Abstract
European eels are dangerously threatened with extinction. Recent advances tend to show that pollution could, in addition to other already identified factors, contribute to this drama. In a previous report, cadmium (Cd) pre-exposure was found to strongly stimulate the pituitary-liver-gonad axis of maturing female silver eels, leading, lastly, to oocytes atresia and eels mortality. The present work was performed to get more insights into the effects of Cd pre-exposure on eels’ ovaries. The transcription levels of various genes involved in mitochondrial metabolism, in the cellular response to metal (metallothioneins, MTs) and oxidative stress (catalase, CAT) were investigated. Our results show that ovarian growth is associated with an up-regulation of mitochondrial genes. However, Cd pre-exposure was found to significantly impair this up-regulation. Such findings could explain, at least in part, why oocytes of Cd pre-contaminated eels could not reach final maturation. Concerning MTs, despite the end of the experiment was marked by a strong increase in their gene transcription levels in both eel groups, MTs protein content was found to increase only in the case of Cd pre-contaminated eels. Since this increase in MTs protein content was associated with a massive entry of Cd in gonads, our findings suggest that MTs mRNA, that are normally accumulated in oocytes to cope with the future needs, can be activated and translated in response to Cd exposure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the last three decades, European eel populations (Anguilla anguilla, Linnaeus, 1758) have suffered a dramatic decline. Glass eel recruitment has dropped throughout their entire European distribution area and the European eel stock is now considered to be critically endangered (Stone 2003). Even if over fishing of glass eels at the mouth of many European rivers, habitat loss and physical obstacles to continental migration (e.g. hydropower turbines) are recognized to be the most important factors of this decline, recent reports tend to show that the notoriously complex life cycle of European eels puts them at special risk from pollution (Robinet and Feunteun 2002; Palstra et al. 2006; Pierron et al. 2007a; Pierron et al. 2008). The biological cycle of the European eel (see Tesch 2003 or van Ginneken and Maes 2005) comprises four life stages, two metamorphoses and two trans-Atlantic migrations. Reproduction of the European eel takes place in the Sargasso Sea from where larvae drift back towards the European coasts following oceanic currents. After metamorphosis of the larvae into glass eels, the organisms reach the juvenile growth phase stage (yellow eel) in continental habitats. This stage can last from several years to more than 20 years, depending on the hydrosystem, and ends with a second metamorphosis called silvering which prepares the future genitors (silver eels) to their transoceanic reproductive migration. However, when silver eels leave the European coasts, their gonads are still immature and maturation is blocked at a prepubertal stage (Vidal et al. 2004). This implies that gonad development must occur during their 5,500 km migration, i.e. a 5–6 months period marked by swimming activity and starvation that ends with the spawning and the death of the genitors. In natural conditions, the prepubertal stage is the last known stage, as mature European eels have never been caught in the wild. From an ecotoxicological point of view, their long somatic growth phase makes them particularly propitious to accumulate large amounts of persistent contaminants such as metals (Durrieu et al. 2005) and organic compounds (Bordajandi et al. 2003; Roche et al. 2002). Moreover, since silver eels undergo a natural period of starvation at the silver stage, previously accumulated pollutants can be remobilized and redistributed, leading to the appearance of delayed toxic events (Palstra et al. 2006; Pierron et al. 2008).
In a recent work, we have investigated the impact of cadmium (Cd), a widespread, non essential and highly toxic metal, on the reproductive capacities of female European silver eels (Pierron et al. 2008). In order to mimic the European eel life cycle and especially their reproductive migration, we captured female sliver eels during the start of their reproductive migration and exposed the half of them to Cd. Thereafter, both unexposed (controls) and Cd pre-contaminated eels were forced to swim in uncontaminated seawater and artificially matured by weekly injections of carp pituitary extracts (CPE; Dufour et al. 2003; Durif et al. 2006). Our results have revealed an endocrine stimulating effect of Cd pre-exposure on the pituitary-gonad-liver axis, an effect mainly marked by an increase in (1) pituitary luteinizing hormone gene transcription level (lh subunit lh-β and gp-α), (2) vitellogenesis (at both gene transcription and protein levels) and (3) ovarian growth. This was followed, after 20 weeks of swimming and hormonal treatment, by a strong phenomenon of oocytes atresia and fish mortality affecting only Cd pre-contaminated eels. These effects occurred before oocytes could reach full maturation and were associated with a large entry of both vitellogenin and Cd into the ovaries. Indeed, a redistribution of previously stored cadmium, even from the low Cd levels of control eels, was observed during sexual maturation.
Here, our main goal was to get more insights into the effects potentially triggered by Cd pre-exposure on gonad tissue. More precisely, we have investigated the possible effect of Cd pre-exposure on the ovarian mitochondrial metabolism; the mitochondrion being a well known key intracellular target of Cd toxicity (Wang et al. 2004; Sokolova 2004; Risso-de Faverney et al. 2004; Takaki et al. 2004; Belyaeva et al. 2008). The transcription level of genes encoding for mitochondrial ribosomal 12S RNA as well as proteins involved in the mitochondrial respiratory chain (NADH dehydrogenase subunit 5, nd5; cytochrome C oxidase subunit 1, cox1; ATP synthase subunit 6–8, atp6-8 and cytochrome C, cytc) were assessed in gonads of both control and Cd pre-contaminated maturing eels. As mitochondria dysfunctions triggered by Cd are often associated with the production of reactive oxygen species (ROS), we have also monitored the transcription level of the gene encoding for catalase (cat), an enzyme involved in the fight against oxidative stress (Scandalios 2005). The analysis was completed by the determination of the gene transcription level and the ovarian protein content of a protein involved in metal sequestration and response to oxidative stress, the metallothioneins (MTs).
Materials and methods
Experimental design
The experimental design was first described in details in Pierron et al. (2008). Briefly, in order to mimic as well as possible the biological cycle and especially the reproductive migration of European eels, the experimental protocol associated two complementary steps. First, fifty-two female silver eels were caught in unpolluted site (Loire river, France) during their continental downstream migration, which represents the start of their reproductive migration. Half of the animals was submitted to aqueous Cd exposure over 30 days at a concentration of 14.8 ± 0.4 μg l−1 (mean ± SE, n = 12). In parallel, the other half was maintained in the same physiological conditions but in absence of Cd, thus constituting a control group. Thereafter, both control and Cd pre-contaminated eels were individually identified with colored elastomer tags and randomly placed in two swim tanks filled with natural uncontaminated seawater (salinity 30.5 ± 0.2‰). In order to mimic the reproductive migration of European eels, all organisms were submitted to a water current to force them swimming at a speed of about 30–40 cm s−1, representing a distance of 5,500 km covered in 6 months. To induce gonad maturation, both control and Cd pre-contaminated eels received a perivisceral injection a week of carp pituitary extract (CPE) at a dose equivalent to 20 pituitary powder∙kg body weight−1, according to a method previously described (Durif et al. 2006).
With this protocol, five control and pre-contaminated eels were sacrificed for analysis just after the first step of Cd exposure and after 8, 18 and 22 CPE injections. However, due to excess mortality, only four pre-exposed eels could be analysed at the last sampling time.
During the two steps, animals were submitted to natural photoperiod and were not fed as eels undergo a natural period of starvation at the silver stage.
Sampling procedure
At each sampling time, fish were weighed, measured and killed by severing the medulla oblongata. For ovaries, samples needed for Cd determination, histological investigations and gene transcription analyses were collected according to a standardized method, 4 cm in front of the anus. These samples and the rest of the ovaries were weighed to calculate the gonadosomatic index (GSI expressed as a percentage, (gonad weight/total body weight) × 100). Mean GSI data as well as mean metal burdens and results of histological investigations have already been published and available in Pierron et al. (2008).
Metallothionein quantification
The level of metallothionein proteins in gonads was determined by mercury-saturation assay as previously described, using cold inorganic mercury (Gonzalez et al. 2006). Results are expressed in nmol of Hg-binding sites present in the whole ovarian tissue (wet weight, ww). Since the exact quantity of Hg-binding sites per metallothionein molecule is unknown for Anguilla anguilla, metallothionein burdens cannot be expressed in nmol metallothionein in the whole ovarian tissue.
Quantitative real time RT-PCR
For each gene, specific primers previously developed for Anguilla anguilla were used (cf. Pierron et al. 2007b). Due to the high protein and lipid content of gonad tissue in these maturing organisms, for each silver eel, total RNAs were extracted from two pieces of 60 mg of tissue. Moreover, prior to RNA extraction, tissue homogenates were treated with one volume of phenol/chloroform/isoamylic alcohol (25/24/1) followed by another treatment with one volume of pure chloroform. Thereafter, in order to obtain a sufficient amount of RNAs for subsequent gene transcription analyses, homogenates from the two pieces of gonad tissue of the same individual were mixed and total RNAs were extracted using the Absolutely Total RNA Miniprep kit (Stratagene, Netherlands), according to the manufacturer’s instructions. RNAs quality was evaluated by electrophoresis on a 1% agarose gel and their concentrations were determined by spectrophotometry. First-strand cDNA was synthesized from 5 μg of total RNA using the Stratascript First-Strand Synthesis System (Stratagene, Netherlands) according to the manufacturer’s instructions. After extraction and reverse transcription, amplification of cDNA was monitored using the DNA intercaling dye SyberGreen I. Real-time PCR reactions were performed in a Light-Cycler® (Roche, Switzerland) following the manufacturer’s instructions (one cycle at 95°C for 10 min and 50 amplification cycles at 95°C for 5 s, 60°C for 5 s and 72°C for 20 s). Each 20 μl reaction contained 2 μl reverse transcribed product template, 1 μl of master mix including the SyberGreen I fluorescent dye (Roche, Switzerland), and the specific primer pairs at a final concentration of 300 nM each primer. The reaction specificity was determined for each reaction from the dissociation curve of the PCR product. The dissociation curve was obtained by following the SyberGreen fluorescence level during a gradual heating of the PCR products from 60 to 95°C. For genes encoding for 12S, cat and mts, their gene transcription levels were normalized according to the β-actin gene transcription. Concerning mitochondrial genes encoding for enzymes involved in the mitochondrial respiratory chain (cox1, atp6-8, cytc and nd5), their gene transcription levels were normalized according to the mitochondrial 12S gene transcription.
Data treatment
All values are presented as mean ± S.E. Comparisons among groups were performed using analysis of variance (ANOVA), after checking assumptions (normality and homoscedasticity of the error term). If significant effects were detected, Least Square Deviation test was used to separate means. When assumptions were not met, we used log and Box-Cox data transformation (Peltier et al. 1998) or non-parametric Kruskall-Wallis test. In this latter case, the U-Mann-Whitney test was used to separate means. For all the statistical results, a probability of P < 0.05 was considered significant.
The relationship between GSI and ovarian 12S, cox1, atp6-8, cytc or nd5 gene transcription levels as well as the relationship between ovarian Cd and MTs protein contents were investigated using the non-parametric Spearman (r) rank correlation test due to the non linearity in the data.
All computations were performed using the STATISTICA version 6.1 software.
Results
Change in mitochondrial gene transcription
Concerning the gene transcription level of 12S, no significant differences were observed between control and Cd-pre-contaminated eels, and this, throughout the maturation phase (Fig. 1a). For both control and Cd-pre-contaminated eels, 12S gene transcription level was found to progressively increase after 8 CPE injections. However, we must note that this increase was more pronounced during the last 4 weeks of hormonal treatment. Indeed, 12S gene transcription level was increased, in mean, 10- and 13-times between the 8th and the 18th (10 weeks) CPE injection and between the 18th and the 22th (4 weeks) CPE injection, respectively. From the beginning to the end of the experiment, 12S gene transcription was, in mean, significantly increased 114-times. Interestingly, for both control and Cd-pre-contaminated eels, change in 12S gene transcription level during the maturation phase was found to be significantly correlated with increasing GSI of animals (Fig. 1b;Table 1).
Change in basal transcription level of the gene encoding for 12S in gonads of control (solid line, black squares) and Cd pre-contaminated (dotted line, white circles) female silver eels as function of (a) the number of weekly injection of carp pituitary extract (CPE) received by maturing organisms (mean ± S.E., n = 5) or (b) the gonado-somatic index (GSI) of maturing organisms (n = 40)
In the case of control eels, the transcription level of genes encoding for proteins involved in the mitochondrial respiratory chain (cox1, atp6-8, cytc and nd5) were found to progressively increase throughout the maturation phase (Fig. 2). However, as was the case for 12S gene, we must note that the most important increase in gene transcription level was observed during the last 4 weeks of the experiment. Between the 18th and the 22th CPE injection, the gene transcription levels of cox1, atp6-8, cytc and nd5 were, in mean, increased 57-times. As a comparison, their gene transcription levels were increased, in mean, 2.3- and 48-times between time 0 and the 8th CPE injection and between the 8th and the 18th CPE injection, respectively. Moreover, as was the case for 12S gene, the gene transcription levels of cox1, atp6-8, cytc and nd5 in control eels were found to be significantly correlated with increasing GSI of animals (Table 1). Such a pattern was, however, not observed in the case of Cd pre-contaminated eels. Indeed, no significant correlations between cox1, atp6-8, cytc or nd5 gene transcription levels and the GSI of animals could be observed (Table 1). Corollary, the gene transcription levels of cox1, atp6-8, cytc or nd5 were found to be significantly lower than those determined in control eels after 18 and 22 CPE injections.
Change in basal transcription level (mean ± S.E., n = 5) of genes encoding for proteins involved in the mitochondrial respiratory chain (cox1, atp6-8, cytc, nd5) and for catalase (cat) in gonads of control (solid line, black squares) and Cd pre-contaminated (dotted line, white circles) female silver eels experimentally matured by weekly injection of carp pituitary extract (CPE). For each sampling time, * denotes a significant effect of cadmium pre-exposure (two ways analysis of variance, P < 0.05)
Concerning cat gene, whereas no significant change in its transcription level was observed throughout the maturation phase in control eels, its transcription level was found to significantly increase in Cd pre-contaminated eels during the last 4 weeks of hormonal treatment. This increase was so important that its transcription level was, at the end of the experiment, significantly 9.4-fold higher in Cd pre-contaminated eels than this determined in control eels.
Metallothioneins gene transcription and protein burden
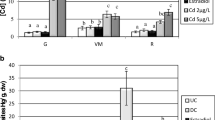
A T = 0, i.e. just after Cd exposure, the gene transcription level of mts was found to be significantly 2.1-fold lower in the ovary of Cd pre-contaminated eels in comparison to controls (Fig. 3). However, such a difference was not observed at the protein level. Indeed, no significant differences in MTs protein burdens were observed between control and Cd pre-contaminated eels at this time. A similar pattern was also observed after 18 CPE injections. Thereafter, during the last 4 weeks of hormonal treatment, mts gene transcription level was found to drastically and significantly increase in both control and Cd pre-contaminated eels. During this period, mts gene transcription level was increased 54- and 345-times in control and Cd pre-contaminated eels, respectively. Despite mts gene transcription level was significantly 2.2-fold more important in controls in comparison to Cd pre-contaminated eels after 18 CPE injections, no significant differences could be observed after 22 CPE injections. At the protein level, such a significant increase was observed only in the case of Cd pre-contaminated eels. Whereas MTs burden did not significantly increased (P = 0.21) during the last 4 weeks of hormonal treatment in the case of control eels, this value was significantly increased 4.8-times in Cd pre-contaminated eels. Interestingly, ovarian MTs protein burdens of both control and Cd pre-contaminated eels were significantly correlated with ovarian Cd burdens (Fig. 4). Such a relationship was, however, not observed at the transcriptional level (r = 0.02, P = 0.91).
Change in (a) basal transcription level (mean ± S.E., n = 5) of genes encoding for metallothioneins (mts) and (b) metallothionein protein content (expressed as nmol of Hg-binding sites present in the whole ovarian tissue, wet weight, mean ± S.E., n = 3) in gonads of control (solid line, black squares) and Cd pre-contaminated (dotted line, white circles) female silver eels experimentally matured by weekly injection of carp pituitary extract (CPE). For each sampling time, * denotes a significant effect of cadmium pre-exposure (two ways analysis of variance and Kruskall Wallis test, P < 0.05)
Relationship between metallothioneins (MTs) content (expressed as μmol of Hg-binding sites present in the whole ovarian tissue, wet weight) and Cd content in gonads of control (black squares) and Cd pre-contaminated (white circles) female silver eels experimentally matured by weekly injection of carp pituitary extract (n = 40)
Discussion
In a previous work (see Pierron et al. 2008), we have reported a stimulating effect of Cd pre-exposure on the pituitary-gonad-liver axis of experimentally maturing silver eels, leading to an early and enhanced vitellogenesis. From 20 weeks of hormonal treatment, this was followed by strong phenomena of oocytes atresia and eels mortality affecting only Cd pre-contaminated eels. Significantly, these devastating effects of Cd were observed in organisms that presented metal concentrations in the main organs of Cd bioaccumulation, the liver and the kidney, still below those observed in eels from Cd contaminated hydrosystems. Indeed, after 30 days of Cd exposure, average Cd concentrations (data not shown) in the liver and the kidney reached means of 1.7 ± 0.4 and 10.6 ± 2.7 μg g−1 (dry weight, dw), respectively, in the case of Cd-exposed eels versus 0.9 ± 0.1 and 5.9 ± 0.2 μg g−1 (dw), respectively, in the controls, i.e. in eels from the Loire river (France). For comparison, Cd concentrations in the liver and the kidneys of yellow eels (aged 6–14 years) inhabiting the Gironde estuary (Durrieu et al. 2005), which is characterized by a historic Cd pollution, reach means of 5 ± 0.8 and 34.2 ± 5.1 μg g−1 (dw), respectively. Here, our main goal was to get more insights into the specific effects potentially triggered by Cd pre-exposure on gonad tissue, mainly on the mitochondrial respiratory chain, a primary site of Cd toxicity (Wang et al. 2004; Sokolova 2004; Risso-de Faverney et al. 2004; Takaki et al. 2004; Belyaeva et al. 2008), by means of qRT-PCR gene transcription analysis.
Concerning the effect of hormonal treatment on 12S gene transcription, our data show a progressive and strong increase in its transcription level in both control and Cd pre-contaminated eels during the maturation phase and this, without significant differences between the two groups of animals. Moreover, for both control and Cd pre-contaminated eels, a significant correlation was found between 12S gene transcription level and GSI of animals. As the GSI of animals increased in response to hormonal treatment, the gene transcription level of 12S increased. Such an increase in mitochondrial gene transcription level could reflect an increase in the number of mitochondria in gonad tissue. This assumption appears indeed consistent with previous ultrastructural investigations carried out on gonad tissue of experimentally maturing female European silver eels. Authors reported a proliferation of mitochondria in gonad ooplasm after hormonal treatment with CPE (Burzawa-Gérard et al. 1994). Such a report was also established from oocytes of female Anguilla japonica artificially matured with salmon pituitary extracts (Kayaba et al. 2001). Whereas few and undeveloped mitochondria were observed in gonad of immature/untreated animals (oocytes at the oil droplet stage), a large number of developed mitochondria were observed in the course of vitellogenesis in oocytes of maturing eels. As a consequence, it appears reliable to hypothesize that the increase in 12S gene transcription level that we observed in the present study reflects an increase in the number of mitochondria in gonads of maturing silver eels. Such an increase in mitochondria number could be linked to the build-up of mitochondrial activity for enhanced ATP production; such increase in ATP production could aim to prepare oocytes for (1) vitellogenin incorporation (e.g. development of the zona radiata and microvillosities) and reshuffle, (2) steroidogenesis and (3) final maturation (i.e., germinal vesicle migration and breakdown; Habibi and Lessman 1986; Burzawa-Gérard et al. 1994; Dufour et al. 2003; Kwon et al. 2005). We must note, however, that our results could not exclude that these mRNA are stored in ooplasm and translated at a later time, mainly during embryogenesis, a mechanism used by several organisms (Vassalli and Stutz 1995; Seydoux 1996; Stebbins-Boaz and Richter 1997).
Interestingly, for control eels, the increase in 12S gene transcription level in response to hormonal treatment was associated with an increase in cox1, atp6-8, cytc and nd5 gene transcription levels. Thus, the transcription level of genes encoding for proteins involved in the mitochondrial respiratory chain appears to be up-regulated by hormonal treatment. Such a phenomenon was first described in gonads of artificially maturing female Anguilla japonica as well as in naturally and artificially maturing New Zealand eels (Anguilla australis and Anguilla dieffenbachii). The transcription level of the gene encoding for cytochrome b (CYTB), a protein involved in the electron transport in the mitochondrial respiratory chain (as CYTC in the present study), was found to significantly increase from early to late vitellogenesis (Lokman et al. 2003). Similarly, a large-scale gene transcription analysis by means of serial analysis of gene expression (SAGE) revealed high levels of ATP synthase transcripts in zebrafish ovarian fully-grown follicules. Complementary, the proteomic analysis carried out on the same tissue revealed high levels of the corresponding proteins, thus supporting the fact that ATP synthase mRNAs are effectively translated into proteins (Knoll-Gellida et al. 2006). Concerning the Cd effect, whereas our previous results have shown a stimulating effect of Cd pre-exposure on the pituitary-gonad-liver axis of European silver eels, our present results show, at the opposite, an impairment of the transcription level of genes involved in the mitochondrial respiratory chain. Indeed, after 18 and 22 CPE injections, mean transcription levels of cox1, atp6-8, cytc and nd5 were found to be significantly lower than those determined in controls eels. This is, however, consistent with a previous work carried out on gills of European glass eels. The transcription level of these same genes was found to be down-regulated in response to aqueous Cd exposure (Pierron et al. 2007b). Analogously to that found here, the most important effect of Cd exposure was observed on nd5 transcription level. Additionally, such effect of Cd on cox1 gene transcription was also described in liver of carp experimentally exposed to low concentrations of a mixture of waterborne and dietary Cd (Reynders et al. 2006). A decrease in cox2 and cox4, at both transcriptional and protein levels, was also reported during in vitro investigations performed on human MDA-MB231 cells exposed to low Cd concentrations (Cannino et al. 2008). Thus, such a down-regulation of genes involved in the mitochondrial respiratory chain could be the result of a direct effect of Cd on gonads, rather than an indirect effect of the metal mediated by changes in circulating hormone levels (see Pierron et al. 2008). Additionally, as ovaries were not the only organs that have significantly accumulated Cd during the maturation phase, it is possible that similar events occurred in other tissues of eels. This could lead to the appearance of toxic events in various tissues of eels (notably in kidneys, see Pierron et al. 2008). In the particular case of ovaries, the appearance of such Cd effects could explain, at least in part, why oocytes of Cd pre-contaminated eels could not reach final maturation and subsequently why these oocytes were subjected to atresia at the end of the experiment. Indeed, several studies carried out on fish, mammalian and amphibian oocytes have highlighted the vital role of oxidative phosphorylation during final maturation (Brachet et al. 1975; Habibi and Lessman 1986; Wycherley et al. 2005; Johnson et al. 2007). For example, the use of inhibitors or uncouplers of oxidative phosphorylation was found to abolish germinal vesicle migration and breakdown in ovaries of female goldfish (Habibi and Lessman 1986). However, as previously described in our first article, atresia phenomenon could have several origins. Among the different hypotheses previously developed, we have proposed that Cd could have directly triggered apoptosis of oocytes. Indeed, the pro-apoptotic effect of Cd, an effect mediated by mitochondrial dysfunctions and subsequent ROS production, is now well recognized (Risso-de Faverney et al. 2004; Poliandri et al. 2006). This hypothesis is consistent with the fact that the end of the experiment was marked, only in the case of Cd pre-contaminated eels, by a significant increase in cat transcription level; the transcription level of which being known to be up-regulated by ROS (Scandalios 2005). Alternatively, since we observed a strong stimulating effect of Cd pre-exposure on LH subunits genes transcription levels, Cd could indirectly, by altering circulating hormones levels, trigger an overstimulation and subsequently atresia of oocytes (see Pierron et al. 2008). Our present results cannot permit to favour one hypothesis over another, certainly because atresia phenomenon was the resultant of multiple dysfunctions, associating direct and indirect effects of Cd on sexual tissues.
The end of the experiment was also marked by a strong increase in the gene transcription level of mts. However, in the case of control eels, such an increase in mts gene transcription level could not be observed at the protein level. Such a discrepancy between mts gene transcription and MTs protein content was already observed and described in oocytes of lizard Podarcis sicula (Riggio et al. 2003). Whereas a significant and progressive accumulation of mts mRNA was observed from pre-vitellogenic to vitellogenic oocytes and eggs, no significant accumulation of MTs proteins could be observed. Analogously, whereas SAGE analysis carried out on zebrafish ovaries revealed high levels of mt2 transcripts, the concomitant proteomic analysis failed to reveal significant amounts of the corresponding proteins (Knoll-Gellida et al. 2006). As already evoked, such mRNA accumulation could aim to provide sufficient materials needed for embryogenesis. Indeed, a number of studies indicate that translationally-inactive mRNAs are commonly present in oocytes and eggs. These maternal mRNAs are translated after fertilization to cope with the needs of embryogenesis (Spirin 1994; Vassalli and Stutz 1995; Stebbins-Boaz and Richter 1997). In the particular case of MTs, activation of mRNAs after fertilization could aim to cope with the needs of metalloproteins that store and donate essential metals, notably copper and zinc which are essential for embryogenesis (Kambe et al. 2008). Interestingly, and this, at the opposite to control eels, the significant increase in mts transcripts observed at the end of the experiment was effectively marked by a concomitant and significant increase in ovarian MTs protein content of Cd pre-contaminated eels. Since this increase in protein burden was associated with a massive entry of Cd in gonads of Cd pre-contaminated eels (see Pierron et al. 2008), it could be suggested that the significant metal accumulation observed in gonads at the end of the experiment have stimulated the translation of MTs mRNAs. In support of this assumption, the MTs protein content of both control and Cd pre-contaminated eels was found to be significantly correlated with the Cd content of gonads. Moreover, a similar pattern was observed in lizard oocytes. Indeed, as described above, whereas increasing mts gene transcription levels during oogenesis was not associated with an increase in MTs protein content, a single injection of Cd chloride in lizard female triggered an ovarian MTs protein synthesis (Riggio et al. 2003). Such findings suggest that Cd could activate the translation of normally stored and untranslated MTs mRNA. Alternatively, since the end of the experiment was marked by a concomitant and significant increase in both MTs protein content and cat gene transcription level in gonads of Cd pre-contaminated eels, an effect of oxidative stress on the translational activation of MTs mRNA cannot be ruled out. In our case, we have to note, however, that this increase in ovarian MTs protein content was insufficient to completely prevent the appearance of Cd-induced toxic events in gonads of Cd pre-contaminated eels, thus suggesting that a significant part of the accumulated Cd is associated with sensitive fractions of the cell such as mitochondria (Campbell et al. 2005).
Conclusion
This study tends to show how internal stores of Cd could be released during fish migrations at levels high enough to be toxic. In our experimental, Cd released was found to seriously disrupt development of sexual tissues of maturing female silver eels, leading to oocytes atresia. In addition to previous reported changes in pituitary hormonal gene transcription or on vitellogenesis, our present results tend to show that this Cd released could also impair ovarian mitochondrial metabolism and consequently, compromise full oocyte development. In addition, this work provides new insights in ovarian mts gene transcription and translation regulation during sexual maturation. Our results tend to show, that albeit normally weakly represented in eel’s ovaries, MTs protein synthesis can be induced by Cd. However, it must be underlined that an important limitation of our work relies not only on the use of hormonal treatment but also from the regime of Cd contamination. Our experimental exposure scenario was indeed very different from what wild eels experience during their growth in aquatic ecosystems. In other words, an acute contamination by dissolved Cd over one month cannot be, in term of organotropism or intracellular partitioning for example, strictly representative of a contamination that proceed over several years, at a lower pressure of contamination and by both the direct and the trophic route of exposure. However, the fact that we observed a significant remobilization and redistribution of Cd during the course of the experiment, and also in control individuals (see Pierron et al. 2008), reinforces the potential occurrence of phenomena that we observed under natural conditions.
References
Belyaeva EA, Dymkowska D, Wieckowski MR, Wojtczak L (2008) Mitochondria as an important target in heavy metal toxicity in rat hepatoma AS-30D cells. Toxicol Appl Pharmacol 231(1):34–42. doi:10.1016/j.taap.2008.03.017
Bordajandi LR, Gomez G, Fernandez MA, Abad E, Rivera J, Gonzalez MJ (2003) Study on PCBs, PCDD/Fs, organochlorine pesticides, heavy metals and arsenic content in freshwater fish species from the River Turia (Spain). Chemosphere 53(2):163–171. doi:10.1016/S0045-6535(03)00417-X
Brachet J, Schutter PD, Hubert E (1975) Studies on maturation in Xenopus laevis oocytes III Energy production and requirements for protein synthesis. Differentiation 3:3–14. doi:10.1111/j.1432-0436.1975.tb00840.x
Burzawa-Gérard E, Baloche S, Leloup-Hatley J, Le Menn F, Messaouri H, Nunez-Rodriguez J, Peyon P, Roger C (1994) Ovogenèse chez l’anguille (Anguilla anguilla L): ultastructure de l’ovaire à différents stades de développement et implication des lipoprotéines au cours de la vitellogenèse. Fr Peche Piscic 335:213–233. doi:10.1051/kmae:1994014
Campbell PGC, Giguère A, Bonneris E, Hare L (2005) Cadmium-handling strategies in two chronically exposed indigenous freshwater organisms–the yellow perch (Perca flavescens) and the floater mollusc (Pyganodon grandis). Aquat Toxicol 72(1–2):83–97. doi:10.1016/j.aquatox.2004.11.023
Cannino G, Ferruggia E, Luparello C, Rinaldi AM (2008) Effects of cadmium chloride on some mitochondria-related activity and gene expression of human MDA-MB231 breast tumor cells. J Inorg Biochem 102(8):1668–1676. doi:10.1016/j.jinorgbio.2008.04.002
Dufour S, Burzawa-Gérard E, Le Belle N, Sbaihi M, Vidal B (2003) Reproductive endocrinology of the European eel, Anguilla Anguilla. In: Aida K, Tsukamoto K, Yamauchi K (eds) Eel biology. Springer Verlag, Tokyo, pp 373–383
Durif C, Dufour S, Elie P (2006) Impact of silvering stage, age, body size and condition on reproductive potential of the European eel. Mar Ecol Prog Ser 327:171–181. doi:10.3354/meps327171
Durrieu G, Maury-Brachet R, Girardin M, Rochard E, Boudou A (2005) Contamination by heavy metals (Cd, Zn, Cu, Hg) of hight fish species in the Gironde estuary (France). Estuaries 28:581–591. doi:10.1007/BF02696069
Gonzalez P, Baudrimont M, Boudou A, Bourdineaud J-P (2006) Comparative effects of direct cadmium contamination on gene expression in gills, liver, skeletal muscle and brain of the zebra fish (Danio rerio). Biometals 19(3):225–235. doi:10.1007/s10534-005-5670-x
Habibi H, Lessman C (1986) A study of goldfish oocyte meiosis in vitro: effects of 2, 4-dinitrophenol and adenosine-5-triphosphate. Fish Physiol Biochem 1(4):197–205. doi:10.1007/BF02311136
Johnson MT, Freeman EA, Gardner DK, Hunt PA (2007) Oxidative metabolism of pyruvate is required for meiotic maturation of murine oocytes in vivo. Biol Reprod 77:2–8. doi:10.1095/biolreprod.106.059899
Kambe T, Weaver BP, Andrews GK (2008) The genetics of essential metal homeostasis during development. Genesis 46:214–228. doi:10.1002/dvg.20382
Kayaba T, Takeda N, Adachi S, Yamauchi K (2001) Ultrastructure of the oocytes of the Japanese eel Anguilla japonica during artificially induced sexual maturation. Fish Sci 67:870–879. doi:10.1046/j.1444-2906.2001.00335.x
Knoll-Gellida A, André M, Gattegno T, Forgue J, Admon A, Babin PJ (2006) Molecular phenotype of zebrafish ovarian follicle by serial analysis of gene expression and proteomic profiling, and comparison with the transcriptomes of other animals. BMC Genomics 7:46. doi:10.1186/1471-2164-7-46
Kwon HC, Choi SH, Kim YU, Son SO, Kwon JY (2005) Androgen action on hepatic vitellogenin synthesis in the eel, Anguilla japonica is suppressed by an androgen receptor antagonist. J Steroid Biochem Mol Biol 96:175–178. doi:10.1016/j.jsbmb.2005.02.010
Lokman P, Kazeto Y, Ijiri S, Young G, Miura T, Adachi S, Yamauchi K (2003) Ovarian mitochondrial cytochrome b mRNA levels increase with sexual maturity in freshwater eels (Anguilla spp). J Comp Physiol B. Biochem Syst Environ Physiol 173(1):11–19
Palstra AP, van Ginneken VJT, Murk AJ, van den Thillart GEEJM (2006) Are dionxin-like contaminants responsible for the eel (Anguilla anguilla) drama? Naturwissenschaften 93:145–148. doi:10.1007/s00114-005-0080-z
Peltier MR, Wilcox CJ, Sharp DC (1998) Technical note: application of the Box-Cox data transformation to animal science experiments. J Anim Sci 76(3):847–849
Pierron F, Baudrimont M, Bossy A, Bourdineaud J-P, Brèthes D, Elie P, Massabuau J-C (2007a) Impairment of lipid storage by cadmium in the European eel (Anguilla anguilla). Aquat Toxicol 81(3):304–311. doi:10.1016/j.aquatox.2006.12.014
Pierron F, Baudrimont M, Gonzalez P, Bourdineaud J-P, Elie P, Massabuau J-C (2007b) Common pattern of gene expression in response to hypoxia or cadmium in the gills of the European glass eel (Anguilla anguilla). Environ Sci Technol 41(8):3005–3011. doi:10.1021/es062415b
Pierron F, Baudrimont M, Dufour S, Elie P, Bossy A, Baloche S, Mesmer-Dudons N, Gonzalez P, Bourdineaud J-P, Massabuau J-C (2008) How cadmium could compromise the completion of the European eel’s reproductive migration. Environ Sci Technol 42(12):4607–4612. doi:10.1021/es703127c
Poliandri AHB, Machiavelli LI, Quinteros AF, Cabilla JP, Duvilanski BH (2006) Nitric oxide protects the mitochondria of anterior pituitary cells and prevents cadmium-induced cell death by reducing oxidative stress. Free Radic Biol Med 40(4):679–688. doi:10.1016/j.freeradbiomed.2005.09.021
Reynders H, van der Ven K, Moens LN, van Remortel P, De Coen WM, Blust R (2006) Patterns of gene expression in carp liver after exposure to a mixture of waterborne and dietary cadmium using a custom-made microarray. Aquat Toxicol 80(2):180–193. doi:10.1016/j.aquatox.2006.08.009
Riggio M, Trinchella F, Filosa S, Parisi E, Scudiero R (2003) Accumulation of zinc, copper, and metallothionein mRNA in lizard ovary proceeds without a concomitant increase in metallothionein content. Mol Reprod Dev 66:374–382. doi:10.1002/mrd.10365
Risso-de Faverney C, Orsini N, de Sousa G, Rahmani R (2004) Cadmium-induced apoptosis through the mitochondrial pathway in rainbow trout hepatocytes: involvement of oxidative stress. Aquat Toxicol 69(3):247–258. doi:10.1016/j.aquatox.2004.05.011
Robinet TT, Feunteun EE (2002) Sublethal effects of exposure to chemical compounds: a cause for the decline in atlantic eels? Ecotoxicology 11(4):265–277. doi:10.1023/A:1016352305382
Roche H, Buet A, Ramade F (2002) Relationships between persistent organic chemicals residues and biochemical constituents in fish from a protected area: the French National Nature Reserve of Camargue. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 133(3):393–410. doi:10.1016/S1532-0456(02)00122-9
Scandalios JG (2005) Oxidative stress: molecular perception and transduction of signals triggering antioxidant genes defenses. Braz J Med Biol Res 38:995–1014. doi:10.1590/S0100-879X2005000700003
Seydoux G (1996) Mechanisms of translational control in early development. Curr Opin Genet Dev 6:555–561
Sokolova IM (2004) Cadmium effects on mitochondrial function are enhanced by elevated temperatures in a marine poikilotherm, Crassostrea virginica Gmelin (Bivalvia: Ostreidae). J Exp Biol 207:2639–2648. doi:10.1242/jeb.01054
Spirin AS (1994) Storage of messenger RNA in eukaryotes: envelopment with protein, translational barrier at 5 side, or conformational masking by 3 side? Mol Reprod Dev 38(1):107–117. doi:10.1002/mrd.1080380117
Stebbins-Boaz B, Richter JD (1997) Translational control during early development. Crit Rev Eukaryot Gene Expr 7(1–2):321–333
Stone R (2003) Freshwater eels are slip-sliding away. Science 302:221–222. doi:10.1126/science.302.5643.221
Takaki A, Jimi S, Segawa M, Hisano S, Takebayashi S, Iwasaki H (2004) Long-term cadmium exposure accelerates age-related mitochondrial changes in renal epithelial cells. Toxicology 203(1–3):145–154. doi:10.1016/j.tox.2004.06.005
Tesch F-W (2003) The eel Blackwell Publishing fifth edition: 408 p
van Ginneken VJT, Maes GE (2005) The European eel (Anguilla anguilla, Linnaeus), its lifecycle, evolution and reproduction: a literature review. Rev Fish Biol Fish 15:367–398. doi:10.1007/s11160-006-0005-8
Vassalli JD, Stutz A (1995) Translational control awakening dormant mRNAs. Curr Biol 5(5):476–479. doi:10.1016/S0960-9822(95)00095-9
Vidal B, Pasqualini C, Le Belle N, Holland MCH, Sbaihi M, Vernier P, Zohar Y, Dufour S (2004) Dopamine inhibits luteinizing hormone synthesis and release in the juvenile European eel: a neuroendocrine lock for the onset of puberty. Biol Reprod 71:1491–1500. doi:10.1095/biolreprod.104.030627
Wang Y, Fang J, Leonard SS, Krishna R, Murali K (2004) Cadmium inhibits the electron transfer chain and induces reactive oxygen species. Free Radic Biol Med 36(11):1434–1443. doi:10.1016/j.freeradbiomed.2004.03.010
Wycherley G, Kane MT, Hynes AC (2005) Oxidative phosphorylation and the tricarboxylic acid cycle are essential for normal development of mouse ovarian follicles. Hum Reprod 20(10):2757–2763. doi:10.1093/humrep/dei132
Acknowledgments
We wish to thank Bruno Etcheberria and Henry Bouillard for their help and technical assistance in all aspects of this study. Fabien Pierron was supported by a grant from the French Ministry of Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pierron, F., Baudrimont, M., Dufour, S. et al. Ovarian gene transcription and effect of cadmium pre-exposure during artificial sexual maturation of the European eel (Anguilla anguilla). Biometals 22, 985–994 (2009). https://doi.org/10.1007/s10534-009-9250-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-009-9250-3