein P in selenium delivery to target tissues. Biochem J 370(Pt 2):397–402
Sen Gupta R, Kim J, Gomes C, Oh S, Park J, Im WB, Seong JY, Ahn RS, Kwon HB, Soh J (2004a) Effect of ascorbic acid supplementation on testicular steroidogenesis and germ cell death in cadmium-treated male rats. Mol Cell Endocrinol 221(1–2):57–66
Sen Gupta R, Sen Gupta E, Dhakal BK, Thakur AR, Ahnn J (2004b) Vitamin C and vitamin E protect the rat testes from cadmium-induced reactive oxygen species. Mol Cells 17(1):132–139
Siu ER, Mruk DD, Porto CS, Cheng CY (2009) Cadmium-induced testicular injury. Toxicol Appl Pharmacol (in press). doi:10.1016/j.taap.2009.01.028
Stadtman TC (2000) Selenium biochemistry. Mammalian selenoenzymes. Ann N Y Acad Sci 899:399–402
Takiguchi M, Yoshihara S (2006) New aspects of cadmium as endocrine disruptor. Environ Sci 13(2):107–116
Waalkes MP (2000) Cadmium carcinogenesis in review. J Inorg Biochem 79(1–4):241–244
Wayland M, Gilchrist HG, Marchant T, Keating J, Smits JE (2002) Immune function, stress response, and body condition in arctic-breeding common eiders in relation to cadmium, mercury, and selenium concentrations. Environ Res 90(1):47–60
Xu LC, Sun H, Wang S, Song L, Chang HC, Wang XR (2005) The roles of metallothionein on cadmium-induced testes damages in Sprague-Dawley rats. Environ Toxicol Pharmacol 20:83–87
Yadav N, Khandelwal S (2008) Effect of Picroliv on cadmium induced testicular damage in rat. Food Chem Toxicol 46(2):494–501
Yang JM, Arnush M, Chen QY, Wu XD, Pang B, Jiang XZ (2003) Cadmium-induced damage to primary cultures of rat Leydig cells. Reprod Toxicol 17(5):553–560
Yiin SJ, Chern CL, Sheu JY, Lin TH (1999) Cadmium induced lipid peroxidation in rat testes and protection by selenium. Biometals 12(4):353–359
Yu RA, Chen XM (2004) Effects of selenium on rat hepatocellular DNA damage, apoptosis and changes of cell cycle induced by cadmium in vivo. Zhonghua Yu Fang Yi Xue Za Zhi 38(3):155–158
Yu X, Hong S, Faustman EM (2008) Cadmium-induced activation of stress signaling pathways, disruption of ubiquitin-dependent protein degradation and apoptosis in primary rat Sertoli cell-gonocyte cocultures. Toxicol Sci 104(2):385–396
Zhou YJ, Zhang SP, Liu CW, Cai YQ (2009) The protection of selenium on ROS mediated-apoptosis by mitochondria dysfunction in cadmium-induced LLC-PK(1) cells. Toxicol In Vitro 23(2):288–294
Acknowledgment
This work was supported by a grant from the Bureau of Education of Heilongjiang Province (No. 10551038).
Author information
Authors and Affiliations
Corresponding author
Additional information
Jin-Long Li and Rui Gao contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, JL., Gao, R., Li, S. et al. Testicular toxicity induced by dietary cadmium in cocks and ameliorative effect by selenium. Biometals 23, 695–705 (2010). https://doi.org/10.1007/s10534-010-9334-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-010-9334-0