Abstract
Freshwater estuaries may be important control points but have received limited research attention, emblematic of a general under-appreciation of these ecosystems and the services they provide. These ecotone environments exist at the interface of rivers flowing into large lakes, where seiches cause mixing of lotic and lentic waters within flooded river deltas. We assessed the dissolved inorganic nitrogen (DIN) retention and processing controls in the Saint Louis River Estuary (SLRE), which receives inputs from rivers, urban sources, and Lake Superior. Nitrate (NO3–N) was the dominant form of DIN and was consistently highest in the lower estuary due to seiche-delivered Lake Superior water and nitrification of ammonium from urban sources. The estuary transitioned from a net NO3–N source during high flows to a net sink during summer baseflow conditions. NO3–N availability controlled site-specific denitrification rates while sediment organic matter explained the spatial pattern in denitrification potential. As the estuary shifted from a riverine state to one with more lake influence, seiches delivered Lake Superior NO3–N to the lower portion of the estuary, alleviating the final denitrification control and activating the estuary’s ‘denitrification pump’. This amplified removal condition is maintained by critically delivered NO3–N during periods of warm temperatures and long residence times. Often these controls are unsynchronized in streams where NO3–N is typically lowest during summer baseflow. Similar ephemeral biogeochemical processes are likely found within other seiche-prone lakes where organic-rich sediments accumulate at river mouths and are supplied with chemically distinct lake water during low flow periods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Humans have greatly increased the global circulation of dissolved inorganic nitrogen (DIN) (Galloway et al. 2004; Howarth et al. 2012), resulting in diverse environmental consequences that include species loss, climate modification, and coastal and freshwater eutrophication (reviewed by Erisman et al. 2013). Although phosphorus (P) is often identified as the main nutrient limiting primary production in lakes (Carpenter 2008; Schindler et al. 2008), nitrogen (N) is limiting or co-limiting in many freshwater ecosystems (Elser et al. 2007; Lewis et al. 2011), and management for P alone may lead to elevated DIN through decreased rates of microbial denitrification (Finlay et al. 2013). Thus, understanding the controls on DIN processing and identifying aquatic ecosystems with high removal capacity are crucial for mitigating human-driven increases in DIN circulation.
Estuaries are important control points between continents and coastal ocean zones that can remove substantial fractions of the total incoming N (e.g., Nixon et al. 1996; Smyth et al. 2013). Estuarine ecosystems support numerous N transformation pathways (e.g., nitrification, uptake, fixation), resulting in divergence between inputs and exports. While several transformation pathways ultimately consume DIN, removal is mainly attributed to sediment denitrification because it produces nitrogen gas (N2) that most organisms cannot use. In general, greater areal denitrification rates occur in aquatic ecosystems with high nitrate (NO3–N) and organic matter supply and long water residence times (Seitzinger et al. 2006); all of which often occur in estuarine environments. Estuaries can remove large amounts of incoming NO3–N via denitrification (Smyth et al. 2013), especially if they receive inputs from wastewater treatment plants (Bonaglia et al. 2014) or other anthropogenic sources (Fulweiler and Heiss 2014); thus potentially reducing coastal eutrophication risk (Garnier et al. 2006). Estuaries are in a unique position to modify DIN exports from upstream lotic systems and under the right hydrologic conditions DIN from downstream water bodies (e.g., Wankel et al. 2009).
Biogeochemical research on freshwater estuaries lags behind their marine analogs. Freshwater estuaries exist at the intersection of rivers and large lakes and are numerous along the margins of the Laurentian Great Lakes (Larson et al. 2012; Sierszen et al. 2012). Compared to many other large lakes (e.g., Baikal, African rift lakes), the Great Lakes basin has low topographic relief, permitting the expanse of estuarine environments. Most research on freshwater estuaries has therefore focused on the Great Lakes, although estuarine conditions are likely to occur in more lakes globally (e.g., see Einarsson and Lowe 1968; Gardner et al. 2006; Kabeya et al. 2008).
In large lakes, internal seiches cause periodic water level fluctuations (Mortimer and Fee 1976) that can drive lake water upstream (Morrice et al. 2011). The mixing of river and lake waters outside of the lake basin proper creates the distinctive freshwater estuarine environment. Even though water level fluctuations in freshwater estuaries are typically an order of magnitude less than in marine systems (Trebitz 2006), seiches act similarly to ocean tides in delivering chemically and physically distinct lake water to upstream habitats and influencing the exchange of carbon (Bouchard 2007) and N (Morrice et al. 2004) between lotic and lentic environments. Thus, similar to their marine counterparts, processes such as DIN retention may be amplified in these mixing zones. On the other hand, the smaller magnitude of water exchange may reduce the influence of freshwater estuaries on nutrient inputs. The relatively few studies assessing nutrient removal in freshwater estuaries suggest these systems can reduce DIN outflowing into downstream lakes (Tomazek et al. 1997; Morrice et al. 2004; McCarthy et al. 2007); however, questions remain regarding the timing, magnitude, and biogeochemical processes responsible (Sierszen et al. 2012).
This study was motivated by the general question- Do freshwater estuaries retain DIN, similar to many marine estuaries? To this end, we examined the potential of the Saint Louis River Estuary (SLRE) to modulate DIN inputs into Lake Superior, where NO3–N concentrations have been increasing over the past century (Sterner et al. 2007). We investigated: (1) the spatial and temporal patterns of DIN in the SLRE and its role as a source, sink, or transporter of DIN, (2) the spatial patterns of nitrification and denitrification in the SLRE, and (3) the environmental factors regulating denitrification in this system.
Methods
Site description
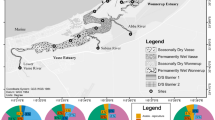
The Saint Louis River Estuary (SLRE) is located at the western tip of Lake Superior (46.74°N, 92.13°W) (Fig. 1). The estuary was formed as post-glaciation isostatic rebound caused Lake Superior water levels to rise, flooding the final 35 km of the Saint Louis River (Herdendorf 1990). The Saint Louis River drains 9868 km2 of northern Minnesota and Wisconsin and is the second largest tributary to Lake Superior. River flows are characteristically brown in color due to allochthonous inputs of organic carbon from the forest- and wetland-dominated watershed (Stephens and Minor 2010) and starkly contrast the clear waters of Lake Superior. The water level of the entire estuary fluctuates with Lake Superior as lake seiches propagate throughout the ~50 km2 SLRE (Loken 2014). Lake Superior has multiple seiche modes, but the 7.9 h seiche has the greatest relative amplitude that can raise water levels 5–30 cm (Mortimer and Fee 1976; Sorensen et al. 2004). Two outlets connect the SLRE to Lake Superior (Fig. 1), which allow water to circulate, thus increasing the opportunity for lake mixing compared to estuaries connected by a single channel. Nearshore Lake Superior currents typically flow counterclockwise (Beletsky et al. 1999) making the northeastern entry (Duluth entry) more prone to lake infiltration, and the southwestern entry (Superior entry) the main estuary outlet.
Saint Louis River Estuary sampling stations. Numbered stations were sampled for spatiotemporal water chemistry and sediments. Stars indicate sampling stations used for end member mixing model. ‘Water chemistry only’ stations were sampled twice during each year to assess the mixing model; ‘sediment only’ stations were sampled once for organic matter content and denitrification analysis. Station 1 is the most upstream station. Dashed line between stations 5 and 6 indicates the boundary between lower and upper estuary for comparative analyses and the approximate extent of Lake Superior infiltration detected during 2 year study
The majority of the SLRE is encircled by the cities of Duluth, MN and Superior, WI and has endured extensive anthropogenic impact. Roughly 60 % of the historic estuary has either been dredged or filled (Saint Louis River Alliance 2013) with more alteration occurring in the lower half of the estuary where much of the shoreline is hardened and a dredge channel is maintained to support commercial shipping activity. Additionally, the lower estuary receives inputs from impervious surface drainages and treated wastewater from regional municipalities. In contrast, the upper estuary retains a scenic river character with frequent backwater wetlands and is partially managed by environmental organizations. The shoreline supports dense macrophyte communities due to the consistent water levels and shallow bathymetry. The transition between the developed lower estuary and natural upper estuary is quite striking and occurs ~10 km from the Duluth entry into Lake Superior (Fig. 1).
Hydrology
Using discharge data from the USGS stream gauge (USGS site 04024000), we approximated the mean water residence time (WRT) of water traveling through the SLRE. The Scanlon gauge is 28 km upstream from Saint Louis River entry into the SLRE and not affected by seiches. Estuary volume was calculated assuming a mean estuary depth of 2 m (56 % of the estuary is <2 m depth; Bellinger et al. 2014) and a total area of 50 km2. We estimated WRT by dividing the approximate volume of the SLRE by Saint Louis River discharge, assuming no additional inputs to the system. Although other rivers flow into the SLRE, we omit them from our hydrologic estimates because of their limited influence on the estuary’s water budget. The Saint Louis River drains 85 % of the estuary catchment; other sources include the Nemadji River (11 %) and several small tributaries (<4 %) (http://nhd.usgs.gov). Discharge from the Nemadji River enters the SLRE directly across from the southeastern channel to Lake Superior (Fig. 1) and predominantly flows unimpededly into the lake. While our estimates of WRT may be prone to error because of additional water inputs, they nonetheless provide a useful relative comparison of hydrologic conditions during baseflow and high flow periods.
For a more detailed assessment of water movements within the estuary, we estimated the amount of water derived from river, lake, and urban sources for 23 sites during high and baseflow conditions. During 4 of our sampling surveys (two each year), water chemistry was determined at our core sampling stations (described below), an additional 15 in-estuary stations, and 3 end member sites (Table 1; water chemistry methods described in following sub-section). End member stations included the Saint Louis River (river), Lake Superior (lake), and near (within 100 m) the effluent discharge site of the Western Lake Superior Sanitary District (WLSSD) wastewater treatment plant (urban) (Fig. 1). Nearshore lake water was collected off the northeast side of the Duluth entry channel jetty to avoid sampling estuary discharge as lake currents typically flow from the north. End member sources had distinct chemical compositions, which we used to generate mixing models that estimated the relative contribution of river, lake, and urban water to each estuary station. For each spatially intensive sampling event (four total), we fit two mixing models using the concentrations of two conservative solutes to solve for the proportion of river (RW i ), lake (LW i ), and urban (UW i ) water at each station i according to Eqs. 1–3:
where A R , A L , A U , and A i are the measured concentrations of solute A for river, lake, urban, and station i respectively; and B R , B L , B U , and B i are the measured concentrations of solute B for river, lake, urban, and station i respectively. For each model, we used different combinations of conservative solutes to assign a contribution from river, lake and urban sources to each estuary station. In total, we estimated the contribution of the three end members to 23 in-estuary stations on four dates.
For each sampling event, the mixing model was first fit using magnesium (Mg) and sodium (Na); the second was fit separately using dissolved organic carbon (DOC) and Na. The source water partitions of the two model outputs were averaged and the total contribution of the three sources was forced to sum to 100 %. We used Na to assess the contribution of urban inputs, as it is often elevated in urban influenced aquatic ecosystems (Verbanck et al. 1989; Fitzpatrick et al. 2007). Both DOC and Mg varied greatly between the Saint Louis River and Lake Superior sources (see Results) and were used to partition water between river and lake inputs. Mg is expected to behave conservatively (Hill 1993) and has been previously used to assess river–lake mixing in the SLRE (Hoffman et al. 2010). While DOC is a less conventional conservative solute, it has been used in another Lake Superior freshwater estuary (Trebitz et al. 2002). Additionally, we observed a low breakdown rate (1.3 μg C day−1) (Loken 2014) and minimal water column primary production (0.1–0.9 mg O2 L−1 day−1) (Small, unpublished data) and felt confident using DOC as an indicator of river–lake mixing within the water residence time of the SLRE. Further, rather than using a single solute (i.e., Mg or DOC), we combined these model outputs to generate a more robust prediction in the event of non-conservative mixing of a single solute and to buffer each solute’s influence on partitioning sources.
Water chemistry
We collected water samples from 9 estuary stations to represent a gradient from river to lake on 13 dates between April 2012 and September 2013 (i.e., core stations; Fig. 1). Stations 1–5 represented upper estuary sites, while stations 6–9 were lower. Stations were situated near the thalweg, but were shifted laterally to avoid traffic within the shipping channel. Sampling occurred approximately monthly during the open water season when sites were accessed by boat, and once during winter ice cover when a subset of sites were visited on foot. To confirm that the water column was not stratified, we obtained a vertical profile of temperature, conductivity, and dissolved oxygen using a YSI 6600 or EXO2 multi-parameter sonde (Yellow Spring, OH).
Surface water from each station was collected into an HDPE carboy and processed in the lab within 10 h of collection. We processed samples in the lab (instead of on the boat) to expedite sample collection so that all stations could be visited within a single day (or within 2 days for spatial intensive surveys—see above). Integrated water samples were taken from 0 to 2 m using a peristaltic pump or an integrated water sampler and stored in a cooler to maintain ambient temperature. Samples for dissolved solute analysis were filtered through a 0.45 μm Geotech® capsule filter.
Cation samples were filtered into acid-washed HDPE bottles, acidified to 1 % with ultrapure hydrochloric acid (HCl), and stored at room temperature until analysis. Cations were analyzed simultaneously on a Perkin-Elmer model 4300 DV ICP spectrometer at the North Temperate Lakes Long-term Ecological Research (NTL-LTER; http://lter.limnology.wisc.edu) facility. A single DIN sample from each station was filtered into new 20 mL plastic scintillation vials and frozen, then analyzed within 4 months. Nitrate plus nitrite nitrogen (referred to as NO3–N) and ammonium plus ammonia nitrogen (referred to as NH4–N) were analyzed in parallel by automated colorimetric spectrophotometry, using an Astoria-Pacific Astoria II segmented flow autoanalyzer (http://lter.limnology.wisc.edu). Duplicate DOC samples were filtered into acid-washed 24 mL glass vials, acidified to 0.1 % HCL, capped with septa leaving no headspace, and stored at 4 °C. Within 3 weeks, DOC was analyzed on a Shimadzu TOC analyzer and reported as means. Samples for natural abundance dual isotopic analysis of δ18O–NO3 and δ15N–NO3 were filtered into acid-washed HDPE bottles and frozen. Isotope samples were analyzed using the denitrifier method at the Colorado Plateau Stable Isotope Analytical Laboratory at Northern Arizona University (http://www.isotope.nau.edu). Isotopes of δ18O–NO3 and δ15N–NO3 were reported as the per mil (‰) deviation from VSMOW and air standards, respectively. Natural abundance isotopic composition was only assessed on samples collected on 30 July 2012.
Reactive N dynamics
The capacity of the estuary to modify DIN was considered at the whole-system scale and by investigating rates and controls of specific N transformations (nitrification and denitrification). First, the potential for whole-system retention or generation of DIN was assessed using the end member mixing model described earlier. Predicted concentrations of calcium (Ca), potassium (K), NO3–N, and NH4–N were generated for each station using Eq. 2 and the predicted end member contributions (see above). Ca and K, which generally behave conservatively (Hill 1993; Fitzpatrick et al. 2007) and were distinct among river, lake, and urban sources (see Loken 2014), were used to test mixing model performance based on root mean squared error (RMSE). Deviations between model-based predictions and observations were used to infer the estuary’s net effect (production or consumption) for non-conservative solutes, namely NH4–N and NO3–N. The mixing model predicted DIN at each station based on conservative mixing of sources; predictions greater than observed values indicated net consumption of that solute. Conversely, predictions less than observations implied net production. The predicted estuary net effect on dissolved solutes incorporated both transformations within the water column and solutes exchanged with the sediments. Similarly, measured δ15N–NO3 was compared to predicted values to identify NO3–N transformations along the estuary’s longitudinal axis. Because isotopic fractions do not mix conservatively (e.g., Middelburg and Nieuwenhuize 2001), we weighted the δ15N–NO3 contribution of each end member by its NO3–N concentration to predict the isotopic composition for each station. While isotope analysis was only done for 2012 baseflow samples, DIN solutes were modeled for a high- and low-flow date in both years to identify changes in the estuary’s DIN retention capacity under contrasting WRT conditions.
Water column nitrification rates were determined on 30 July 2013 for a subset of the water chemistry sampling stations (n = 12) that represented the full spatial extent and previously observed NH4–N range of the estuary. Water from each station was transferred to 333 mL polycarbonate bottles within 10 h of collection and spiked with 15NH4Cl to achieve a concentration of 0.03 µmol 15NH4 L−1. Samples were incubated at ambient temperature (20 °C) in a dark cooler for 20 h. Pre- and post-incubation samples were filtered through 0.45 µm filters and analyzed for NO3–N, NH4–N and δ15N–NO3. Nitrification rates were determined based on changes in NO3–N, NH4–N, and δ15N–NO3 according to methods outlined in Small et al. (2013). Analysis for each station was performed in duplicate and reported as the mean.
Sediments were collected on 8 of the water chemistry survey dates from stations 2–9 to determine spatial and temporal patterns of denitrification and sediment organic content. We also collected a single sediment sample from additional lower (n = 17) and upper (n = 6) stations on 19 June 2012 and 24 June 2013, respectively, to increase the spatial extent of our survey. In total, 56 and 42 individual sediment collections were made in 2012 and 2013, respectively. Sediments were collected from the upper 5–20 cm of the benthic zone using an Ekman dredge. At least 500 mL of benthic material was transferred to 1-L widemouth Nalgene containers and used in denitrification rate experiments (see below). Fifteen mL of the uppermost sediment layer was transferred into sterile 100 mL disposable plastic screw-top containers to be analyzed for sediment organic content. Sediments were stored in a cooler while on the boat and transferred to 4 °C within 6 h. Sediment organic matter was determined as percent loss-on-ignition (LOI), i.e., mass loss of 2.0 ± 0.2 g dried homogenized sediment after combustion (Meyers and Teranes 2001). LOI sediments were dried at 60 °C for at least 48 h, homogenized, and combusted at 550 °C for 4 h.
We determined actual (DeN) and potential (DEA) sediment denitrification rates in the laboratory using the acetylene block technique modified from Groffman et al. (1999). Acetylene diminishes the microbial efficiency of the final denitrification step (conversion of nitrous oxide (N2O) to N2), and the production of N2O is used as an indicator of denitrification. Acetylene also inhibits nitrification, making our estimates under represent in situ denitrification as they do not account for activity coupled to nitrification (Seitzinger et al. 1993). Although this method has some limitations, it provides a cost-effective approach for comparing among multiple sites and identifying controls. Additionally, the acetylene block method dominates the denitrification literature, allowing us to compare SLRE rates to other published results (Groffman et al. 2006).
We determined DeN and DEA rates in parallel within 48 h of collection. We incubated 40 ± 2 g of wet sediment saturated with 40 ± 0.1 mL of estuary water in 125 mL glass Wheaton® bottles at 20 °C. DEA incubations were spiked with glucose and NO3-N to a final concentration of 40 mg C L−1 and 100 mg N L−1, respectively; DeN incubations were given no amendments. All incubations were augmented with 10 mg L−1 chloramphenicol to inhibit microbial proliferation (Smith and Tiedje 1979). Samples were capped with rubber septa, flushed with helium (He) for 5 min to remove oxygen (O2), and injected with 10 mL acetylene. We allowed the acetylene 30 min to fully diffuse into the sediment slurry before taking the initial headspace sample (t 0). Samples were placed on a shaker table in the dark for 2.6 h then sampled the final headspace (t 1). The change in headspace N2O partial pressures (pN2O final − pN2O initial ) was used to determine the denitrification rate using the Bunsen correction and the ideal gas law according to Eq. 4:
where n = 2 (mol N reduced per mol N2O produced), V g is the volume of headspace, V l is the total liquid volume (media + soil wet volume), α is the temperature-dependent Bunsen coefficient (0.554), dw is the sediment dry weight, t is the incubation time, R is the ideal gas constant and T is the temperature of the reaction. For both t 0 and t 1 samples, 10 mL of headspace was withdrawn from incubation bottles and injected into a He-flushed 12 mL gas-tight glass vials (Exetainers®) sealed with rubber septa. We determined pN2O and pO2 in parallel on a gas chromatograph equipped with an electron capture detector (ECD) and thermal conductivity detector (TCD) using methods outlined in Spokas et al. (2005). Gas samples with O2 concentrations greater than 5 % were removed from analysis due to potential gas leakage. Denitrification rates were standardized to sediment dry mass. Samples collected on or before 6 June 2013 were incubated in triplicate; samples collected after were incubated in duplicate. To assess the spatial and temporal patterns of DeN and DEA, we used a two-way ANOVA with station and date as factors. We assessed relationships between denitrification and ambient water column NO3–N and sediment LOI using linear regressions after log transforming DeN, DEA, and LOI to meet normality assumptions.
Denitrification controls were further investigated by amending sediments with combinations of NO3–N and two types of organic carbon: glucose and natural organic matter (NOM; supplied by the International Humic Substance Society). On two dates in 2013, we incubated sediments from five of our core stations that spanned a gradient of sediment organic content with the following amendments: NO3–N only, NO3–N and glucose (DEA), NO3–N and NOM, glucose only, NOM only, and no amendments (DeN). The two carbon treatments were intended to test for possible effects of carbon quality, with NOM representing a recalcitrant, humic-rich carbon source similar to allochthonous materials in the SLRE to contrast the labile glucose treatment. Both carbon sources were amended to 40 mg C L−1, and NO3–N was amended to 100 mg N L−1. Sediments were incubated in parallel (see above). To assess the effects of amendments and sediment organic content (and any interaction) on denitrification, we generated a multiple linear regression model that included carbon (3 levels) and NO3–N (2 levels) as categorical variables along with LOI (continuous) and all interaction terms.
Results
Hydrology and conservative solutes
Flow in the Saint Louis River had a seasonal pattern characterized by high discharge in April and May associated with spring snow melt (Fig. 2a) that declined to baseflow by mid-July and persisted through October. In both years, precipitation in June caused large floods, with the 2012 event being the maximum discharge on record for the 104 years of flow monitoring at the Scanlon station (Czuba et al. 2012). During high flow conditions, WRT estimates indicated that the estuary turned over approximately every 1–5 days. During summer baseflow, estimated WRT was between 40 and 80 days (Fig. 2a).
Saint Louis River hydrology and SLRE water chemistry. Saint Louis River (USGS gauge 04024000) discharge (black) and estimated mean water residence time (WRT; orange) (a). Estuary concentrations of dissolved inorganic nitrogen (b, e) and conservative solutes (c, d, f). Upper estuary stations (1–5) are grey, hollow symbols; lower stations (6–9) are colored and filled. Station 1 reflects Saint Louis River conditions and the blue horizontal dashed line indicates mean Lake Superior concentration (n = 6). Mean Lake Superior NH4–N concentration was 26 µg L−1 (not plotted for clarity). Ice covered the estuary from Nov 2012 until May 2013. One sampling event (28 Feb 2013) occurred during ice cover and only 5 stations were sampled. Black arrows indicate dates of mixing model analysis under high flow (HF) and baseflow (BF) conditions. (Color figure online)
End member mixing modeling results highlighted differences in water sources among stations and between high and low flow sample dates (Fig. 3). Mixing models using Mg and DOC as tracers of river water performed similarly. The mean standard error between model assignments of end member contributions was 1.9 % (range 0.0–5.3 %). As expected, at all stations the majority of estuary water was river-derived during high flow dates (1 May 2012 and 4 June 2013), and at upper estuary stations on all dates. Urban and lake sources were more prominent during the summer baseflow dates (30 July 2012 and 2013). The urban signal was particularly apparent at station 8, while the lake contribution declined with distance from Lake Superior.
Partitions of end member sources to in-estuary stations during high (a) and baseflow (b) conditions. Water partitions were calculated from end member models and averaged over both high flow and baseflow sampling dates. Station 9 is located closest to Lake Superior; station 8 is near the wastewater treatment plant
Water chemistry was relatively uniform throughout the estuary during periods of high flow (April–June; Fig. 2), then began to diverge among sites, especially in the lower estuary and during late summer of both years. Although variable throughout the year, Mg and DOC were generally greatest in the upper estuary and always lower in the lake than in the estuary (Fig. 2). In both years, the largest spatial gradient in estuary Mg and DOC occurred in September, indicating more lake water delivery after prolonged baseflow conditions with stations closest to Lake Superior having the most ‘lake-like’ signal (Fig. 2). Na concentrations were also consistently higher in the estuary than the lake, and showed distinct patterns at station 8, which also had the largest urban signal (Fig. 3). No vertical stratification was observed for any dates at any stations.
Reactive nitrogen dynamics
On most dates and sites, NO3–N was the dominant form of DIN in the SLRE, accounting for 80 % (SD = 23) of the DIN pool. Throughout the estuary, NO3–N concentrations were elevated during winter and spring snowmelt and after large rain events (Fig. 2b). At these times, concentrations approached and occasionally exceeded the nearshore Lake Superior average (299 µg N L−1, SD = 25, n = 6). No spatial differences were apparent among upper stations, while lower sites had higher and more variable NO3–N during baseflow dates in both years. On the last sampling date of each year, there was a spatial gradient in NO3–N (similar to Mg and DOC), with higher concentrations occurring closer to Lake Superior. NH4–N ranged between 0 and 736 µg N L−1, with greatest concentrations routinely occurring at station 8 on dates when Na was also high (Fig. 2). Station 9 was also enriched in NH4–N compared to the upper estuary, but was not as high as station 8. Lastly, one three occasions, a single upper estuary site (station 3 or 4) had an elevated NH4–N concentration.
Predicted concentrations from the three-way mixing model were strongly correlated with observations for Ca (r = 0.90) and K (r = 0.95) in the SLRE (Fig. 4). Low RMSE for Ca (1.48) and K (0.15) indicated reliable model performance and conservative solute behavior. In contrast, DIN did not behave conservatively (Fig. 4) based on poor model fits for both NO3–N (RMSE = 110.7) and NH4–N (RMSE = 317.5). After accounting for the major water sources to the estuary, our model implied that the SLRE was a net source of NO3–N during high flow conditions (Fig. 4). During baseflow, some stations remained net producers, but more changed roles and became net consumers of NO3–N. During high flow, conservative mixing alone underestimated NO3–N by an average of 95 μg NO3–N L−1. Conversely, in late July (i.e., baseflow), estuary-wide predictions were 9.5 μg NO3–N L−1 greater than observations, suggesting modest NO3–N consumption. During all sampling events, there was no clear spatial pattern for net generation or consumption of NO3–N. Predictions for NH4–N based on conservative mixing generally supported net consumption but were highly variable (Fig. 4d) due to occasionally high observations or predictions for stations near the wastewater treatment plant and station 4.
Mixing model predictions versus observed concentrations of calcium (a), potassium (b), nitrate (c), and ammonium (d). Predicted concentrations generated from end member mixing model for in-estuary stations during high flow conditions (open symbols) and baseflow (filled symbols) in 2012 (triangles) and 2013 (circles). Points falling near the 1:1 line behaved conservatively while observations above or below indicated net consumption or production, respectively. Outliers in ammonium (d) plot were from stations surrounding the wastewater treatment plant and proximate to station 4
Using dual isotopic analysis, we identified differences in NO3–N among river, lake and estuary water collected on 30 July 2012. Lake Superior δ18O–NO3 and δ15N–NO3 values were consistent with previous studies (Finlay et al. 2007). Compared to the lake, the Saint Louis River was similar in δ18O–NO3 (and therefore not useful to assess mixing or fractionating) and enriched in δ15N–NO3 (Fig. 5a). Lake Superior and Saint Louis River δ15N–NO3 bracketed those from estuary stations, with lower stations similar to Lake Superior, though slightly less enriched. Observed δ15N–NO3 was consistently lower than predictions from the mixing model, suggesting an influence of nitrification within the SLRE (Fig. 5b).
Nitrate isotopic analysis and nitrification during baseflow. Observed nitrate isotopic composition from 30 July 2012 (a). Deviations between model-based predictions and observations of δ15N–NO3 from 30 July 2012 (b). Water column nitrification rates from 30 July 2013 (c). Distance to lake calculated from station to closest entry point. Panels a and b reference samples collected in 2012; panel c from 2013
Water column nitrification rates in July 2013 were variable but higher in the lower estuary than at upper sites, consistent with the NO3–N isotope results from the previous year. Rates ranged from 0.10 to 0.46 µg N L−1 day−1 in the upper estuary and from 0.89 to 8.88 µg N L−1 day−1 in the lower (Fig. 5c). The highest rates occurred below station 7 (~7 km from Lake Superior) in the more urbanized portion of the estuary where DIN was often high (Fig. 2). The nitrification rate for nearshore Lake Superior was 0.07 µg N L−1 day−1 and was within ranges reported for offshore surface waters (Small et al. 2013).
Denitrification and sediment composition varied spatially but not temporally. Sediment DeN rates averaged 1.2 (SD = 1.2) and 2.4 (SD = 7.9) µg N g−1 day−1 in 2012 and 2013, respectively. No difference in DeN occurred among stations, but differences existed among dates in 2013 (Fig. 6; F = 12.23, df = 3, P < 0.001); however, no clear seasonal trend emerged. DEA was always greater than DeN, averaging 11.8 (SD = 9.8) and 11.5 (SD = 7.7) µg N g−1 day−1 in 2012 and 2013, respectively. Rates were more variable in 2012 than 2013 and were lower on the final two sampling dates of 2012. Comparing our core sampling stations, DEA was consistently highest closer to Lake Superior (Fig. 6) and significantly different among stations in 2012 and 2013 (F = 3.45, df = 7, P = 0.01 and F = 14.75, df = 7, P < 0.001, respectively). Sediment LOI was also greater at our lower estuary core stations and had similar spatial patterns as DEA (Fig. 7). But when we include our additional sediment samples (Table 1; Fig. 1), the spatial differences for both LOI and DEA disappear as both were highly variable across the entire estuary (Fig. 7).
Sediment denitrification rates with no amendments (DeN; a, b) and with nitrate and glucose amendments (DEA; c, d). Note the change in y-axis scale between DeN and DEA. Station 1 is furthest upstream, and station 9 is most proximate to Lake Superior. Denitrification rates are reported as means with error bars representing standard deviation among lab replicates. No sediments were analyzed from station 1 in 2013
Sediment loss-on-ignition (LOI; a) and potential denitrification (DEA; b) for core stations (grey) and all sites (white) visited in 2 year study (see Table 1). Lower estuary includes all stations within 10 km or Lake Superior
Relationships with potential drivers differed for DeN and DEA. Ambient NO3–N concentrations were weakly correlated with DeN (t = 1.89, df = 67, P = 0.06, R 2 = 0.05) but not with DEA (t = −1.33, df = 72, P = 0.19) (Fig. 8a). In contrast, sediment composition was strongly positively correlated with DEA (t = 17.6, df = 95, P < 0.001, R 2 = 0.76) but not DeN (t = 1.15, df = 73, P = 0.25) (Fig. 8b). Finally, amendment experiments revealed a positive response to addition of NO3–N (F = 398.4, df = 1, P < 0.001) but not organic carbon (Fig. 9). This lack of response to carbon held for both labile (glucose) and recalcitrant (NOM) treatments (F = 1.69, df = 2, P = 0.20). The regression model also indicated a positive synergistic effect between NO3–N and LOI (Fig. 9; F = 313.9, df = 1, P < 0.001), as sediments with high LOI had the greatest response to added NO3–N amendments. No other interactions were significant (P > 0.2).
Relationships between potential denitrification controls and measured rates; a water column nitrate (NO3–N) and b sediment loss-on-ignition (LOI) for all samples collected in 2012–2013. Note that denitrification and LOI are plotted on log axis. DeN (open symbols) incubations were given overlying estuary water with no amendments; DEA (closed symbols) were amended with glucose and NO3–N. Linear regression model for DeN and NO3–N (shown in dash) was weakly significant (t = 1.89, df = 67, P = 0.063, r 2 = 0.05) and model for DEA and LOI (solid line) was highly significant (t = 17.6, df = 95, P < 0.001, r 2 = 0.76). Models comparing DEA versus NO3–N and DeN versus LOI were not significant (P > 0.1)
Nitrate control of sediment denitrification rate. Sediment samples from a subset of core stations were incubated with combinations of nitrate and two carbon types- glucose and Suwannee River natural organic material (NOM) standard. Denitrification rates are reported as means ± SDs among lab replicates and ordered by loss-on-ignition (LOI). Nitrate, LOI, and a nitrate:LOI interaction had significant effects (P < 0.001), while carbon (nor any carbon interactions) had an effect (P > 0.2)
Discussion
Both hydrologic and biotic processes regulated temporal and spatial patterns in DIN chemistry of the SLRE. During the estuary’s river-like periods, NO3–N removal capacity appeared to be minimal as water moved quickly through the system, minimizing the opportunities for processing. As discharge declined, the mixing gradient between river and lake expanded, allowing more water column NO3–N to be removed. During this baseflow period, lake seiches pushed lake water furthest up the estuary, providing NO3–N-rich lake water to the lower estuary. Thus, there was an alignment of optimal denitrification conditions (sufficient NO3–N, organic-rich sediments, warm temperatures, and long WRT) during summer baseflow within the critical mixing zone of Lake Superior. Delivery of NO3–N via seiches enhanced denitrification through mechanisms not present in many river systems in which periods of high removal capacity are not usually synchronized with times of high NO3–N delivery.
Hydrology
The Saint Louis River regularly supplied the majority of the water to the SLRE, although more urban and lake water was detected within the lower estuary reach during baseflow conditions (Fig. 3). Beyond the mixing model results, the spatial gradient in Mg (and DOC) indicated that the contribution of Lake Superior to the estuary’s water budget was greatest in September (Fig. 2c). At this time, the Mg signal diverged at station 6, suggesting that lake water infiltrated ~10 km into the estuary. Likely, lake water intrusion continually expands up the estuary until ice formation or a sufficient precipitation event occurs.
Some constraints and assumptions of the mixing model approach must be recognized that may explain the small partitioning of lake water to upper stations (Fig. 3). Although both Mg (Morrice et al. 2004; Hoffman et al. 2010) and DOC (Trebitz et al. 2002) have been used to separate lake and river contributions to Lake Superior estuaries, both can develop minor spatial gradients without lake mixing. If river concentrations change rapidly, the river end member at the date of sampling does not accurately represent the river signal that has traveled through the estuary. Concentrations along the estuary gradient reflect the source water that entered the estuary at different times (as well as mixing of different source waters). Because river Mg concentrations generally increased during the study, our estimates of lake mixing based on Mg may be an overestimate. Similarly, DOC-based predictions may be imprecise, as this pool may change as a result of metabolism or photo-oxidation. However, another study in a freshwater estuary of Lake Superior used DOC to assess river and lake mixing (Trebitz et al. 2002), and we observed a low DOC breakdown rate and minimal water column gross primary production (Small, unpublished data). Additionally, Wollheim et al. (2015) suggest that terrestrially-derived DOC behaves somewhat conservatively in river systems. Therefore, we suggest DOC may be used as a conservative tracer in other systems with similar hydrologic conditions, organic matter sources, and metabolic processing. As with any conservative tracer, DOC and Mg have their limitations; but both provided a reasonable estimate of the estuary’s water budget, and mixing model results were well-validated with additional conservative solutes (Ca and K).
Urban sources contribute more water during baseflow. Our calculated water budget (Fig. 3) provides meaningful relative comparisons among stations, but each assigned contribution may overestimate urban sources. We inferred the ‘urban’ signal by collecting estuary water near the wastewater discharge location (i.e., not directly from the treatment plant). Thus our urban samples were diluted with estuary water, which made our mixing model assign more urban water to balance each station’s Na budget. Over the study period, flows from the WLSSD water treatment facility averaged 1.6 m3 s−1 and were relatively constant during the year (typically between 1 and 2 m3 s−1; WLSSD, unpublished data). When river flows were high, wastewater effluent contributed less than 1 % of the estuary’s water budget, in comparison to extreme baseflow when it approached 20 % of river discharge. As river flows declined in late summer, the relative contribution of urban water increased, especially around station 8, where water often contained extreme concentrations of Na and NH4–N (Fig. 2).
Due to large summer rains, our baseflow water budget (Fig. 3) underrepresents the extent of the estuary’s water chemistry spatial gradient. In both years large June rains postponed the onset of baseflow (Fig. 2a). Had our end member sampling surveys occurred later in the season (i.e., September) or in years with less spring precipitation, we would have expected the relative proportion of both urban and lake contributions to be larger. In September of both years, the largest spatial gradient in estuary Mg and DOC occurred (Fig. 2), indicating greater infiltration of lake water into the SLRE. Likely, lake water penetration into the SLRE is a function of the cumulative discharge over a preceding interval of time (i.e., weeks–months). With prolonged baseflow conditions, the relative contribution of river inputs will decrease, as other sources (urban, lake, etc.) contribute a larger fraction of the estuary’s water budget.
Water chemistry
Estuary DIN concentrations varied more than conservative solutes, suggesting active N cycling within the SLRE. Highest NO3–N concentrations occurred during spring melt and after large rain events, consistent with flushing observed in other temperate watersheds (e.g., Ohte et al. 2004; Pellerin et al. 2012) and another SLRE study (Johnston et al. 2001). During baseflow, NO3–N diverged among stations (Fig. 2b), with higher concentrations observed in the lower estuary attributable to delivery of lake-derived NO3–N to these sites. NH4–N concentrations were often greatest at stations in the lower estuary that also had elevated in Na (Fig. 2), suggesting inputs from urban sources. Likely, some of this urban-supplied NH4–N was delivered to the lake, as the mean concentration at our nearshore Lake Superior station was ~10X greater than reported values from offshore sites (Kumar et al. 2007). Water chemistry data from the Western Lake Superior Sanitary District (WLSSD) indicated that NH4–N was the dominant form of DIN (98 %) in effluent discharged from their treatment facility near station 8 during 2012–2013, which had NH4–N concentrations ~100X that of the estuary (mean = 3.92 mg N L−1, range = 0–9.62, n = 27) (WLSSD, unpublished data). Additionally, station 4 (and other nearby sampling stations) had elevated NH4–N on some dates. Possible sources include non-sampled point sources, sediments from historic industries (i.e., U.S. Steel superfund site), or nearby backwater wetlands, which have been shown to supply NH4–N in other lotic wetland systems (Stanley and Ward 1997). In most estuarine studies, sediments release NH4–N to the water column, attributable to mineralization of organic matter (Nixon 1981; Enoksson 1993; Foster and Fulweiler 2014). Upper estuary sediments outside the main channel contain more organic matter compared to the thalweg (Fig. 7a). Perhaps these organic-rich sediments contribute a small flux of NH4–N to surface waters, which can only be detected in the main channel under certain hydrologic conditions. However, NH4–N concentrations were relatively low for the entire estuary indicating that supplied NH4–N was rapidly mixed, assimilated, or nitrified.
Results from our mixing model that assess the estuary’s net effect on DIN (i.e., Fig. 4) should be interpreted cautiously. During high flow, the model indicated NO3–N production, which may be the result of a non-sampled high NO3–N tributary or due to changing concentrations in the river. Our model assumes that the river end member is constant and no other tributaries supply water, both of which are not true. While the effect of changing concentrations in the river can produce errors in both directions (i.e., production or consumption), the influence of tributaries will likely only increase production estimates as non-sampled sources that contribute high volumes of low concentration water are doubtful. Therefore predictions of overall consumption are more robust for baseflow than for high flow. While the model has its limitations, it produced similar trends in subsequent years, which suggest a seasonal transition in the estuary’s overall effect on NO3–N.
Our mixing model identified changes in the estuary’s net effect on NO3–N, but interpreting changes in NH4–N were problematic due to a few extreme observations and predictions (Fig. 4). For the most part, model predictions were greater than observations indicating overall NH4–N consumption within the estuary, likely due to nitrification or assimilatory processes. But given the variability of wastewater effluent, complex mixing dynamics, and the highly reactive nature of NH4–N (Mulholland et al. 2000; Peterson et al. 2001), these results are somewhat limited and not discussed further. Instead, we focus our assessment on NO3–N, given its majority contribution to the DIN pool.
While end member inputs were dynamic and there may have been additional sources that were not included in our calculations, the mixing model provides a reasonable assessment of the net-effect on NO3–N within the context of this study. In general, we found a transition in estuary-wide NO3–N dynamics, characterized by NO3–N release during high flow spring months and NO3–N retention in late summer when WRT and contributions from non-river sources were greatest. In addition to mixing model-based inferences, water chemistry from September further supports this transition as NO3–N concentrations were much lower than expected based on mixing (Fig. 2b). With declining discharge in late summer, more seiche-delivered and recently produced NO3–N is likely being removed as the estuary transitions to a more ‘lake-like’ system. Our observed seasonal pattern is consistent with another, but significantly smaller Lake Superior freshwater estuary in which maximum DIN retention also occurred during summer baseflow (Morrice et al. 2004). Notably, maximum retention coincided with long WRT and warmer temperatures, increasing the potential for biotic processes to act on NO3–N (Seitzinger et al. 2006) and during a period when inputs from the lake enriched lower estuary NO3–N.
Nitrification
Multiple lines of evidence point to the role of nitrification in shaping SLRE DIN dynamics. On the day that water samples were collected for isotopic analysis, upper estuary stations were comprised of 93–99 % river water (Fig. 3) and therefore should have converged with the river δ15N–NO3 in the absence of any transformations. But all estuary stations had lower δ15N–NO3 than model-based predictions (Fig. 5b), pointing to the influence of biologically mediated NO3–N production (Barnes and Raymond 2010) during baseflow. The greatest divergence in observed and model predictions occurred between stations 6 and 8, where measured water column nitrification rates were also highest (Fig. 5c), and where NH4–N inputs from urban sources were often elevated (Fig. 2e). Given the well-oxygenated water column (Bellinger et al. 2014; Loken 2014), NH4–N appears to be rapidly nitrified, explaining why NH4–N did not persist further downstream. Nitrification of urban NH4–N inputs would contribute to NO3–N enrichment in the lower estuary. However, the estuary acted as an overall NO3–N sink when we performed our isotopic analysis (Fig. 4), indicating that any enrichment effect was overridden by a non-fractionating NO3–N consumption process.
If nitrification were the dominant N transformation in the estuary, we would expect to observe longitudinal increases in NO3–N beyond those attributable to lake inputs. This pattern occurred during high flow dates. While mixing model results demonstrated that urban sources of water are less important during high flow periods, mineralization of benthic organic matter may provide an alternative source of NH4–N to the water column. Fringing wetlands dominate much of the upper estuary, which have been shown to release NH4–N following flow increases in other systems (Stanley and Ward 1997). If nitrification influence on DIN dynamics persists during high flow, it may contribute to the estuary-wide NO3–N source behavior. During low-flow periods, we observed high nitrification rates and estuary-wide loss of NO3–N, suggesting increased turnover of the NO3–N pool. Thus the relative role of nitrification on the NO3–N pool likely declines as the SLRE shifts to a net sink when residence times and temperatures are high.
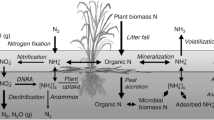
While our study focused on the sources and fate of water column DIN, we must also consider sediment processes that affect estuary-wide N dynamics. Mineralization of benthic organic matter produces NH4–N, which can contribute to DIN enrichment directly by diffusing into the water column or indirectly by fueling sediment nitrification. In general, sediment nitrification is the main source of NO3–N to oxic portions of the sediments, where it can be coupled to denitrification (Seitzinger et al. 2006). While coupled nitrification/denitrification likely removes a large portion of N from the SLRE, it should not alter water column DIN concentrations, as organic N is transformed to N2 gas entirely within the benthos. In another SLRE study, Bellinger et al. (2014) found a negative correlation between sediment nitrification and denitrification, indicating that the two processes may be temporally or spatially decoupled. Thus under some conditions, sediment nitrification could be a source of NO3–N to the water column. At other times, the denitrification demand for NO3–N exceeds what is produced in the sediments, resulting in a chemical gradient that draws NO3–N downward and removes it from the water column. Thus the estuary’s alternating source/sink NO3–N behavior may reflect changes in the net flux of DIN across the sediment water interface. We encourage direct measures of benthic N fluxes in future investigations, which might more accurately assess the estuary’s overall DIN retention capacity.
Sediment denitrification
SLRE sediments supported modest denitrification activity that lacked distinct temporal pattern (Fig. 6), falling within ranges reported for the estuary and one of its tributaries (Johnston et al. 2001; Bellinger et al. 2014). The lack of a temporal pattern probably resulted from methods used in our denitrification assay. All sediments were incubated at a common temperature, which is known to be a strong driver (Piña-Ochoa and Álvarez-Cobelas 2006). Our methods also limit our ability to accurately assess in situ denitrification activity as the acetylene block technique does not account for denitrification coupled to sediment nitrification (Seitzinger et al. 1993). While our rates do not represent the estuary’s actual activity, they can be conceived as a minimum in situ rate, allow for spatiotemporal comparison of the denitrifying potential, and be used to assess drivers of this process.
For sediment denitrification to alter water column NO3–N, NO3–N must first diffuse into sediments. Bellinger et al. (2014) suggested that NO3–N diffusion into the sediments sustained the SLRE denitrifying community because sediment nitrification did not generate sufficient NO3–N to balance denitrification rates. Diffusion (and subsequent denitrification) is a non-fractionating process and would allow nitrification to modify water column δ15N–NO3 without increasing overall NO3–N concentrations. While we did not observe a temporal trend in denitrification (Fig. 6), rates in aquatic ecosystems generally increase with temperature (Piña-Ochoa and Álvarez-Cobelas 2006) if limiting reactants (i.e., NO3–N and organic C) are available. We suggest that the importance of denitrification increases during baseflow, overriding NO3–N producing processes and shifts the estuary to an overall NO3–N sink.
Differences between DeN and DEA and amendment experiments revealed strong limits on this process. Denitrification rates responded strongly to added NO3–N but not C (Fig. 9), as has been reported for a variety of aquatic ecosystems (e.g., Forshay and Stanley 2005; Wall et al. 2005; Roach and Grimm 2011). The relationship between DeN and ambient NO3–N was weak though (Fig. 8a), signaling that water column nutrient availability poorly predicts actual denitrification rates. In theory, sediments with high rates would ultimately consume overlying NO3–N, thus weakening any relationship with water column concentration. Additionally, a portion of the SLRE’s in situ denitrification is coupled to sediment nitrification, which our methods do not capture and may explain the lack of correlation to overlaying NO3–N concentrations. An alternative control may explain the overall spatiotemporal pattern. The estuary gradually widens in proximity to Lake Superior, resulting in reduced water velocities and increased accumulation of fine material along the estuary’s main flow path. Our core stations were chosen near the thalweg and do not reflect the variability of sediment composition across the entire estuary (Fig. 7a). Differences in sediment organic matter at our core sampling stations likely caused the apparent spatial gradient in DEA observed in Fig. 6. Although carbon amendments did not increase denitrification rates, sediments with high organic matter content (i.e., LOI) had greater potential to remove NO3–N (Fig. 8) and were most responsive to added NO3–N (Fig. 9). These results suggest that while NO3–N may be the proximate controller of denitrification, sediment organic content acts as a larger-scale constraint on DIN-removal capacity across the estuary. Thus, redistribution or removal of sediments in this ecosystem by floods, restoration activities, or dredging to maintain shipping channels has the potential to alter the capacity and location of DIN removal in the SLRE and other freshwater estuaries.
Alternative NO3–N removal pathways may also shape DIN dynamics. We expect autotrophic uptake to be a minor pathway of NO3–N removal; water column gross primary production rates in the SLRE were 0.1–0.9 mg O2 L−1 day−1 (Small, unpublished data)- near the lowest reported for aquatic ecosystems (Hoellein et al. 2013) perhaps due to strong light limitation in the estuary’s highly colored waters (Small, unpublished data; Philips et al. 2000). Additional NO3–N reduction pathways, such as dissimilatory nitrate reduction to ammonium (DNRA) and anaerobic ammonium oxidation (anammox), may also play a role in reducing NO3–N loads (see reviews by Burgin and Hamilton 2007; Giblin et al. 2013). In some marine estuaries, DNRA and denitrification account for similar magnitudes of NO3–N losses (Koop-Jakobsen and Giblin 2010; Bonaglia et al. 2014). The SLRE has comparatively low concentrations of NO3–N, thus denitrifying microbes should not be oversaturated with NO3–N and should outcompete DNRA microbes (see Burgin and Hamilton 2007). Additionally, if DNRA capacity was high in the SLRE, the newly generated NH4–N was not transported downstream and thus did not affect overall DIN loading to Lake Superior. In the spirit of attributing NO3–N loss to other pathways, we performed a single set of anammox rate experiments. These limited results from only two stations on a single survey suggest 0–10 % of NO3–N loss could be attributed to anammox (Loken, unpublished data). Although additional NO3–N reduction pathways exist, we focus on denitrification because of its ability to remove NO3–N from the system, rather than transforming it to other forms of available N.
Conclusions
The answer to our initial question—Are freshwater estuaries control points for DIN retention, similar to marine estuaries?—appears to be: ‘sometimes’ for the SLRE. Denitrification is the likely mechanism for reducing DIN from river water traveling to the lake as well as DIN inputs from the lake. During high flow periods with short WRT, the DIN dynamics within the SLRE were qualitatively similar to a river, dominated by unidirectional flow and a limited opportunity to reduce water column DIN and according to our model produced NO3–N. Declining discharge shifted the system from a river-dominated state to one more similar to a marine estuary, characterized by prolonged WRT and mixing of waters derived from up- and down gradient (i.e., river and lake) sources. A clear point of distinction between the two phases is the alignment of favorable conditions for denitrification: warm temperatures, prolonged WRT, and critically, delivery of NO3–N-rich water from Lake Superior. This steady input of NO3–N due to seiche-driven flow reversals coupled with amenable conditions created a ‘denitrification pump’ in the lower estuary, shifting the whole system from a net source to a sink of DIN. This stands in contrast to many streams and rivers in which the largest NO3–N concentrations pass through the channel during high flow and cold months (e.g., Ohte et al. 2004; Pellerin et al. 2012) during non-optimal denitrification conditions. Subsequently, when rivers warm NO3–N concentrations decline, resulting in reduced actual denitrification (e.g., Pattinson et al. 1998) even though potentials are elevated due to the increased temperatures (Piña-Ochoa and Álvarez-Cobelas 2006). Similar disconnects occur in backwaters of the Mississippi River, where hydrologic NO3–N delivery is minimal during low-flow summer periods and results in low actual denitrification when potentials are highest (Richardson et al. 2004). Therefore during the SLRE’s optimal removal period, seiche-mediated delivery of NO3–N enhances the ‘denitrification pump’, ultimately overcoming estuary-produced NO3–N resulting in a switch to an overall DIN sink.
Among different freshwater estuaries, this DIN-removal capacity, and the control point behavior in general, is expected to be a function of the ratio of river flow to seiche volume coupled with the geomorphology of the estuary (Morrice et al. 2004). Given the smaller amplitude of seiches relative to ocean tides, upstream mixing by seiches should be more frequently cancelled out by river flows, making the river–lake mixing state a more ephemeral condition in freshwater estuaries. Nonetheless, these mixing zones are abundant throughout the Laurentian Great Lakes (Sierszen et al. 2012), and probably occur along edges of other seiche-prone lakes. The limited attention they have received to date is emblematic of a general under-appreciation of the ecosystems and the services they provide (Larson et al. 2012; Sierszen et al. 2012). DIN retention is likely to occur in freshwater estuaries (Morrice et al. 2004; McCarthy et al. 2007; Knuth and Kelly 2011), along with other ecosystem processes that have been reported for marine estuaries (e.g., see Bouchard 2007). Removal of DIN from aquatic ecosystems will become increasingly important with N saturation of terrestrial ecosystems and impacts of climate change (Baron et al. 2012). This study showcases the need to properly address the role of freshwater estuaries as crucial constriction points linking uplands with large lakes and mitigating human impacts to downstream aquatic ecosystems.
References
Barnes RT, Raymond PA (2010) Land-use controls on sources and processing of nitrate in small watersheds: insights from dual isotopic analysis. Ecol Appl 20:1961–1978
Baron JS, Hall EK, Nolan BT, Finlay JC, Bernhardt ES, Harrison JA, Chan F, Boyer EW (2012) The interactive effects of excess reactive nitrogen and climate change on aquatic ecosystems and water resources of the United States. Biogeochemistry 114:71–92
Beletsky D, Saylor JH, Schwab DJ (1999) Mean circulation in the Great Lakes. J Great Lakes Res 25:78–93
Bellinger BJ, Jicha TM, Lehto LP, Seifert-Monson LR, Bolgrien DW, Starry MA, Angradi TR, Pearson MS, Elonen C, Hill BH (2014) Sediment nitrification and denitrification in a Lake Superior estuary. J Great Lakes Res 40:392–403
Bonaglia S, Deutsch B, Bartoli M, Marchant HK, Brüchert V (2014) Seasonal oxygen, nitrogen and phosphorus in benthic cycling along an impacted Baltic Sea estuary: regulation and spatial patterns. Biogeochemistry 119:139–160
Bouchard V (2007) Export of organic matter from a coastal freshwater wetland to Lake Erie: an extension of the outwelling hypothesis. Aquat Ecol 41:1–17
Burgin AJ, Hamilton SK (2007) Have we overemphasized the role of denitrification in aquatic ecosystems? A review of nitrate removal pathways. Front Ecol Environ 5:89–96
Carpenter SR (2008) Phosphorus control is critical to mitigating eutrophication. Proc Natl Acad Sci 105:11039–11040
Czuba CR, Fallon JD, Kessler EW (2012) Floods of June 2012 in northeastern Minnesota: U.S. Geological Survey Scientific Investigations Report 2012–5283
Einarsson E, Lowe B (1968) Seiches and set-up on Lake Winnipeg. Limnol Oceanogr 13:257–271
Elser JJ, Bracken MES, Cleland EE, Gruner DS, Harpole WS, Hillebrand H, Ngai JT, Seabloom EW, Shurin JB, Smith JE (2007) Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Let 10:1135–1142
Enoksson V (1993) Nutrient recycling by coastal sediments: effects of added algal material. Mar Ecol Prog Ser 92:245–254
Erisman JW, Galloway JN, Seitzinger S, Bleeker A, Dise NB, Petrescu AMR, Leach AM, de Vries W (2013) Consequences of human modification of the global nitrogen cycle. Phil Trans R Soc B 368:20130116
Finlay JC, Sterner RW, Kumar S (2007) Isotopic evidence for in-lake production of accumulating nitrate in Lake Superior. Ecol Appl 17:2323–2332
Finlay JC, Small GE, Sterner RW (2013) Human influences on nitrogen removal in lakes. Science 342:247–250
Fitzpatrick ML, Long DT, Pijanowski BC (2007) Exploring the effects of urban and agricultural land use on surface water chemistry, across a regional watershed, using multivariate statistics. Appl Geochem 22:1825–1840
Forshay KJ, Stanley EH (2005) Rapid nitrate loss and denitrification in a temperate river floodplain. Biogeochemistry 75:43–64
Foster SQ, Fulweiler RW (2014) Spatial and historic variability of benthic nitrogen cycling in an anthropogenically impacted estuary. Front Mar Sci 1:1–16
Fulweiler RW, Heiss EM (2014) A decade of directly measured sediment N2 fluxes: what can Narragansett Bay tell us about the global ocean nitrogen budget? Oceanography 27:184–195
Galloway JN, Dentener FJ, Capone DG, Boyer EW, Howarth RW, Seitzinger SP, Asner GP, Cleveland CC, Green PA, Holland EA, Karl DM, Michaels AF, Porter JH, Townsend AR, Vorosmarty CJ (2004) Nitrogen cycles: past, present, and future. Biogeochemistry 70:153–226
Gardner JT, English M, Prowse TD (2006) Wind-forced seiche events on Great Slave Lake: hydrologic implications for the Slave River Delta, NWT, Canada. Hydrol Process 20:4051–4072
Garnier J, Cébron A, Tallec G, Billen G, Sebilo M, Martinez A (2006) Nitrogen behaviour and nitrous oxide emission in the tidal Seine river estuary (France) as influenced by human activities in the upstream watershed. Biogeochemistry 77:305–326
Giblin AE, Tobias CR, Song B, Weston N, Banta GT, Rivera-Monroy VH (2013) The importance of dissimilatory nitrate reduction to ammonium (DNRA) in the nitrogen cycle of coastal ecosystems. Oceanography 26:124–131
Groffman PM, Holland EA, Myrold DD, Robertson GP, Zou X (1999) Denitirification. In: Robertson GP, Bledsoe CS, Coleman DC, Sollins P (eds) Standand soil methods long-term ecological research. Oxford University, New York, pp 272–288
Groffman PM, Altabet MA, Böhlke JK, Butterbach-Bahl K, David MB, Firestone MK, Giblin AE, Kana TM, Nielsen LP, Voytek MA (2006) Methods for measuring denitrification: diverse approaches to a difficult problem. Ecol Appl 16:2091–2122
Herdendorf CE (1990) Great Lakes estuaries. Estuaries 13:493–503
Hill AR (1993) Base cation chemistry of storm runoff in a forested headwater wetland. Water Resour Res 29:2663–2673
Hoellein TJ, Bruesewitz DA, Richardson DC (2013) Revisiting Odum (1956): a synthesis of aquatic ecosystem metabolism. Limnol Oceanogr 58:2089–2100
Hoffman JC, Peterson GS, Cotter AM, Kelly JR (2010) Using stable isotope mixing in a Great Lakes coastal tributary to determine food web linkages in young fishes. Estuar Coasts 33:1391–1405
Howarth R, Swaney D, Billen G, Garnier J, Hong B, Humborg C, Johnes P, Mörth C-M, Marino R (2012) Nitrogen fluxes from the landscape are controlled by net anthropogenic nitrogen inputs and by climate. Front Ecol Environ 10:37–43
Johnston CA, Bridgham SD, Schubauer-Berigan JP (2001) Nutrient dynamics in relation to geomorphology of riverine wetlands. Soil Sci Am J 65:557–577
Kabeya N, Kubota T, Shimizu A, Nobuhiro T, Tsuboyama Y, Chann S, Tith N (2008) Isotopic investigation of river water mixing around the confluence of the Tonle Sap and Mekong rivers. Hydrol Process 22:1351–1358
Knuth ML, Kelly JR (2011) Denitrification rates in a Lake Superior coastal wetland. Aquat Ecosyst Health Manag 14:414–421
Koop-Jakobsen K, Giblin AE (2010) The effect of increased nitrate loading on nitrate reduction via denitrification and DNRA in salt marsh sediments. Limnol Oceanogr 55:789–802
Kumar S, Sterner RW, Finlay JC, Brovold S (2007) Spatial and temporal variation of ammonium in Lake Superior. J Great Lakes Res 33:581–591
Larson JH, Trebitz AS, Steinman AD, Wiley MJ, Mazur MC, Pebbles V, Braun HA, Seelbach PW (2012) Great Lakes rivermouth ecosystems: scientific synthesis and management implications. J Great Lakes Res 39:513–524
Lewis WM, Wurtsbaugh WA, Paerl HW (2011) Rationale for control of anthropogenic nitrogen and phosphorus to reduce eutrophication of inland waters. Environ Sci Technol 45:10300–10305
Loken LC (2014) Hydrologic and biotic controls of nitrogen cycling in a Lake Superior freshwater estuary. MS Thesis, University of Wisconsin, Madison
McCarthy MJ, Gardner WS, Lavrentyev PJ, Moats KM, Frank J, Klarer DM (2007) Effects of hydrological flow regime on sediment-water interface and water column nitrogen dynamics in a Great Lakes coastal wetland (Old Woman Creek, Lake Erie). J Great Lakes Res 33:219–231
Meyers PA, Teranes JL (2001) Sediment organic matter. In: Last WM, Smol JP (eds) Tracking environmental change using lake sediments, vol 2., Physical and geochemical methods. Kluwer, Dordrecht, pp 239–269
Middelburg JJ, Nieuwenhuize J (2001) Nitrogen isotope tracing of dissolved inorganic nitrogen behaviour in tidal estuaries. Estuar Coast Shelf Sci 53:385–391
Morrice JA, Kelly JR, Trebitz AS, Cotter AM, Knuth ML (2004) Temporal dynamics of nutrients (N and P) and hydrology in a Lake Superior coastal wetland. J Great Lakes Res 30:82–96
Morrice JA, Trebitz AS, Kelly JR, Sierszen ME, Cotter AM, Hollenhorst T (2011) Determining sources of water to Great Lakes coastal wetlands: a classification approach. Wetlands 31:1199–1213
Mortimer CH, Fee EJ (1976) Free surface oscillations and tides of Lakes Michigan and Superior. Philos Trans R Soc Lond B 281:1–61
Mulholland PJ, Tank JL, Sanzone DM, Wollheim WM, Peterson BJ, Webster JR, Meyer JL (2000) Nitrogen cycling in a forest stream determined by a 15N tracer addition. Ecol Monogr 70:471–493
Nixon SW (1981) Remineralization and nutrient cycling in coastal marine sediments. In: Nielsen B, Cronin L (eds) Estuaries and nutrients. Humana Press, New Jersey, pp 111–138
Nixon SW, Ammerman JW, Atkinson LP, Berounsky VM, Billen G, Voicourt WC, Boynton WR, Church TM, Ditoro DM, Elmgren R, Garber JH, Giblin AE, Jahnke RA, Owens NJP, Pilson MEQ, Seitzinger SP (1996) The fate of nitrogen and phosphorus at the land-sea margin of the North Atlantic Ocean. Biogeochemistry 35:141–180
Ohte N, Sebestyen SD, Kendall C, Shanley JB, Wankel SD, Doctor DH, Boyer EW (2004) Tracing sources of nitrate in snowmelt runoff using a high-resolution isotopic technique. Geophys Res Lett 31:L21506. doi:10.1029/2004GL020908
Pattinson SN, Garcia-Ruiz R, Whitton BA (1998) Spatial and seasonal variation in denitrification in the Swale-Ouse system, a river continuum. Sci Total Environ 210:289–305
Pellerin BA, Saraceno JF, Shanley JB, Sebestyen SD, Aiken GR, Wollheim WM, Bergamaschi BA (2012) Taking the pulse of snowmelt: in situ sensors reveal seasonal, event and diurnal patterns of nitrate and dissolved organic matter variability in an upland forest stream. Biogeochemistry 108:183–198
Peterson BJ, Wollheim WM, Mulholland PJ, Webster JR, Meyer JL, Tank JL, Martí E, Bowden WB, Valett HM, Hershey AE, McDowell WH, Dobbs WK, Hamilton SK, Gregory S, Morrall DD (2001) Control of nitrogen export from watersheds by headwater streams. Science 292:86–90
Philips EJ, Cichra M, Aldridge FJ, Jembeck J, Hendrickson J, Brody R (2000) Light availability and variations in phytoplankton standing crops in a nutrient-rich blackwater river. Limnol Oceanogr 45:916–929
Piña-Ochoa E, Álvarez-Cobelas M (2006) Denitrification in aquatic environments: a cross-system analysis. Biogeochemistry 81:111–130
Richardson WB, Strauss EA, Bartsch LA, Monroe EM, Cavanaugh JC, Vingum L, Soballe DM (2004) Denitrification in the Upper Mississippi River: rates, controls, and contribution to nitrate flux. Can J Fish Aquat Sci 61:1102–1112
Roach WJ, Grimm NB (2011) Denitrification mitigates N flux through the stream–floodplain complex of a desert city. Ecol Appl 21:2618–2636
Saint Louis River Alliance (2013) Saint Louis River Aliance. http://stlouisriver.org/. Accessed 9 March, 2013
Schindler DW, Hecky RE, Findlay DL, Stainton MP, Parker BR, Paterson MJ, Beaty KG, Lyng M, Kasian SEM (2008) Eutrophication of lakes cannot be controlled by reducing nitrogen input: results of a 37-year whole-ecosystem experiment. Proc Natl Acad Sci 105:11254–11258
Seitzinger SP, Nielsen LP, Caffrey J, Christensen PB (1993) Denitrification measurements in aquatic sediments: a comparison of three methods. Biogeochemistry 23:147–167
Seitzinger SP, Harrison JA, Böhlke JK, Bouwman AF, Lowrance R, Peterson B, Tobias C, Van Drecht G (2006) Denitrification across landscapes and waterscapes: a synthesis. Ecol Appl 16:2064–2090
Sierszen ME, Morrice JA, Trebitz AS, Hoffman JC (2012) A review of selected ecosystem services provided by coastal wetlands of the Laurentian Great Lakes. Aquat Ecosyst Health Manag 15:92–106
Small GE, Bullerjahn GS, Sterner RW, Beall BFN, Brovold S, Finlay JC, McKay RML, Mukherjee M (2013) Rates and controls of nitrification in a large oligotrophic lake. Limnol Oceanogr 58:276–286
Smith MS, Tiedje JM (1979) Phases of denitrification following oxygen depletion in soil. Soil Biol Biochem 11:261–267
Smyth AR, Thompson SP, Siporin KN, Gardner WS, McCarthy MJ, Piehler MF (2013) Assessing nitrogen dynamics throughout the estuarine landscape. Estuar Coasts 36:44–55
Sorensen J, Sydor M, Huls H, Costello M (2004) Analyses of Lake Superior seiche activity for estimating effects on pollution transport in the St. Louis River Estuary under extreme conditions. J Great Lakes Res 30:293–300
Spokas K, Wang D, Venterea R (2005) Greenhouse gas production and emission from a forest nursery soil following fumigation with chloropicrin and methyl isothiocyanate. Soil Biol Biochem 37:475–485
Stanley EH, Ward AK (1997) Inorganic nitrogen regimes in an Alabama wetland. J North Am Benthol Soc 16:820–832
Stephens BM, Minor EC (2010) DOM characteristics along the continuum from river to receiving basin: a comparison of freshwater and saline transects. Aquat Sci 72:403–417
Sterner RW, Anagnostou E, Brovold S, Bullerjahn GS, Finlay JC, Kumar S, McKay RML, Sherrell RM (2007) Increasing stoichiometric imbalance in North America’s largest lake: nitrification in Lake Superior. Geophys Res Lett 34:L10406. doi:10.1029/2006GL028861
Tomazek JA, Gardner WS, Johengen TH (1997) Denitrification in sediments of a Lake Erie coastal wetland (Old Woman Creek, Huron, Ohio, USA). J Great Lakes Res 23:403–415
Trebitz AS (2006) Characterizing seiche and tide-driven daily water level fluctuations affecting coastal ecosystems of the Great Lakes. J Great Lakes Res 32:102–116
Trebitz AS, Morrice JA, Cotter AM (2002) Relative role of lake and tributary in hydrology of Lake Superior coastal wetlands. J Great Lakes Res 28:212–227
Verbanck M, Vanderborght J-P, Wallast R (1989) Major ion content of urban wastewater: assessment of per capita loading. Res J Water Pollut Control Fed 61:1722–1728
Wall LG, Tank JL, Royer TV, Bernot MJ (2005) Spatial and temporal variability in sediment denitrification within an agriculturally influenced reservoir. Biogeochemistry 76:85–111
Wankel SD, Kendall C, Paytan A (2009) Using nitrate dual isotopic composition (δ15N and δ18O) as a tool for exploring sources and cycling of nitrate in an estuarine system: Elkhorn Slough, California. J Geophys Res 114:G01011. doi:10.1029/2008JG000729
Wollheim WM, Stewart RJ, Aiken GR, Butler KD, Morse NB, Salisbury J (2015) Removal of terrestrial DOC in aquatic ecosystems of a temperate river network. Geophys Res Lett 42:6671–6679
Acknowledgments
We thank everyone (especially Shon Schooler, Tracey Ledder, and Kim Duernberger) at the Lake Superior National Estuary Research Reserve (LSNERR) for providing logistical support. Thanks to Ryan Hassemer, Nolan Klein, Lauren Reuss, Isaac Bergstrom and others for field and lab work. Thanks to Liz Runde and NTL-LTER (DEB-0822700) at University of Wisconsin and Sandy Brovold at the University of Minnesota-Twin Cities for lab analysis. Thanks to Joe Mayasich at the Western Lake Superior Sanitary District for providing data. Thanks to John Crawford, Nora Casson, Anett Trebitz, and two anonymous reviewers for comments on earlier drafts. This work is the result of research sponsored by the Minnesota and Wisconsin Sea Grant College Programs supported by the NOAA office of Sea Grant, United States Department of Commerce, under Grant No. NA10OAR4170069. The U.S. Government is authorized to reproduce and distribute reprints for government purposes, notwithstanding any copyright notation that may appear hereon. This paper is journal reprint No. JR628 of the Minnesota Sea Grant College Program. Additional support was provided to LC Loken by the University of Wisconsin-Madison—Anna Grant Birge Award.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Maren Voss.
Rights and permissions
About this article
Cite this article
Loken, L.C., Small, G.E., Finlay, J.C. et al. Nitrogen cycling in a freshwater estuary. Biogeochemistry 127, 199–216 (2016). https://doi.org/10.1007/s10533-015-0175-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-015-0175-3