Abstract
A study was made of the aquatic environment, tissue nutrient composition and surface phosphatase activities of the aquatic moss Warnstorfia fluitans in Brandon Pithouse Stream, a small acidic stream in N-E England. The water, which originates from an underground spring, had been pH 2.6 for at least 30 years, but about 3.9 during the present study. The moss was by far the most abundant phototroph during all this period. Seasonal changes in aqueous nitrogen and phosphorus fractions were measured over a 2-year period near the source. Most of the filtrable N and P were at times organic, but the very high N:P ratio (even if organic N is excluded) suggests that only organic phosphate is likely to be important for the moss. There was a high peak in organic phosphate in late spring in both study years. Surface phosphomonoesterase (PMEase) and phosphodiesterase (PDEase) activities were highly correlated in the field and in axenic culture, though there were some differences in response to environmental factors. Axenic material showed higher PMEase and PDEase activities when grown with organic P than with inorganic P. Although the data suggest that internal P content is an important factor influencing phosphatase activities, PDEase activity was especially marked when the moss was grown with the diester, DNA, as P source, indicating that at least one of its surface phosphatases can also respond directly to the environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A number of moss species have been shown to form surface phosphatase enzymes in aquatic environments (Turner et al., 2001, 2003). The internal phosphate content appears to be a key factor influencing phosphatase development of these mosses (Press & Lee, 1983; Whitton et al., 2005). In the two widespread northern hemisphere species whose surface phosphatase activities have received the most study, Rhynchostegium riparioides (Christmas & Whitton, 1998a) and Fontinalis antipyretica (Christmas & Whtton, 1998b), there is a low level of surface PMEase (phosphomonoesterase) activity when the moss is phosphate-rich, but this increases markedly with increasing phosphate-limitation (Christmas & Whitton, 1998a). Surface PDEase (phosphodiesterase) activity is apparently absent under phosphate-rich conditions and is induced later than PMEase activity; hence the greater the phosphate-limitation, the greater the relative importance of PDEase. However, not all aquatic phototrophs respond in the same way. For instance, in some cyanobacteria there is no PMEase activity under phosphate-rich conditions and PDEase activity is induced at the same time as PMEase activity (Whitton et al., 2005).
The concentrations of nutrient elements and phosphatase activities of mosses can provide a record of the nutritional status of the environment (Steinman & Boston, 1993; Steinman, 1994; Garcia-Alvaro et al., 2000; Whitton et al., 2002). As the aquatic mosses whose phosphatase activities have been reported in detail mostly come from streams and small rivers where peat is important in the catchment, but with the pH of the water typically near neutral, it was decided to investigate the surface phosphatase activities of a moss from a quite different environment, an acidic stream. Acid mine drainage water is low in soluble (inorganic) phosphate because of the precipitation by aluminium (Gross, 2001) and further removal of soluble inorganic phosphate occurs when the water rises above about pH 3.0 in waters rich in iron, leading to precipitation of iron “oxides”. A further problem in some drainage waters is the presence of arsenate at concentrations likely to interfere with inorganic phosphate uptake. The availability of, and ability to use, organic phosphates is therefore likely to be especially important in highly acidic waters derived from mine wastes, though not in some volcanic waters, where inorganic phosphate may be quite high (Brock, 1978). Warnstorfia fluitans (Hedw.) Loeske was chosen for the study reported here, because observations have been made on its occurrence in an acidic stream (Brandon Pithouse Stream) over a long period (Hargreaves et al., 1975; Gimeno et al., 1998).
A preliminary study at this site in 1999 (Ellwood et al., 2002) showed that PMEase and PDEase activities were low from January to July, but high for the rest of the year, when the tissue N:P ratio (by mass) exceeded 10. The aim of the project was to determine the factors influencing the PMEase and PDEase activity of a moss from a moss from a highly acidic stream. Sufficient data were obtained to permit comparison with R. riparioides and F. antipyretica, including seasonal changes. Axenic material was included in the study in order to ensure that key results were not an artefact due to the presence of periphytic bacteria or fungi.
Sample site
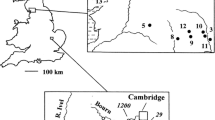
Brandon Pithouse Stream (NZ 222405, 210 m a.s.l.) is 8 km south-west of Durham City, N-E England. (The name of the stream relates to a deep coal mine several hundred meters from the site during the first half of the 20th century, but this probably had no influence on the stream.) The stream rises from a piped drain in a field and forms part of the catchment of Red Burn, a second-order tributary of the River Wear. The pH of the water at source was 2.6 and remained at this value for several hundred metres when first measured by B.A.W. in the late 1960s (Hargreaves et al., 1975). (Records for W. fluitans were listed under its synonym, Drepanocladus fluitans.) Physiological studies were reported on several algae from this site (Hargreaves & Whitton, 1976), but not on phosphate. At this period the first few metres of the stream were dominated by large growths protonema, almost certainly the protonema of W. fluitans, though further downstream this moss occurred as leafy shoots (B.A. Whitton, unpublished data). Towards the end of the 1990s the pH of the water started to rise due to activities of an opencast mining company in the catchment; it was about pH 3.5 in 1999 (Ellwood et al., 2002). Although the pH had risen further by 2002, Warnstorfia remained the most abundant phototroph in this stream ever since it was first studied.
Soils in the catchment are a mixture of Cambic Stagno Gleys and disturbed soils. Until largely removed by the mining company, the catchment contained seams of a high-grade coal with low sulphur content. It seems probable that the acidity of the stream results from oxidation of iron pyrite rather than sulphur in the coal.
Materials and methods
Field
A range of physical and chemical variables was recorded on each sampling, which was conducted monthly from March to December 1999 and then twice a month until February 2001. Although sampling was 2 months out of phase with the calendar year, for simplicity the data are reported as 1999 or 2000, unless there is a need to highlight information for the months which do not fall within that calendar year. At the source the entire flow of the spring is piped, which allows measurement by conversion of the time taken to fill a 5-dm3 plastic measuring cylinder (m−3 s−1). Temperature and conductivity were measured with a WTW (Wissenschaftliche Technische Werkstätten, Weilheim, Germany) model LF 320 meter with a TetraCon 325 probe. Calibration with BDH (BDH Chemicals Ltd, Poole, UK) standard solutions (36 and 1,413 μS cm−1) was carried out monthly. Dissolved oxygen was measured with a WTW model OXI 196 fitted with an EOT 196-probe. Calibration was done before each set of measurements using a WTW Oxical-S sleeve, with checks to ensure no membrane damage or air bubbles. The pH was measured in the field with a WTW meter (model pH 196 with a WTW SenTix electrode type 41–3). Calibration was performed prior to measurements using the standard BDH pH buffers: 4.0, 7.0, and 9.22. Values for mean pH were determined after antilogarithmic conversion.
Plastics and glassware were soaked in 2% (v/v) Decon® (Decon Laboratories Ltd., Hove, UK) solution for at least 24 h and rinsed four times in deionised water. All water samples for analysis of dissolved nutrients were passed at the site through 0.45 μm cellulose-acetate membranes, (previously soaked in 10% HCl and rinsed in deionised water) held in a Millipore Swinnex filter housing. All water samples for total nutrient analysis were collected in bottles primed with stream water. All moss shoots were taken from submerged plants in positions with obvious flow. The shoots were washed by repeat rinses in a plastic bag with water from the stream, then drained of excess water and placed in a sterile plastic bag, stored in a cool box with ice and transported to the laboratory.
Analysis
Water for P analysis was passed through a cation-exchange column (Amberlite resin, IR-120 (H), 14–52 mesh, particle size 0.30–1.18 mm) to remove iron and possibly other metals, which precipitate during digestion. Anions were not separated, since tests with standard additions indicated no interference.
Ammonium, nitrate and phosphate fractions were analysed using a Skalar Sanplus segmented flow analyser (Skalar Analytical, Breda, The Netherlands; 1995). The use of 3 and 5-cm path length cuvettes gave limits of detection of approximately 2 μg l−1 for N and P, respectively. TN, FON, TP, FOP were estimated following alkaline persulphate digestion of filtered and unfiltered samples (Langer & Hendrix, 1982; Lambert & Maher, 1994).
Oven-dried (105°C) and pre-weighed moss shoots (about 15 mg) were prepared for digestion by immersion overnight in a mixture of sulphuric acid, selenium, potassium sulphate and salicylic acid prior to digestion in order to prevent any loss of NO3–N during the digestion step (Novazmsky et al., 1983; Houba et al., 1989). Digestion procedures broadly followed the methods of Walinga et al. (1989) using a Skalar 5620/40 digestion block (5600 controller). The clear digest was diluted to 250 ml in deionised water and N (as ammoniacyl-N) and P (as phosphate-P) were then measured. In order to confirm that digestion was complete, standard reference material (Platyhypnidium riparioides = Rhynchostegium riaparioides: No. 61, individual No. 272; Community Bureau of Reference, ECC) of known N and P contents was also digested and analysed.
Culture
Standard sterile culture procedures were adopted for isolation of an axenic, clonal culture and subsequent experiments with this. Solutions unsuitable for autoclaving were passed through pre-sterilised membrane filters (Whatman, 0.2 μm). All vessels were soaked (24+ h) in a 2% v/v deionised water/Deacon 90 solution, stoppered (non-absorbent cotton wool), capped with foil and then autoclaved for 45 min at 1 × 105 Pa.
An axenic culture was obtained by adopting the following procedure for a number of capsules of the moss. A capsule, together with 1 cm of seta for ease of handling, was taken from field material. On return to the laboratory the capsule (with calyptra still in place) was surface sterilised by immersing it in a small volume of 70% v/v ethanol for 30 s in a sterile petri dish. It was then transferred to 20% v/v sodium hyperchlorite solution with 0.05% Tween-20 (polyoxyethylenesorbitan monolaurate) for 15 min. The capsule was next washed five times in autoclaved deionised water, followed by suspension in a drop of water and dissection to release the spores. The suspension was pipetted into several flasks of sterile liquid medium and incubated for a period of about a month whilst the spores developed through a prolific protonemal stage into young shoots. Individual shoots from each flask were then tested for the presence of contaminants with four different bacterial and fungal growth media. These plates were incubated at 20°C for at least 1 week. Those flasks which appeared to be contaminant-free were also checked carefully by light microscopy. Only shoots taken from flasks, which tests indicate were axenic, were used for the subsequent experiments.
The growth medium was buffered (pH 3.6) Chu 10 D, a version of the Chu No. 10 medium (Chu, 1942) modified by Grainger et al. (1989). Standard growth conditions were 20°C, with constant illumination from above (50 ± 3 μmol photon m−2 s−1). Each flask was shaken daily by hand to maintain the shoots submerged. Material for experiments was grown in Chu 10 D medium containing 4 mg l−1 N and 20 μg l−1 P for 7 days to standardise the intracellular P concentrations. The shoots were then cut to a 3-cm length and re-suspended in 250-ml Erlenmeyer flasks containing 100 ml of Chu 10 D medium.
The ability to use organic phosphate as a P source for growth was tested with glucose 1-phosphate representing PME, or DNA representing PDE (herring sperm, degraded free acid type IV, 400 Kbp fragments). These tests were compared with shoots incubated in medium with inorganic phosphate at the same P concentration. Three replicates for each treatment were used. The medium included 4 mg l−1 N (supplied as CaNO3) and 0.25, 0.5 or 1.0 mg l−1 P. The possibility of autodegradation of the organic phosphates was ruled out by FRP analysis of medium incubated under similar conditions, but without shoots.
Phosphatase assays
Assays were conducted using the methods of Turner et al. (2001) with one of the analogue substrates, pNPP, bis-pNPP or MUP. Assays on field material were always made within 24 h of collection (usually quicker). Each assay consisted of five 2-cm shoot apices, with four replicates for each measurement. The shoots were placed in glass vials, with 2.9 ml medium containing the appropriate buffer: pH 3.0–6.5; DMG; pH 7.0–8.0, HEPES; pH 8.5–10.0, glycine. The vials were placed in a shaking water bath (ca. 100 strokes min−1) at 20°C and about 20 μmol photon m−1 s−1 and left for 20 min to equilibrate. The assays were then made for 20 min using 0.1 ml substrate to give a final concentration of 100 μM. Assays with axenic material were performed on the entire excised shoot (approximately 3 cm).
To terminate the assay and develop the product colour 2.5 ml assay mixture was immediately removed and added to 0.25 ml terminator solution (Fedde & Whyte, 1990) in a test tube and mixed. This step was precisely timed to gain accurate representation of activity. Following analysis, the material was dried at 105°C for 24+ h and then weighed to 0.00001 g on a microbalance.
In order to establish the pH optimum, assays were conducted at 0.5 and 1.0 intervals within the pH range of 3.0–10.0 for 20 min, at 20°C using 100 μM substrate concentration. A study was also made of the short-term response to temperature over the range 0–50°C, using 10°C intervals. Each population was assayed for 20 min at 100 μm pNPP/bis-pNPP concentration, to pH 5.5 and 10°C intervals from 0 to 50°C.
Comparisons were made between the phosphatase activities of whole shoots (axenic) and 2-cm apical tips (field) in order to establish whether results for field material might be influenced by residual bacterial activity in the field material.
Results
Stream water
There were obvious changes in nutrient concentrations during the 2-year period (Fig. 1), with a peak in FOP occurring in late spring in both years, but otherwise no obvious similarity between the 2 years. Mean annual concentration of NH4–N was significantly higher (P < 0.01) in 2000 (303 ± 76 μg N l−1) than in 1999 (176 ± 60 μg N l−1). Overall, inorganic N was more important than organic N, whereas the converse was true for phosphate. The ratio of filtrable N:P (by mass) was almost always very high, whether or not organic N was included (Table 1).
Morphology
Axenic material was quite similar morphologically to the field population, but axenic shoots were less robust and there was rare branching. Further observations on the field population showed that branching was more frequent during periods of low flow. From the basal part of the apical tips of the axenic shoots used in the investigation a small number of rhizoids where also observed, however this was noted for a few shoots only. In the field the shoots occur in very dense beds (shoot number to unit area), which act as traps for detritus, a potential source of N and P. deriving from the stream water or the shoots themselves. This clumping of shoots, which was not present in the cultures, may also have an effect on morphology. General observations of cultured and field shoots, although there are no available data on the latter, suggest that growth (elongation) is faster in the culture shoots.
Tissue nutrients
Tissue N concentration was almost always higher in 2000 than 1999, with the mean value significantly higher (P < 0.01). In contrast, tissue P concentration did not show a consistent pattern through the year, though the average was not much less than in 1999 (Fig. 2). There was no relationship between tissue N and tissue P; the N:P ratio was mostly much higher in 2000 than 1999. Seasonal trends were not evident and the N and P contents were not significantly correlated (P > 0.05; Fig. 2).
The mean values for tissue N and P were lower in laboratory culture than in the field (Table 2). However, during growth in batch culture, the tissue P concentration was higher on day 3 than at the beginning of the experiment or at any time subsequently (Fig. 3). The effect was apparent even with 0.25 mg l−1 P in the medium, though most obvious with 1 mg l−1. As a consequence, the lowest N:P ratio occurred on day 3 (Table 2). However, by day 30 the tissue N:P was lower in culture than the mean value for field material (Table 2).
Influence of P source (Pi, glucose-1-phosphate, DNA) and concentration (0.25, 0.5, 1.0 mg l−1 P) on N and P concentrations, N:P ratio (by mass) and growth (shoot length) of shoots of axenic Warnstorfia. Incubation conditions 20°C and 50 μmol photon m−2 s−1. Shoots sampled on days 0, 3, 5, 10, 17, 24, 31
Use of organic substrates
The moss grew on glucose-1-phosphate and DNA almost as well as Pi (Fig. 3), with some indication of the response being more rapid in the sequence Pi ≥ G-1-P > DNA e.g. relative concentrations of tissue P on day 3. There was an obvious lag in the increase of shoot length with DNA at all three P concentrations tested, though the increase per unit time was similar later.
Phosphatase activities
PMEase and PDEase activities were detectable throughout the sampling and experimental period (Fig. 4); there were only a couple of measurements which approached the detection limit in the field. The majority of field measurements gave quantitatively similar PMEase results with pNPP and MUP, except during the latter part of 1999 and the first two months of 2000, which showed higher values using MUP (Fig. 4). PMEase hydrolysis of pNPP ranged from 11.4 to 101.5 μmol pNP g−1 d.wt h−1, whilst hydrolysis of MUP ranged from 10.1 to 174.5 μmol MU g−1 d.wt h−1. PDEase activity ranged from 2.0 to 97.8 μmol pNP g−1 d.wt h−1. In 1999, PMEase and PDEase activity showed low values in spring and early summer and high values from late summer to early winter. No pattern of seasonal change was evident in 2000. PMEase (pNPP and MUP) and PDEase activities were strongly correlated (pNPP and bis-pNPP, r = 0.67, MUP and bis-pNPP, r = 0.73; P < 0.01 in both cases).
The axenic material showed higher PMEase and PDEase activities when grown with organic P than with Pi (Fig. 3). PMEase activity showed a slight increase when PDE (i.e. DNA) was the source and the converse was also true. However, the increase in PDEase activity was greater when shoots were cultured with DNA than with glucose-1-phosphate (Fig. 3).
The influence of environmental factors was tested on field material during a standard 20-min assay. There was a linear increase in PMEase and PDEase activities between 0 and 50°C (r values; PMEase 0.96, PDEase 0.94: data not shown). Above 60°C there was visible cell damage at the end of the assay, while activity at 70°C was less than at 60°C. The Q 10 value (ratio of activity at a given temperature t and the activity at t + 10) peaked between 10 and 20°C and then decreased as temperature increased. The range of Q 10 values for PMEase (1.0–3.0) was larger than PDEase (1.4–2.11), showing PMEase to be more influenced by temperature.
An experiment to determine any possible effect of light showed that there was a marked effect on PMEase but not on PDEase activity. In the dark (0 μmol photons m−2 s−1) PMEase activity was nearly 2-fold higher than in natural light (13 μmol photons m−2 s−1). There was no significant change in PDEase activity (P < 0.05) under the light conditions tested. However, a repeat experiment using another sample of field material and comparing the effect dark and low level natural light showed the opposite response for PMEase activity, with activity in the dark about 60% of that in the light.
The pH profile for PMEase and PDEase activities was similar for field and axenic material, including the optimum pH; however, the optimum was apparently slightly higher for PDEase (5.5) than PMEase (5.0) (Fig. 5). There was no visible cell damage at either of the pH limits tested (3.0, 10.0). A repeat experiment using 1 μM MUP showed no difference in the form of the response curve from that of 100 μM MUP over most of the pH range, but there were marked differences at pH 3.0, especially for PDEase activity, where there was a second peak at pH 3.0 using 1 μM substrate.
PMEase and PDEase activities showed a hyperbolic relationship with substrate activity (Fig. 6). Linear transformations of the data gave mean K m values of 321 and 138 μM, and V max values of 318 and 117 μmol pNP g−1 d.wt h−1 for PMEase and PDEase, respectively. These values indicate that potential reaction rate of PMEase is higher, but with a lower substrate affinity, than PDEase. The K m and V max values for PMEase of the axenic population were lower than the field material (87.2 μM and 35.8 μmol pNP g−1 d.wt h−1, respectively).
There was no significant correlation between phosphatase activities and stream water nutrient status (P > 0.05; data not shown). However, there were significant inverse relationships between tissue N and stream water P and between tissue P and stream water N (Table 3). Tissue N was also significantly positively related to stream water NO3–N. In the seasonal study the PMEase and PDEase activities were inversely related to tissue P concentration and PDEase was inversely related to tissue N concentration. The only significant correlation with tissue N:P was PDEase activity of axenic shoots cultured in Pi (Table 4). Activity of the axenic shoots showed inverse relationships with cellular N and P and positive relation with cellular N:P.
Discussion
Filtrable organic phosphate concentration was higher than that of filtrable inorganic phosphate in all 24 months. The large peaks of organic phosphate in the spring of both years are similar to the peaks reported for other streams in northern England at this time of year (e.g. Livingstone & Whitton, 1984). The study showed not only that W. fluitans can produce surface enzymes hydrolysing organic phosphates, but that these permit the organism to obtain phosphate from these sources. It seems less likely that filtrable organic N is important, because the aqueous N:P ratio of the water was usually very high even when only inorganic N is considered. The differing hydrolytic rates of the two phosphomonoesters substrates (pNPP and MUP) suggest that there may be a range of specific PMEases or a broadly specific PMEase with various substrate affinities. Differences in response to pNPP and MUP have been reported in some, but not all, studies of surface phosphatase activity of aquatic phototrophs (Whitton et al., 2005).
The response of phosphatase activities to environmental factors was quite similar in field and axenic material, suggesting that any influence of periphytic bacteria was negligible compared with activity of the moss. Marked changes were observed in PMEase and PDEase activities of the field population during the 2-year period. PDEase activity was high from late summer to early winter 1999 at a time when the tissue N:P ratio was especially high, suggesting that the increased activity was a response to P limitation. PMEase assayed with MUP was also high at this time, but not that assayed with pNPP, raising the possibility that a further PMEase becomes active under some conditions. However, the greater activity shown with MUP was apparent during a period when N:P was relatively low (Fig. 3), rather than the converse as might be expected. Both field and laboratory results indicate that PDEase activity is often as high as PMEase activity assayed with pNPP, though less in the stream material during the period when PMEase activity assayed with MUP was very high. Upland populations of the aquatic moss F. antipyretica sometimes also show higher PDEase than PMEase activity (Christmas & Whitton, 1998b). This contrasts with the shoots of terrestrial mosses, where (surface) PDEase activity is usually less and sometimes (Palustriella commutata var. commutata and Polytrichum commune) absent (Turner et al., 2001). As some terrestrial mosses possess rhizoids penetrating the substratum, perhaps these provide a site for PDEase activity, which seems especially likely in a species such as P. commune, which possesses primitive conducting tissue (Proctor, 2000). Rhizoid development on Warnstorfia was so sparse it seems unlikely these contributed much to the observed PDEase activity. The effect of light on phosphatase activity indicated the importance of light for PMEase activities of Warnstorfia, but more detailed study is required on the influence of other factors on its response to light. On the other hand, it seems that PDEase activity is not light dependant.
There appears to be a threshold value for tissue P concentration, below which activity starts to increase markedly. This was about 2 mg P g−1 d.wt in field material and 1.5 mg P g−1 d.wt in culture, perhaps reflecting the differences in average tissue P concentration between field and cultured material (Table 2). Further study is needed to clarify what factors influence this. Possibilities include the differing ionic environment, since this may influence nutrient uptake (Glime & Vitt, 1984) and the presence of a current passing water over the moss in the field. The higher tissue N of the field population during the second year may be influenced by the changes in the aquatic environment that occurred as a result of mining activity within the catchment area. Notably, there was a greater contribution of NH4–N to TIN, which rose from 13% to 37%, and also an increase in water pH (see Fig. 1 and Table 1). As the apical tips of aquatic bryophytes generally reflect their chemical environment (Brown, 1984) then the increase in tissue N could be expected given the change in environmental conditions. Warnstorfia appears to be slightly less responsive to P limitation than F. antipyretica and R. riparioides (Christmas & Whitton 1998a), where PMEase activity increased when the tissue P concentration fell below 3 mg P g−1 d.wt.
As already reported (for 1999: Ellwood et al., 2002), there were no significant relationships between phosphatase activity of the field population and any of the measured ambient variables. If some feature of internal P content is the factor involved in inducing synthesis of PMEase and PDEase, a close relationship might not be expected because activity would reflect aqueous P concentration over a period representing the response time of the moss to changing P status. A lag in the response would be expected, as with PMEase of Rhynchostegium riparioides (Christmas & Whitton, 1998a). The results contrast with the situation for the stream alga Stigeoclonium, where PMEase activity was inversely related to tissue P measured on the same day (Gibson & Whitton, 1987). However, Stigeoclonium grows much more rapidly than Warnstorfia, so the prior period of days influencing activity is much less. Another difference might be internal storage and translocation of nutrients, which is reported for some mosses, though mostly with respect to N (Bates, 1994; 2000; Wells & Brown, 1996; Eckstein & Karlsson, 1999). However, this has not yet been investigated in Warnstorfia.
Evidence from the present study suggests that internal phosphate concentration is involved in regulating phosphatase activity of Warnstorfia. Tissue N and P concentrations of field shoots were inversely related to the aqueous P and N, respectively (Table 4). In the batch culture experiment on the influence of initial P concentration, the lowest aqueous P concentration led to the lowest tissue P concentration and the highest PMEase and PDEase activities. However, the external P source also has an effect, because culturing with glucose-1-phosphate led to slightly higher PMEase and culture with DNA to markedly higher PDEase. The enzymes are essential for the moss to make use of the organic phosphate and, at least in the case of PDEase, the high level of activity cannot simply be a reflection of reduced internal tissue P concentration, because the effect was already pronounced by day 3.
It is difficult to assess the extent to which PDEase activity in Warnstorfia is a response to tissue P limitation or to the presence of suitable substrate in the environment, because both factors appear to be important. The monthly sampling in 1999 showed PDEase activity to increase during periods of low FOP, but this may simply be due to insufficient sampling as within-month variation in 2000 showed large differences in activity. In the culture experiment PDEase activity doubled within the first three days of addition of a PDE substrate, but subsequently decreased again as the tissue P concentration increased.
The high temperature optimum in short-term assays of phosphatase activities of Warnstorfia is similar to that found in other studies of surface phosphatase (Whitton et al., 2005). The pH optimum of PMEase activity of Warnstorfia (5.0) is also similar to that of other aquatic mosses, such as F. antipyretica (5.5: Christmas & Whitton, 1998b) and R. riparioides (5.5: Ellwood, 2002). The value of pH 5.0 is within the range sometimes found in the stream water during the present study, but much higher than the value (2.6) occurring from at least the mid-1960s (probably much earlier) and the early 1990s. There was no indication that a substrate concentration (1 μM) more like that occurring in the field leads to an optimum for PMEase activity more like field conditions, as occurs with some other organisms (Whitton et al., 2005). There appeared to be a second peak in PDEase activity at pH 3.0 with 1 μM, but not 100 μM bis-MUP, so perhaps Warnstorfia is particularly efficient at using diesters at low pH. Protonema occurred only sparsely during the present study, but this growth stage dominated the uppermost part of the stream in the 1960s (B.A. Whitton, unpublished data). It would of interest to know whether its phosphatase activities respond differently to pH.
It was possible to apply linear transformations of the Michaelis–Menton equation to the data as for pure enzyme systems. Rates of PMEase and PDEase activity in the natural population were similar, ranging from 303.9 to 101.5 and 30.8 to 97.8 μmol pNP g−1 d.wt h−1, respectively. However, affinity for PME was 3-fold lower than for PDE. It was suggested by Christmas and Whitton (1998b) that the low affinity of PDEase of F. antipyretica sampled from an upland stream could be important for utilising pulses of high concentrations of PDE, rather than continuous low concentrations. If so, the high affinity of PDEase recorded here may be important for continuously low PDE concentrations. The axenic population had much lower PMEase K m values than the field population. As there was no organic P source in the standard medium, a reduction in K m would fit with the theory of affinity of a species adjusting to the type and concentration of the substrate.
Abbreviations
- bis-pNPP:
-
bis-para-nitrophenyl phosphate
- DMG:
-
3-dimethylglutaric acid
- FON:
-
filtrable organic nitrogen
- FOP:
-
filtrable organic phosphorus
- FRP:
-
filtrable reactive phosphorus
- HEPES:
-
N-(2-hydroxyethyl)-piperazine-N′-2-ethanesulfonic acid
- MU:
-
methylumbelliferone
- MUP:
-
methylumbelliferyl phosphate
- pNP:
-
para-nitrophenol
- pNPP:
-
para-nitrophenyl phosphate
- PMEase:
-
phosphomonoesterase
- PDEase:
-
phosphodiesterase
- Pi:
-
inorganic phosphate-P
- TIN:
-
total inorganic nitrogen
- TN:
-
total nitrogen
- TP:
-
total phosphorus
References
Bates, J. W., 1994. Responses of the mosses Brachythecium rutabulum and Pseudoscleropodium purum to a mineral nutrient pulse. Functional Ecology 8: 686–692.
Bates, J. W., 2000. Mineral nutrition, substratum ecology, and pollution. In Shaw A. J. & B. Goffinet (eds), Bryophyte Biology. Cambridge University Press, UK: 248–311.
Brock, T. D., 1978. The habitats. In Brock T. D. (eds), Thermophilic Microorganisms and Life at High Temperatures. Springer-Verlag, New York, 12–38.
Brown, D. H., 1984. Uptake of mineral elements and their use in pollution monitoring. In Dyer A. F. & J. G. Duckett (eds), The Experiment Biology of Bryophytes. Academic Press, London: 229–255.
Christmas, M. & B. A. Whitton, 1998a. Phosphorus and aquatic bryophytes in the Swale–Ouse river system, north-east England. 1. Relationship between ambient phosphorus, internal N:P ratio and surface phosphatase activity. Science of the Total Environment 210/211: 389–399.
Christmas, M. & B. A. Whitton, 1998b. Phosphorus and aquatic bryophytes in the Swale–Ouse river system, north-east England. 2. Phosphomonoesterase and phosphodiesterase activities of Fontinalis antipyretica. Science of the Total Environment 210/211: 401–409.
Chu, S. P., 1942. The influence of the mineral composition of the media on the growth of planktonic algae. 1. Methods and culture media. Journal of Ecology 30: 284–325.
Eckstein, R. L. & P. S. Karlsson, 1999. Recycling of nitrogen among segments of Hylocomium splendens as compared with Polytrichum commune: implications for clonal integration in an ectohydric bryophyte. Oikos 86: 87–96.
Ellwood, N. T. W., 2002. Factors Influencing Phosphatase Activities of Mosses in Upland Streams. Ph.D. Thesis. University of Newcastle-upon-Tyne, UK.
Ellwood, N. T. W., S. M. Haile & B. A. Whitton, 2002. Surface phosphatase activity of the moss Warnstorfia fluitans as an indicator of the nutrient status of an acidic stream. Verhandlung Internationale Vereinigung Limnologie 28: 620–623.
Fedde, K. N. & M. P. Whyte, 1990. Alkaline phosphatase (tissue-non specific isoenzyme) is a phosphoethanolamine and pyridoxal-5′phosphate ectophosphatase: normal and hypophosphatasia fibroblast study. American Journal of Human Genetics 47: 767–775.
Garcia-Alvaro, M. A., J. Martinez-Abaigar, E. Nuñez–Olivera & N. Beaucourt, 2000. Element concentrations and enrichment ratios in the aquatic moss Rhynchostegium riparioides along the River Iregua (La Rioja, Northern Spain). Bryologist 103: 518–533.
Gibson, M. T. & B. A. Whitton, 1987. Hairs, phosphatase activity and environmental chemistry in Stigeoclonium, Chaetophora and Draparnaldia (Chaetophorales). British phycological Journal 22: 11–22.
Gimeno, C., F. Puche & B. A. Whitton, 1998. Effect of pH on shoots and protonema of Warnstorfia fluitans (Hedw.) Loeske. Boletin Sociedad Español Briologia 13: 13–17.
Glime, J. M. & D. H. Vitt, 1984. The physiological adaptations of aquatic musci. Lindbergia 10: 41–52.
Grainger, S. L. J., A. Peat, D. N. Tiwari & B. A. Whitton, 1989. Phosphomonoesterase activity of the cyanobacterium (blue-green alga) Calothrix parietina. Microbios 59: 7–17.
Gross, W., 2001. Ecophysiology of algae living in highly acidic environments. Hydrobiologia 433: 173–180.
Hargreaves, J. W., E. J. H. Lloyd & B. A. Whitton, 1975. Chemistry and vegetation of highly acidic streams. Freshwater Biology 5: 563–576.
Hargreaves, J. W. & B. A. Whitton, 1976. Effect of pH on growth of acid stream algae. British Phycological Journal 11: 215–223.
Houba V., W. van Vark, I. Walinga & J.J. van der Lee, 1989. Plant Analysis Procedures (Part 7, Chapter 2.2). Department of Soil Science and Plant Analysis. Wageningen, The Netherlands.
Lambert, D. & W. Maher, 1994. An evaluation of the efficiency of the alkaline persulphate digestion method for the determination of total phosphorus in turbid waters. Water Research 29: 7–9.
Langer, C. L. & P. F. Hendrix, 1982. Evaluation of a persulphate digestion method for particulate nitrogen and phosphorus. Water Research 16: 1451–1454.
Livingstone, D. & B. A. Whitton, 1984. Water chemistry and phosphatase activity of the blue-green alga Rivularia in Upper Teesdale streams. Journal of Ecology 72: 405–421.
Novozamsky, I., V. J. G. Houba, R. van Eck & W. van Vark, 1983. A novel digestion technique for multi-element analysis. Communications in Soil Science and Plant Analysis 14: 239–249.
Press, M. C. & J. A. Lee, 1983. Acid phosphatase activity in Sphagnum species in relation to phosphate nutrition. New Phytologist 93: 567–573.
Proctor MCF. 2000. Physiological ecology. In Shaw A. J. & B. Goffinet (eds), Bryophyte Biology. Cambridge University Press, Cambridge, 225–247.
Skalar, 1995. The Sanplus Segmented Flow analyser: Soil and Plant Analysis. Instruction Manual: Publ. No. 0102003. Skalar Analytical, Breda, The Netherlands.
Steinman, A. D., 1994. The influence of phosphorus enrichment on lotic bryophytes. Freshwater Biology 31: 53–63.
Steinman, A. D. & H. L. Boston, 1993. The ecological role of aquatic bryophytes in a woodland stream. Journal of the North American Benthological Society 12: 17–26.
Turner, B. L., R. Baxter, N. T. W. Ellwood & B. A. Whitton, 2001. Characterization of the phosphatase activities of mosses in relation to their environment. Plant Cell and Environment 24: 1165–1176.
Turner, B. L., R. Baxter, N. T. W. Ellwood & B. A. Whitton, 2003. Seasonal phosphatase activities of mosses from Upper Teesdale, northern England. Journal of Bryology 25: 203–214.
Walinga, I., W. van Vark, V. J. G. Houba & L. L. van der Lee, 1989. Plant Analysis Procedures, Part 7. Department of Soil Science and Plant Nutrition, Wageningen Agricultural University, The Netherlands, 138–141 pp.
Wells, J. M. & D. H. Brown, 1996. Mineral nutrient recycling within shoots of the moss Rhytidiadelphus squarrosus in relation to growth. Journal of Bryology 19: 1–17.
Whitton, B. A., E. Clegg, M., Christmas, J. J. Gemmell & P.J. Robinson, 2002 Development of Phosphastase Assay for Monitroing Nutrients in Rivers – Methodology Manual for Measurement of Phosphatase Activity in Mosses and Green Algae. Environment Agency of England and Wales STRE106-E-P 53 pp. (Distributed by WRc, Frankland Road, Swindon, Wilts SN5 8YF, UK).
Whitton, B. A., A. M. Al-Shehri, N. T. W. Ellwood & B. L. Turner, 2005. Ecological aspects of phosphatase activity in cyanobacteria, eukaryotic algae and bryophytes. In Turner B. L., E. Frossard & D. S. Baldwin, (eds), Organic Phosphorus in the Environment. CAB International, Wallingford, UK: 205–241.
Acknowledgements
N.T.W.E. was supported by a studentship from the UK Engineering and Physical Sciences Research Council. The authors are also most grateful to H.J. Banks Ltd for other financial support and Mr C. Harle for access to the site.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: S. M. Thomaz
Rights and permissions
About this article
Cite this article
Ellwood, N.T.W., Whitton, B.A. Phosphatase activities of the aquatic moss Warnstorfia fluitans (Hedw.) Loeske from an acidic stream in North-East England. Hydrobiologia 575, 95–107 (2007). https://doi.org/10.1007/s10750-006-0283-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-006-0283-0