Abstract
Wetlands of northern Belize, distributed along a salinity gradient, are strongly phosphorus limited and dominated largely by three species of emergent macrophytes: Eleocharis cellulosa, Cladium jamaicense, and Typha domingensis. We assessed changes in root and sediment phosphatase activities of each species to simultaneous changes of nutrients (N, P) and salinity in a mesocosm experiment. Phosphorus and nitrogen treatment effects on both root and sediment phosphatase were highly significant for all the species, while salinity significantly affected root phosphatase activity in Cladium only. All species showed a significant negative correlation between root phosphatase activity and increasing tissue P content until a threshold of 0.2% P, 0.15% P and 0.12% P in Eleocharis, Cladium and Typha, respectively. There was also a significant negative correlation between soil available P and root and sediment phosphatases in all species. Activity of root phosphatases of Eleocharis and Typha were positively correlated with root tissue N. Both root and sediment phosphatases of all three species were positively correlated with soil available N. The strongest (positive) correlation was found between phoshatase activites and N/P ratios. The results confirmed that these systems are P-limited and that extracellular phosphatases respond to P enrichment by decreasing their activities. Expression of root phosphatase activity by dry root weight, sediment volume, or whole plant biomass gave very different relative results across nutrient treatments and species, suggesting that root phosphatase activities need to be interpreted in a wider context that considers root density.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phosphorus is frequently the most limiting element for plant and microbial growth and development. To cope with this limitation, plants and microorganisms have evolved a range of strategies for P acquisition in nutrient-deficient environments (Aerts and Chapin 2000; Vance et al. 2003; Oberson and Joner 2005). Human changes in the global P budget have lead to eutrophication of previously oligotrophic ecosystems (Bennett et al. 2001; Martinez-Crego et al. 2006), thus it is important to understand how adaptations to low P change when the P-limitation is removed.
One of the P-acquisition strategies employed by microorganisms, as well as plants, involves production and secretion of phosphatase enzymes responsible for release of P from organic P-esters. Two categories of plant phosphatases can be distinguished based on their relative substrate selectivity: (1) specialized enzymes with clear substrate specificity that are believed to have distinct metabolic functions (Duff et al. 1994), and (2) non-specific phosphatases such as root-secreted extracellular phosphomonoesterases that occur in a variety of species. Extracellular phosphatases are usually located on the outer surface of epidermal cells and root apical meristems and they appear relatively stable for hours to days (Vance et al. 2003). The expression of the root-surface and sediment phosphatase activity is regulated by the extent of P limitation to plant and microbial growth (Wright and Reddy 2001; Phoenix et al. 2003). In the presence of adequate supplies of orthophosphate, the enzyme is usually repressed, while under P-limitation it is stimulated. Enhanced enzyme activity has also been reported as a response to increased nitrogen availability and subject to stressors, namely salinity (Duff et al. 1994). The majority of reports on root or sediment phosphatase activities focus on the potential role of these enzymes in improving P economy of agricultural crops (Tarafdar and Claassen 1988; Asmar and Gissel-Nielsen 1997; Tarafdar and Gharu 2006) and on exploring possibilities of use of the phosphatases as biomarkers indicating the level of water eutrophication (Hernandez et al. 2002; Newman et al. 2003; Martinez-Crego et al. 2006; Ellwood and Whitton 2007).
As part of a research project studying changes in ecosystem processes and community structure following nutrient additions, we conducted a mesocosm experiment to evaluate the response of three macrophyte species, Eleocharis cellulosa Torr., Cladium jamaicense Crantz and Typha domingensis Pers., and cyanobacterial mats to factorial combinations of low, medium and high salinity, P, and N. The results focusing on the impact of increased nutrient availability on the growth and nutrient uptake of these species are presented in Macek and Rejmánková (2007), while a paper by Rejmánková and Komárková (2005) reports on the response of cyanobacterial mats. Here we focus on changes in root and sediment phosphatase activities in these species resulting from the combined effects of P and N addition and increasing salinity.
We aimed to answer the following questions: (1) How do the root and sediment phosphatase activities respond to changes in P, N and salinity? (2) Does the root phosphatase activity depend more on the external P availability or P tissue content? (3) Are the activities of root and sediment phosphatases correlated? (4) Are there any species specific differences in enzyme activity? We hypothesized that (1) phosphatase activities will decrease with increasing P and the decrease will depend on both plant tissue and sediment P concentrations, (2) the activity of the enzymes will be stimulated by higher sediment and plant N concentration, and (3) the enzyme activity will decrease with increasing salinity. While there is a general consensus in the literature on the enzyme responses to P and N (Sinsabaugh and Moorhead 1994; Christmas and Whitton 1998; Ellwood and Whitton 2007), the reports on phosphatase response to increasing salinity are conflicting and range from a decrease in activity (Nausch 1998; Rejmánková and Komárková 2005) to no response (Sirová et al. 2006) or increased activity (Duff et al. 1994; Hernandez et al. 2002). We did not have a clear prediction for potential differences in phosphatase activities among the investigated species. Kuhn et al. (2002) found significantly greater root phosphatase activity in Cladium as compared to Typha and explained this by a better adaptation of Cladium to P-limiting conditions of peat in the Everglades, USA. However, our unpublished data indicated that all three species exhibit high root phosphatase activity when grown in the P-limited marshes of Belize where P-limitation is accentuated by high availability of calcium and subsequent co-precipitation of available P.
Materials and methods
The investigated species, Eleocharis cellulosa Torr., Cladium jamaicense Crantz and Typha domingensis Pers., are rhizomatous, clonal, non-mycorrhizal macrophytes, and in terms of life strategies sensu Grime (2001), they range from a competitor (Typha) to stress tolerators (Eleocharis and Cladium) (Rejmánková et al. 1996).
Mesocosms
Pre-planted, same age ramets of the three species, originating from a medium salinity marsh, were trimmed to have only the youngest 3–4 leaves (Cladium, Typha) or 2–3 shoots (Eleocharis) and planted in 4-l plastic pots. The initial root biomass was approximately equal for all species (range 0.1–0.2 g DW). The pots were filled with 3,500 cm3 of sediment consisting of a mix of peat, clay and marl, which is typical of many northern Belizean marshes. The mix had a bulk density 0.32 g cm−3, pH 7.3, and contained 16.5% C, 1.22% N, and 0.013% P. Pots were placed in mesocosms (88 cm × 88 cm × 30 cm), and flooded by rain water to reach a water level of ~3 cm above the soil surface. A full factorial design of three salinities, three levels of P and three levels of N was used. One mesocosm was kept as a control with no additions. The mesocosms were located in a grassland area adjacent to a pond on the outskirts of the town of Orange Walk, Belize. Plants were placed in separate pots with four replicate pots per species.
A mixture of salts that reflects the average water ionic composition of marshes in the region (Rejmánková, unpublished data) was used for the salinity treatment. CaSO4 · 2H2O: 0.5 g l−1; MgSO4 · 7H2O: 0.5 g l−1; CaCl2 · 2H2O: 0.5 g l−1; K2SO4 0.5 g l−1; NaCl:2.5 g l−1 were mixed in rain water. The salinity was increased gradually over 2 weeks to reach the final values of low (0.2–0.5 ppt), medium (1–1.5 ppt) and high (4–5 ppt). Salinity was adjusted bi-weekly or after a heavy rain, as needed.
Phosphorus was added as KH2PO4 and nitrogen as NH4NO3. Nutrients were injected with a syringe about 10 cm below the sediment surface of each pot in 6 days intervals (10 additions total). Additions of nutrients over the duration of the experiment corresponded to an annual equivalent of 1 g m−2 y−1, 10 g m−2 y−1, and 20 g m−2 y−1, for low, medium and high N, respectively, and to 0.5 g m−2 y−1, 5 g m−2 y−1, and 10 g m−2 y−1, for low, medium and high P, respectively. These additions were selected to correspond to an ongoing long-term field nutrient addition experiment (Rejmánková et al. 2008). To put these values in perspective, the average extractable sediment P concentration in the marshes in the study area ranges from 0.5 to 1 μg cm−3, while the lowest addition in the mesocosm experiment corresponded to 3.5 μg cm−3. The experiment began with the first addition of nutrients after the final salinities had been established. It lasted from January 30 through March 30, 2002.
Sample collection and nutrient analysis
At the end of the experiment, the plants were harvested and divided into leaves/shoots, rhizomes and roots. Sediment samples were collected with a 2 cm diameter soil corer. Dry leaf/shoot and root tissue was ground and assayed for total N with a Perkin Elmer HCN analyzer. Total P was measured spectrophotometrically using ascorbic acid reduction of phosphomolybdate complex after combustion and consequent acid digestion in 2 N HCl (McNamara & Hill 2000). Interstitial water samples collected for nutrient analysis were placed into HCl-rinsed plastic bottles and stored on ice until processing. The samples for NH4–N, NO3–N and PO4–P were filtered through a 0.45 μm filter within 1 h after sampling and frozen until analysis. They were later analyzed using standard colorimetric methods (Murphy and Rilley 1962; Solorzano 1969; Kamphake et al. 1967, for PO4–P, NH4–N, NO3–N, respectively). Interstitial PO4–P and the sum of NH4–N and NO3–N were used in data analyses as proxies for plant-available P and N.
Phosphatase
Phosphatase activity was measured using methylumbelliferyl phosphate (MUFP) as a substrate (Hoppe 1993). Apical portions of roots, 0.075–0.15 g fresh weight, were washed in distilled water, towel-dried, and pre-incubated for 50 min in 8 ml of pH 8 Tris buffer at 30°C. Bacteria attached to root surface may be significant sources of phosphatase activity. In a preliminary trial we compared the phosphatase activity of roots treated with sodium azide solution to kill the surface bacteria (Kuhn et al. 2002) with activity of roots washed in distilled water only. Since we did not find any significant differences between these two treatments (Eleocharis: 8.87 ± 2.22 SD and 8.75 ± 0.75 SD, Typha 8.48 ± 3.39 SD and 8.56 ± 3.15 SD, Cladium 4.21 ± 1.38 SD and 4.46 ± 0.61 SD for azide and distilled water, respectively), we omitted the sodium azide rinse. Then 2 ml of 5 mM MUFP (final concentration 1 mM) were added and the samples were incubated at 30°C for 40 min. The reaction was terminated by adding 0.5 ml of 0.2 N NaOH. Fluorescence of the methylumbeliferon (MUF) was measured with a Quantech fluorometer equipped with a near-UV lamp, 430 nm emission filter, and 365 nm excitation filter. Ratio of fresh weight/dry weight was assessed on representative samples for each species and the length, surface area and diameter of roots were measured on a root scanner using WIN/Mac RHIZO (Regent Instruments Inc.) interactive image analysis software. Root phosphatase activity was expressed on both a dry-weight and surface-area basis. Sediment phosphatase activity was measured in a slury (1 g fresh weight of sediment was shaken with 10 ml of Tris buffer). One milliliter of this suspension was combined with 3 ml of Tris and 1 ml of 5 mM MUFP (for final concentration of 1 mM MUFP), incubated at 30°C for 60 min, filtered and the fluoresecence immediately measured as above. A portion of the homogenized sample was treated with HgCl2 (4 mM l−1 final concentration), which served as a fixative stopping all enzymatic activity, and used as blank in three replicates to determine non-enzymatic hydrolysis of the substrate.
Data analyses
Data were analyzed using multifactorial ANOVA with species, salinity, N and P as factors. In all analyses, most of the interactions of species × factor were significant, which means that species responded to the treatments differently. Therefore we used only three factors (salinity, N, P) in succeeding analyses, and we evaluated data for each species separately. For post-hoc comparisons we used Scheffé test. Path analysis of direct and indirect effects of sediment phosphorus and nitrogen on root phosphatase activity of Eleocharis cellulosa, Typha domingensis and Cladium jamaicense was performed according to Pedhazur (1997).
Results
Phosphorus and nitrogen treatment effects on both root and sediment phosphatase were highly significant for all the species (Table 1; Figs. 1 and 2). Salinity significantly affected root phosphatase activity in Cladium, a marginal effect was seen in Eleocharis, and phosphatase activity of Typha was not affected by salinity at all. Activities of both root and sediment phosphatases decreased in response to P addition. This decrease was significant between low and medium and low and high P-addition only (Scheffé, P < 0.01), while the differences between medium and high addition were not significant. The exception was the sediment phosphatase activities in Cladium where there was no difference between the low and medium P addition, while the difference between low and high and medium and high treatments was significant (Scheffé, P < 0.05). A significant P × N interactions in root phosphatase of Eleocharis and Typha, and sediment phosphatase of Cladium resulted from the fact that the phosphatase activity was decreasing with increasing P, while it was increasing with increasing N. The significant N × salinity interactions in both root and sediment enzyme activity of Eleocharis is obviously also a result of the two opposite trends, increasing enzyme activity with increasing N and a slight decrease with increasing salinity.
We did not include a treatment of no N and no P addition to the data analyses due to problems with unbalanced design. However, when comparing the lowest nutrient addition treatment to the no addition treatment, there were no significant differences between either root or sediment phosphatases in any of the three species (t-test, P > 0.05, data not shown), although there was an indication of higher root phosphatase activity in Typha (6.8 ± 0.53 SD and 10.7 ± 3.21 SD μmol g DW−1 min−1 for low addition and no addition, respectively) and Cladium (3.33 ± 1.36 SD and 5.13 ± 1.02 SD μmol g DW−1 min−1 for low addition and no addition, respectively).
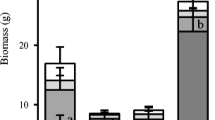
When expressed on the dry weight basis, the activities of root phosphatases were similar for Eleocharis and Typha but lower for Cladium (Figs. 1, 3a). When expressed on the root surface area basis, a completely different picture emerged: activities of root phosphatases of Typha were the lowest while there were almost no differences between Eleocharis and Cladium (Fig. 3b). This was because Typha had the highest specific root area, SRA (root area/root dry weight), of the three species. Since we knew the dry weight of the roots per pot and the volume of sediment in the pot, we also expressed the enzyme activity per volume of soil. In this case, Typha, which grows proliferously and has high root production, displayed two to four times higher activities than the other two species (Fig. 3c).
The effects of various factors of interest on the phosphatase activity was further evaluated by a series of simple correlations (Table 2). All species showed highly significant negative correlation between root phosphatase activity and increasing P content of leaves and roots. There was also highly significant negative correlation between log of plant-available P and root and sediment phosphatases in all species. On the other hand, root phosphatases of both, Eleocharis and Typha were positively correlated with root tissue N. Both root and sediment phosphatases of all three species were positively correlated with log of plant-available N; the correlation with leaf tissue N was either weak or insignificant. Root phosphatase activities were also closely correlated with both root and leaf tissue N/P, and both root and sediment phosphatatses were closely correlated with log of plant available N/P.
Concentrations of sediment nutrients may impact root phosphatase activities directly but may also affect them indirectly through changes in root tissue nutrients. To elucidate the importance of these direct and indirect effects, we used a simple path analysis (Fig. 4). It revealed that the direct negative effect of the plant-available P (coefficient a 41 in Fig. 4) was significant for all three species and is more important than the indirect negative effect through root P. Unexpected was the significant negative effect of plant-available P through root tissue N in Eleocharis and Typha. To explain this, we have to look at the response of tissue N to increases in available P. Root tissue N in both species was negatively correlated with increasing plant-available P (P = 0.002 and P = 0.01, respectively); no such correlation was found for Cladium. Although the simple relationship of root phosphatase and root tissue N was positive (see Table 2), the overall indirect effect of plant-available P was negative. The direct effect of plant-available N on phosphatase activity was insignificant for Eleocharis and Typha but significantly positive for Cladium. Path analysis also indicated an indirect positive effect of increasing plant-available N through root tissue N that was significant for Eleocharis and Typha. There was also an indirect positive effect through root P resulting from the combination of two negative effects: (1) more N in sediments decreased root P, (2) more root P decreased phosphatase. Activities of root and sediment phosphatases were highly correlated in all species (Table 2).
Path diagram of direct (a 41) and indirect (a 21 × a 42 and a 31 × a 43) effects of plant-available phosphorus (P) and nitrogen (N) concentrations on root phosphatase activity of Eleocharis cellulosa, Typha domingensis and Cladium jamaicense. Path coefficients a 21 and a 31 are standardized simple regression coefficients, path coefficients a 42 and a 43 are standardized partial regression coefficients from the multiple regression. r 41 is the overall correlation between plant-available P (N) and phosphatase activity. Path coefficients printed in bold are significant at P < 0.05. If both path coefficients forming a product are significant at P < 0.05, the product is also printed in bold. The residual variable U includes all unmeasured variables that affect the dependent variable and its path coefficient = √1−R2, where R is the proportion of variation in the dependent variable explained by the model
Discussion
Enzyme activity and available phosphorus
The prediction of decreasing phosphatase activity in response to increased amount of available P was confirmed for both root and sediment phosphatases. This was not surprising as similar relationship has been found in various plant species and/or sediments (Kroehler and Linkins 1988; Duff et al. 1994; Wright and Reddy 2001). The closest comparison with sediment phosphatases comes from the paper by Sirová et al. (2006) who measured activities of phosphatases in the layer of benthic cyanobacteria and underlaying sediments in marshes of northern Belize, i.e., same type of sediments as those used in our mesocosms, and compared the enzyme activities among controls and P-enriched plots. They observed a significant suppression of phosphatase activity in sediments of P-addition plots with the highest activities in the P concentration range of 100–500 μg l−1 and a sharp threshold at 500 μg l−1. Similarly, our highest activities were found at the lowest P additions, which had the average interstitial water P concentration of 42 μg l−1. Kuhn et al. (2002) found the highest root phosphatase activities in the range of 10–80 μg l−1 of inorganic P in the interstitial water and a significant decrease at 500 μg l−1. The phosphatase activity of cyanobacterial mats from the mesocosm experiment followed a similar trend, although the absolute values were about 2–3× higher than the sediment enzyme activities (Rejmánková and Komárková 2005). A highly significant decrease in phosphatase activities was also registered in samples of the decomposing litter located in P enriched plots (Rejmánková and Sirová 2007). Wright and Reddy (2001) observed similar trends for the activity of extracellular enzymes in the Everglades wetland soils.
The best comparison to our root phosphatase activities are Kuhn et al. (2002) data on the root phosphatase activities in Typha and Cladium grown in the Everglades peat. In agreement with their results, we found a significant decrease in enzyme activity in response to added P. However, the species response was different. Kuhn et al. (2002) found significantly higher enzyme activity (based on dry weight) in Cladium compared to Typha, which is just the opposite of our results. They explained the species specific differences by the fact that Cladium, a species adapted to oligotrophic conditions, expresses higher phosphatase activities than Typha, a species used to relatively eutrophic conditions. We speculate that the Typha plants used in our experiment originated in a population growing in a nutrient poor marsh and thus were adapted to low P levels. Moreover, even at the nutrient enriched locations, most of the P is still in the organic form (Turner and Newman 2005, Rejmánková, unpublished data), and in order to sustain their vigorous growth, Typha probably has to employ the extracellular enzymes.
Although there was a significant decrease in phosphatase activity at higher P levels, the enzymes were not inhibited completely and activities were detected even in roots from the highest P additions (>2,000 μg l−1 of PO4–P in the interstitial water). This seems in line with the growing body of literature on the possible existence of different enzyme pools, one constitutive, irrepressible by inorganic phosphate levels and one adaptive regulated by the concentration of inorganic P (Kroehler and Linkins 1988; Wetzel 1992; Phoenix et al. 2003).
We also asked whether the root phosphatase activity depends more on the external P availability or P tissue content. The results of simple correlations and path analysis indicated that while increasing concentrations of both, plant-available and tissue P led to a significant decrease in phosphatase activity, the direct effect of plant-available P on enzyme activity was more significant. Contrary to our results, Christmas and Whitton (1998) and Ellwood and Whitton (2007), who studied surface phosphatase activities in aquatic mosses, found the internal P concentration to be responsible for regulation of phosphatase activities, but no significant correlation between phosphatase activity and water P. Similarly, Martinez-Crego et al. (2006) reported a negative correlation between both root and leaf tissue P and extracellular phosphatase activities in seagrass, Posseidonia oceanica, but no relationship with P in water. The investigated mosses and seagrass came from environments, streams and a coastal sea meadow respectively, with water P fluctuating more than in our mesocosm experiment. In such case, the internal P concentration that integrates P availability over a longer period may show a tighter correlation with enzyme activities than water P.
Christmas and Whitton (1998) give a value of 0.3% tissue P as a threshold, below which the phosphatase activity starts increasing. This is a higher concentration than what we found: Eleocharis had the highest threshold (0.2%), followed by Cladium (0.15%) and Typha (0.12%).
Enzyme activity and nitrogen
We found a strong positive correlation between sediment nitrogen and both root and sediment phosphatases. This is in agreement with Johnson et al. (1999) and Phoenix et al. (2003) who found higher enzyme activities in Plantago lanceolata and several calcareous grassland species that were exposed to increased sediment nitrogen. Phoenix et al. (2003) explained the stimulatory effect of increased N availability on phosphatase activity by a phosphate starvation inducible type response—their results suggested that the expression of genes in the proposed phosphate starvation rescue system could be sensitive to shoot and soil N as well as P. An inverse relationship has been reported between tissue N and water/sediment P (Phoenix et al. 2003, Ellwood and Whitton 2007). Similarly, Eleocharis and Typha in our experiment showed a significant decrease in root tissue N with increasing P in the sediments (P = 0.003 and 0.016, respectively). In sediment where inorganic P is increasing, this decrease in N does not have any significant effect on enzyme activity because the negative effect of increasing sediment P is much stronger than the negative effect of decreasing root tissue N (see the path analysis). In environments, such as many wetlands, with abundant supplies of organic P, where root phosphatases are needed to break the organic compounds, plants with higher root tissue N content should be at an advantage.
Enzyme activity and salinity
We did not find any direct response to salinity changes in Eleocharis and Typha, while in Cladium, contrary to our prediction, root phosphatase activity significantly increased at higher salinities. According to Duff et al. (1994), salt stress is among the environmental determinants that elicit increased expression of intra-and/or extracellular phosphatases. Increased salinity represented a significant stress to all three species and decreased their growth (Macek and Rejmánková 2007). Salinity did not have any direct effect on sediment phosphatase, which is in agreement with the study of Sirová et al. (2006) who found that enzymes in wetland sediments in our study area were not significantly impacted by salinity.
Data interpretation
Any direct comparison of absolute values of root enzymatic activities among different species and with data presented by other researchers is difficult because of different methodologies (different substrate type and concentration, different reaction temperatures, different pH). The data interpretation is also complicated by the fact that different authors express the enzyme activities based on different entities (dry weight, fresh weight, root length, protein amount, etc.). Fresh or dry weights are most commonly used to base enzyme activity on (Turner et al. 2001; Phoenix et al. 2003; Playsted et al. 2006). However, there are major differences in specific root area (SRA) among different species and functional types. It follows that if enzyme activities are expressed per unit root weight, then what seems as a significant difference in enzyme activity may only be an artefact caused by SRA differences (Phoenix et al. 2003). The discrepancy caused by different ways of expressing the enzyme activity should serve as a warning: If the enzyme activity is expressed based on dry weight, only species with similar SRA should be compared, otherwise the comparison is meaningless. To make more meaningful speculations about the role of root phosphatase activities of different species, we need to know the root density per sediment volume. As demonstrated by our example (Fig. 3c), when root phosphatase activity was expressed per sediment volume, Typha had by far the largest activity due to its prolific root growth. Its root density was two to four fold that of Eleocharis and Cladium (Macek and Rejmánková 2007). The drawback of expressing the enzyme activity per sediment volume is that there are not many data available on root density for wetland plant species and not all the parts of the roots release the same amount of enzyme. The enzyme activities are concentrated to actively growing root tips and the activity decreases with the distance from the tip (E. Rejmánková, unpublished data).
Cladium has been frequently described as a stress tolerator exhibiting strong morphological constraints and being well adapted to low nutrients (Newman et al. 1996; Lorenzen et al. 2001). It has been argued that for slow growing macrophytes, such as Cladium, the nutrient uptake by their less extended root system is still high enough to supply their growth (Macek and Rejmánková, 2007). Typha, on the other hand, has been characterized as a typical competitor in terms of its plastic opportunistic guerrilla growth strategy, fast and efficient space occupancy and rather wasteful nutrient management (Chiang et al. 2000; Lorenzen et al. 2001). Eleocharis is known to respond rapidly to nutrient addition, but it displays limited rhizome plasticity (Daoust and Childers 2004; Chen et al. 2005; Macek and Rejmánková 2007) and can be considered as an intermediate between the two above species. Figure 3d expresses the root phosphatase activity based on the total final biomass of each species. There were no diferences between Eleocharis and Typha but Cladium exhibited significantly lower root phosphatase activity. This is in agreement with life strategies of these species. Cladium grows slowly, conserves nutrients and apparently also conserves the amount of enzymes it produces, while Typha and Eleocharis release more enzyme relative to their total biomass.
The sediment and root phosphatases of individual species were well correlated. Sediment phosphatases are generally believed to be of microbial origin, but several authors suggest that plant phosphatases may be even more important than phosphatases produced by rhizosphere organisms (Tarafdar and Claassen 1988). According to Asmar and Gissel-Nielsen (1997), the rhizoplane microorganisms contributed about 3% of total activity while the rest comprised root-originated enzymes. Similarly, Colvan et al. (2001) stated that phosphatases in soils are primarily of plant origin and are stabilized through complexation with humic substances. We speculate that since we found a close correlation between sediment and root phosphatases, the sediment phosphatases were of plant origin. The counterargument is that when the phosphatases were expressed on per volume basis, the sediment phosphatases were an order of magnitude higher (data not shown). More sophisticated methodology than what we used would be needed to answer these questions.
In conclusion, we confirmed that sediment and root phosphatases are highly sensitive to changes in sediment N and P in all species, while salinity impacted only Cladium. Collectively all results from various studies from the Belizean marshes support the theory that these systems are P-limited and the extracellular phosphatases respond to P enrichment by decreasing their activities. In terms of root phosphatases though, the root amount increased with P addition and so when the enzyme activity was expressed per sediment volume, the differences between enriched and un-enriched sites became less significant. The information on root phosphatase activities needs to be interpreted in the context of wider information on root density.
References
Aerts R, Chapin FS (2000) The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Adv Ecol Res 30:1–67
Asmar F, Gissel-Nielsen G (1997) Extracellular phosphomono- and phosphodiesterase associated with and released by the roots of barley genotypes: a non-destructive method for the measurement of the extracellular enzymes of roots. Biol Fertil Soils 25:117–122
Bennett EM, Carpenter SR, Caraco NF (2001) Human impact on erodable phosphorus and eutrophication: a global perspective. BioScience 51:227–234
Chen HJ, Mendelssohn IA, Lorenzen B, Brix H, Miao SL (2005) Growth and nutrient responses of Eleocharis cellulosa (Cyperaceae) to phosphate level and redox intensity. Am J Bot 92:1457–1466
Chiang C, Craft CB, Rogers DW, Richardson CJ (2000) Effects of 4 years of nitrogen and phosphorus additions on Everglades plant communities. Aquat Bot 68:61–78
Christmas M, Whitton BA (1998) Phosphorus and aquatic bryophytes in the Swale-Ouse river system, north-east England. 1. Relationship between ambient phosphate, internal N: P ratio and surface phosphatase activity. Sci Total Environ 210:389–399
Colvan SR, Syers JK, O’Donnell AG (2001) Effect of long-term fertilizer use on acid and alkaline phosphomonoesterase and phosphodiesterase activities in managed grassland. Biol Fertil Soils 34:258–263
Daoust RJ, Childers DL (2004) Ecological effects of low-level phosphorus additions on two plant communities in a neotropical freshwater wetland ecosystem. Oecologia 141:672–686
Duff SMG, Sarath G, Plaxton WC (1994) The role of acid-phosphatases in plant phosphorus-metabolism. Physiol Plant 90:791–800
Ellwood NTW, Whitton BA (2007) Phosphatase activities of the aquatic moss Warnstorfia fluitans (Hedw.) Loeske from an acidic stream in north-east England. Hydrobiologia 575:95–107
Grime JP (2001) Plant strategies,vegetation processes, and ecosystem properties. Wiley, Chichester, 417 pp
Hernandez I, Niell FX, Whitton BA (2002) Phosphatase activity of benthic marine algae. An overview. J Appl Phycol 14:475–487
Hoppe HG (1993) Use of fluorogenic model substrates for extracellular enzyme activity (EEA) measurement of bacteria. In: Kemp PF (ed) Handbook of methods in aquatic microbial ecology. Lewis, Boca Raton, pp 423–431
Johnson D, Leake JR, Lee JA (1999) The effects of quantity and duration of simulated pollutant nitrogen deposition on root-surface phosphatase activities in calcareous and acid grasslands: a bioassay approach. New Phytol 141:433–442
Kamphake LJ, Hannah SA, Cohen JM (1967) Automated analysis for nitrate by hydrazine reduction. Water Res 1:205–216
Kroehler CJ, Linkins AE (1988) The root surface phosphatases of Eriophorum vaginatum—effects of temperature, pH, substrate concentration and inorganic phosphorus. Plant Soil 105:3–10
Kuhn NL, Mendelssohn IA, McKee KL, Lorenzen B, Brix H, Miao SL (2002) Root phosphatase activity in Cladium jamaicense and Typha domingensis grown in Everglades soil at ambient and elevated phosphorus levels. Wetlands 22:794–800
Lorenzen B, Brix H, Mendelssohn IA (2001) Growth, biomass allocation and nutrient use efficiency in Cladium jamaicense and Typha domingensis as affected by phosphorus and oxygen availability. Aquat Bot 70:117–133
Macek P, Rejmánková E (2007) Response of emergent macrophytes to experimental nutrient and salinity additions. Funct Ecol 21:478–488
Martinez-Crego B, Romero J, Alcoverro T (2006) The use of surface alkaline phosphatase activity in the seagrass Posidonia oceanica as a biomarker of eutrophication. Mar Ecol 27:381–387
McNamara AE, Hill WR (2000) UV-B irradiance gradient affects photosynthesis and pigments but not food quality of periphyton. Freshw Biol 43:649–662
Murphy J, Rilley JP (1962) A modified single solution method for determination of phosphate in natural water. Anal Chem Acta 27:31–36
Nausch M (1998) Alkaline phosphatase activities and the relationship to inorganic phosphate in the Pomeranian Bight (southern Baltic Sea). Aquat Microb Ecol 16:87–94
Newman S, Grace JB, Koebel JW (1996) Effects of nutrients and hydroperiod on typha, cladium, and eleocharis: implications for everglades restoration. Ecol Appl 6:774–783
Newman S, McCormick PV, Backus JG (2003) Phosphatase activity as an early warning indicator of wetland eutrophication: problems and prospects. J Appl Phycol 15:45–59
Oberson A, Joner EJ (2005) Microbial turnover of phosphorus in soil. In: Turner BL, Frossard E, Baldwin DS (eds) Organic phosphorus in the environment. CABI, Cambridge
Pedhazur EJ (1997) Multiple regression in behavioral research: explanation and prediction, 3rd edn. Hartcourt Brace, Fort Worth
Phoenix GK, Booth RE, Leake JR, Read DJ, Grime JP, Lee JA (2003) Simulated pollutant nitrogen deposition increases P demand and enhances root-surface phosphatase activities of three plant functional types in a calcareous grassland. New Phytol 161:279–289
Playsted CWS, Johnston ME, Ramage CM, Edwards DG, Cawthray GR, Lambers H (2006) Functional significance of dauciform roots: exudation of carboxylates and acid phosphatase under phosphorus deficiency in Caustis blakei (Cyperaceae). New Phytol 170:491–500
Rejmánková E, Komárková J (2005) Response of cyanobacterial mats to nutrient and salinity changes. Aquat Bot 83:87–107
Rejmánková E, Sirová D (2007) Wetland macrophyte decomposition under different nutrient conditions: relationships between decomposition rate, enzyme activities and microbial biomass. Soil Biol Biochem 39:526–538
Rejmánková E, Pope KO, Post R, Maltby E (1996) Herbaceous wetlands of the Yucatan Peninsula: communities at extreme ends of environmental gradients. Int Rev Gesamten Hydrobiol 81:223–252
Rejmánková E, Macek P, Epps K (2008) Wetland ecosystem changes after three years of phosphorus addition. Wetlands (in press)
Sinsabaugh RL, Moorhead DL (1994) Resource-allocation to extracellular enzyme-production—a model for nitrogen and phosphorus control of litter decomposition. Soil Biol Biochem 26:1305–1311
Sirová D, Vrba J, Rejmánková E (2006) Extracellular enzyme activities in benthic cyanobacterial mats: comparison between nutrient-enriched and control sites in marshes of northern Belize. Aquat Microb Ecol 44:11–20
Solorzano L (1969) Determining ammonia in natural waters by the phenolhypochlorite method. Limnol Oceanogr 14:799–801
Tarafdar JC, Claassen N (1988) Organic phosphorus-compounds as a phosphorus source for higher-plants through the activity of phosphatases produced by plant-roots and microorganisms. Biol Fertil Soils 5:308–312
Tarafdar JC, Gharu A (2006) Mobilization of organic and poorly soluble phosphates by Chaetomium globosum. Appl Soil Ecol 32:273–283
Turner BL, Newman S (2005) Phosphorus cycling in wetland soils: the importance of phosphate diesters. J Environ Qual 34:1921–1929
Turner BL, Baxter R, Ellwood NTW, Whitton BA (2001) Characterization of the phosphatase activities of mosses in relation to their environment. Plant Cell Environ 24:1165–1176
Vance CP, Uhde-Stone C, Allan DL (2003) Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol 157:423–447
Wetzel RG (1992) Gradient-dominated ecosystems: sources and regulatory functions of dissolved organic matter in freshwater ecosystems. Hydrobiologia 229:181–198
Wright AL, Reddy KR (2001) Phosphorus loading effects on extracellular enzyme activity in Everglades wetland soil. Soil Sci Soc Am J 65:588–595
Acknowledgements
We thank Ireneo Briceno, and Russell King for their assistance in the field and Joy Futrell for laboratory assistance. We also thank Marcel Rejmanek for his help with the path analysis and Dagmara Sirova for editorial comments. Comments of the editor and three anonymous reviewers helped to improve the manuscript. This research was supported by NSF grant NSF # 0089211 to E.R.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rejmánková, E., Macek, P. Response of root and sediment phosphatase activity to increased nutrients and salinity. Biogeochemistry 90, 159–169 (2008). https://doi.org/10.1007/s10533-008-9242-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-008-9242-3