Abstract
1,4-Dioxane (dioxane) is relatively recalcitrant to biodegradation, and its physicochemical properties preclude effective removal from contaminated groundwater by volatilization or adsorption. Through this microcosm study, we assessed the biodegradation potential of dioxane for three sites in California. Groundwater and sediment samples were collected at various locations at each site, including the presumed source zone, middle and leading edge of the plume. A total of 16 monitoring wells were sampled to prepare the microcosms. Biodegradation of dioxane was observed in 12 of 16 microcosms mimicking natural attenuation within 28 weeks. Rates varied from as high as 3,449 ± 459 µg/L/week in source-zone microcosms to a low of 0.3 ± 0.1 µg/L/week in microcosms with trace level of dioxane (<10 µg/L as initial concentration). The microcosms were spiked with 14C-labeled dioxane to assess the fate of dioxane. Biological oxidizer-liquid scintillation analysis of bound residue infers that 14C-dioxane was assimilated into cell material only in microcosms exhibiting significant dioxane biodegradation. Mineralization was also observed per 14CO2 recovery (up to 44 % of the amount degraded in 28 weeks of incubation). Degradation and mineralization activity significantly decreased with increasing distance from the contaminant source area (p < 0.05), possibly due to less acclimation. Furthermore, both respiked and repeated microcosms prepared with source-zone samples from Site 1 confirmed relatively rapid dioxane degradation (i.e., 100 % removal by 20 weeks). These results show that indigenous microorganisms capable of degrading dioxane are present at these three sites, and suggest that monitored natural attenuation should be considered as a remedial response.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
1,4-Dioxane (dioxane) is a groundwater contaminant of emerging concern due to its potential carcinogenicity (IARC 1999) and its recently discovered widespread occurrence at thousands of sites impacted by chlorinated solvent releases in the US and abroad (Mohr et al. 2010). Dioxane was historically used as a stabilizer for industrial solvents, typically 1,1,1-trichloroethane (1,1,1-TCA), thus explaining this common co-occurrence (Anderson et al. 2012). However, unlike chlorinated solvents, dioxane is hydrophilic and highly mobile in groundwater, and it tends to form much larger plumes at probably thousands of contaminated sites that represent a significant remediation challenge (Mohr et al. 2010).
EPA risk assessments indicate that the drinking water concentration representing a 10−6 cancer risk level for dioxane is 0.35 μg/L (EPA 2010). Although there is no federal regulatory standard for dioxane, stringent drinking water advisory levels have been widely performed in various states. For instance, the California Department of Health (CDPH) has a drinking water notification level of 1 µg/L and a response level of 35 µg/L (California EPA 2014). Moreover, recent national public water quality surveys for the U.S. EPA’s Unregulated Contaminant Monitoring Rule 3 (UCMR3) analyzed about 4,000 water samples collected nationally in 2013, and 12.6 % of them detected dioxane (i.e., >0.07 µg/L) (EPA 2013).
Remediation of dioxane-impacted groundwater often relies on pump and treat approaches using advanced oxidation processes (e.g., UV and hydrogen peroxide) (EPA 2006; DiGuiseppi and Whitesides 2007; Brode et al. 2005). However, in addition to the relatively high cost of such approaches, turbidity of the aqueous streams and hydroxyl radical scavenging significantly inhibit removal efficiencies (Andreozzi et al. 1999). In situ chemical oxidation (ISCO) of dioxane (e.g., with permanganate or persulfate) has also been tested at various sites (Dombrowski et al. 2010; Cronk 2008; Houston et al. 2009). Whereas ISCO may treat source zones, including co-occurring chlorinated solvent dense non-aqueous phase liquids (DNAPLs), it also suffers from site-specific inefficient production of hydroxyl radicals, scavenging of radicals by aquifer minerals or organic matter, and aesthetic impacts to the groundwater (e.g., pink coloring by permanganate) (Huling and Pivetz 2006). Furthermore, neither pump and treat nor ISCO are cost-effective for the remediation of large and dilute dioxane plumes.
Monitored natural attenuation (MNA), which relies primarily on intrinsic bioremediation, is often the most cost-effective approach to manage trace levels of priority pollutants (Macdonald 2000). Although dioxane is generally considered recalcitrant to biodegradation, recent findings by our lab and others suggest that indigenous bacteria that can degrade dioxane might be more widespread than previously assumed (Vainberg et al. 2006; Kim et al. 2007; Li et al. 2010b; Sei et al. 2010; Chiang et al. 2012; Li et al. 2013a, b). Previous research in our lab demonstrated the presence of dioxane degradation and the potential for natural attenuation in microcosms prepared with groundwater and soil from an impacted site in Alaska (Li et al. 2010b). Biodegradation followed zero-order kinetics (i.e., linear decrease in dioxane concentration versus time), indicating lack of significant microbial growth (as expected given the low concentration of dioxane available) and saturated enzymes kinetics that are characteristic of oligotrophic bacteria with high affinity for dioxane (Alvarez and Illman 2006). Previous studies have also surveyed the dioxane degradation potential in various environmental compartments, such as river water, activated sludge, drainage soil, and garden soil samples (Sei et al. 2010). One activated sludge sample exhibited co-metabolism of dioxane (100 mg/L) in the presence of an equal amount of tetrahydrofuran (THF), which is a structural analog of dioxane, whereas dioxane was fully consumed in five of the six drainage soil samples without THF. This illustrates that different systems may harbor different types of dioxane degraders, but dioxane degradation capability is not ubiquitous in nature.
In this work, we conducted microcosm studies that mimic in situ conditions at various monitoring wells from three sites in California (i.e., using local groundwater and aquifer material, and incubated in the dark at site-specific pH) to determine the potential for the indigenous microorganisms to degrade dioxane and assess the spatial variability of degradation kinetics and patterns along the dioxane plume. This is one of the few available studies to illustrate natural attenuation/biodegradation of dioxane in groundwater at a wide range of concentrations (10–10,000 µg/L).
Materials and methods
Site information and sample collection
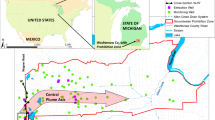
In June 2012, soil cores and groundwater samples were collected from three industrial sites in California (Fig. 1). Site 1 was impacted by 1,1,1-TCA and 1,1-dichloroethene (1,1-DCE) (up to 58 and 4.5 mg/L, respectively). Groundwater dissolved oxygen (DO) ranged from non-detect to 2.9 mg/L. Site 2 was exposed to dioxane from an up-gradient unknown source and contained a small dioxane plume. Downhole DO was 2.3 mg/L. Site 3 aquifer contained a 0.2 mile-long-plume (Fig. 1) in a sandy/silty layer. Downhole DO ranged from 0.1 to 3.1 mg/L. No discernible relationship between DO and distance from the contaminant source was observed at all three sites. Groundwater at all sites had neutral pH.
Groundwater samples were collected using new sterile disposable bailers, and then stored in 5-gallon clean, sterile plastic cubes at 4 °C with headspace minimized. Soil samples were obtained either during well installation by driving a split spoon sampler lined with brass tubes into undisturbed soil or by collection of well bottom sediment during groundwater sample collection.
Microcosm studies
A significant decrease in dioxane concentrations in the groundwater at Site 1 has been observed over last few decades. However, the extent to which biodegradation (rather than dilution or migration) contributes to these decreases is unknown. Moreover, the other two sites have shown relatively stable dioxane concentrations in their monitoring wells. To verify that biodegradation was occurring and assess the associated patterns, the following microcosm sets were prepared.
-
(1)
Biologically active microcosms prepared with groundwater from monitoring wells near the source and in the leading edge of the plume, with dioxane concentrations ranging from 8 to 46,000 µg/L;
-
(2)
Background microcosms (i.e., sample 1–6 and 3–5) prepared with samples from locations up-gradient of the source area with minimal or no impact by dioxane, to bench-mark biodegradation patterns for microcosms prepared with plume samples, which might be acclimated and degrade dioxane faster or with a shorter lag time;
-
(3)
Microcosms prepared with samples from near-source wells of each site and inoculated with the archetype dioxane degrader Pseudonocardia dioxanivorans CB1190 (Parales et al. 1994) to serve as positive controls; and
-
(4)
Sterile negative controls to discern potential dioxane abiotic losses and to provide a baseline for mineralization studies.
For each sampling location, triplicate microcosms were prepared in 250-mL amber glass bottles amended with 150 mL of groundwater plus 50 g of aquifer material (saturated soil or suspended sediments). For monitoring wells 1-1 and 1-2, three layers of soil core samples were obtained, including an upper sand layer (S), middle silt layer (M), and lower sand layer (D). However, groundwater samples were only collected in the upper and lower layers. No groundwater was obtained from the middle layers. Hence, 75 mL groundwater from both the S and D layers were added into these microcosms mimicking dioxane natural attenuation at the middle layers (designated as 1-1M and 1-2M). Two up-gradient background microcosms (i.e., 1-6 and 3-5) only received groundwater with no solid mass amended. For all other monitoring well locations, suspended sediments were obtained from the slurry bottom in the plastic cubes, and condensed by centrifugation. However, microcosms 1-5 were only amended with 10 g of sediment per bottle due to the limited amount obtained. For discussion purposes, the area of highest known groundwater concentrations were assumed to be the source areas, the authors have no specific knowledge of the location of sources.
Negative (sterile) controls were prepared using samples from the monitoring well experiencing the highest contamination level for each site, including 1-1D, 2-1, and 3-1, respectively. Samples were autoclaved separately at 121 °C for 20 min and poisoned with HgCl2 (200 mg/L), to discern biodegradation from potential abiotic losses. P. dioxanivorans CB1190 is a well-characterized Gram-positive actinomycete that is capable of degrading dioxane as sole carbon and energy source (Parales et al. 1994). Therefore, positive controls were prepared with aquifer material (50 g) and groundwater (120 mL) from the same monitoring wells as negative controls, and inoculated with 30 mL of ammonia mineral salt (AMS) media (Li et al. 2010b) containing CB1190 (10 mg/L as total protein concentration) to enhance the biodegradation of dioxane and evaluate whether degradation inhibitors are present at the sites.
All microcosms were incubated for 28 weeks in the dark at the room temperature (i.e., 24 ± 3 °C) under quiescent conditions. At preselected times (e.g., monthly), 0.5-mL liquid samples were removed from the microcosms using 1-mL gas-tight syringes tipped with 25-gauge 1.5-inch needles (Becton Dickson and Co.). Samples were filtered with a 0.2-μm syringe nylon filter (Fisher Scientific, Inc.) and stored at 4 °C until analyzed by GC/MS-SIM combining a novel frozen micro-extraction method developed in our lab (Li et al. 2011). A series dilution with a range from 12.5 to 800 µg/L was used for each calibration. During sample preparation for analysis, samples of predicted concentrations exceeding 1,000 µg/L were diluted 10- or 100-fold to achieve a more precise mass analysis. The detection limit was approximately 1 µg/L.
The zero-order degradation rate was calculated as the slope of the linear regression between incubation time and monitored dioxane concentration data above the detection limit. Similarly, the exponential decay constant by CB1190 in positive controls was estimated by fitting with first-order biodegradation kinetic regime (Alvarez and Illman 2006). Statistical significance among treatments was evaluated by student t test.
Fate of 14C-dioxane
For improved determination of the fate of dioxane (e.g., % mineralization to CO2 and % conversion to bound residue, including possible incorporation into biomass), 100 µL of uniformly 14C-labeled 1,4-dioxane (10-µCi/mL stock solution) were added in the aqueous solution prior to capping the microcosms, resulting in 1 µCi as total radioactivity and 12.8 μg/L as the spiked concentration of 14C labeled dioxane per microcosm. The 14C-labeled 1,4-dioxane was custom-synthesized in August, 2010 by ChemDepo, Inc. (Camarillo, CA) with the purity greater than 99 %. The diluted stock solution was kept in dark at −80 °C to minimize the potential for unintended chemical transformation and decomposition. As shown in Fig. 2a, a test tube containing 1 mL of 1 N NaOH was placed inside the microcosms to trap 14CO2 in the headspace, as previously described (Miller et al. 1996). These test tubes were replaced with new sterile ones containing fresh NaOH during every sampling period of this study. Teflon tapes were wrapped around the screw-top threading to tighten the seal and minimize gas release.
14C in the aqueous solution and 14CO2 in the headspace (trapped in NaOH) were analyzed by mixing 1 mL of filtered water sample/NaOH solution with 10 ml of 14C-scintillation cocktail (UN2924, R.J. Harvey Instrument, NY) and measured by Liquid Scintillation Counter (LSC) using a Beckman LS 6500 (Beckman Instrument Inc., CA). At week 28, one replicate of each treatment was sacrificed, and the biomass together with the aquifer materials was acquired by centrifugation at 5,000 rpm for 5 min. The solids were further rinsed with sterile Millipore water three times to remove unbound 14C-labeled compounds. Then, 1 g of the centrifuged wet solids was combusted at 915 °C for 2 min by an OX600 Biological Oxidizer (R.J. Harvey Instrument, NY) in duplicates, and the effluent was carried out by N2 gas and effervesced through a glass U-tube containing 15 mL of scintillation cocktail. The recovery of the total 14C by this combustion method was determined to be 95.2 ± 2.8 % by spike tests (n = 5). Similar recovery ranges are commonly reported for biological oxidizer-liquid scintillation (BO-LS) analysis (Doucette et al. 2005; Wang et al. 2009). After combustion, the radioactivity in the cocktails was then measured by LSC, and referred as the 14C bound residue (Wang et al. 2009; Li et al. 2010a; Beckles et al. 2007; Yang et al. 2014).
Replication of 1-1S microcosms with new microcosm design
In order to confirm our observation that relatively fast dioxane degradation occurred in these microcosms, groundwater samples were collected again from sampling location 1-1S in March 2013. To achieve a better recovery of 14C, a new microcosm design (Fig. 2b) was selected that utilized a larger NaOH trap attached to the inside-bottom of a glass jar with a lid capable of sealing gas generating media and eliminating the leakage of generated 14CO2. The trap is a 20 mL capless glass vial that accommodates 10 mL of 1 N NaOH solution, and offers easy access for sampling and NaOH replacement during each sampling.
Selected microcosm assays exhibiting relatively fast dioxane biodegradation were repeated for confirmatory purposes. These microcosms were prepared with 75 mL of the newly collected groundwater, and 25 g of aquifer material from sealed brass tubes, which are half of the amount we added in previously prepared microcosms. Then, 1 µCi of 14C-labeled dioxane was spiked into the aqueous phase.
Results and discussion
Evidence of dioxane biodegradation
Significant dioxane removal in biologically active microcosms, but not in sterile controls, demonstrates dioxane biodegradation (Fig. 3). Within 28 weeks of incubation, 12 of 16 microcosms mimicking natural attenuation exhibited significant dioxane removal. This was corroborated in a separate study quantifying the abundance of thmA/dxmA genes, which encode for the active site (i.e., the large α hydroxylase subunit) of THF/dioxane monooxygenases (Li et al. 2013a). Previous molecular and enzymatic studies demonstrated the crucial role of these oxygenases in initiating dioxane metabolism (Li et al. 2013b; Thiemer et al. 2003; Sales et al. 2013).
Degradation (close circles) and cumulative mineralization (open circles) of dioxane in microcosms mimicking natural attenuation and positive and negative controls. Dioxane concentrations lower than the detection limit (i.e., 1 µg/L) were denoted as 0 in the graphs. Error bars represent ± one standard deviation from the mean of triplicate incubations
For Site 1, dioxane was fully depleted by the indigenous microorganisms in all six microcosms prepared with samples from three different geological layers of monitoring well 1-1 and 1-2 till week 24 (Fig. 3). Notably, source-zone microcosm set 1-1S experienced the highest dioxane degradation rates (3,449 ± 459 µg/L/week), suggesting the high abundance of indigenous dioxane degraders. This was verified by qPCR analysis of thmA/dxmA to estimate the concentration of dioxane degrading microorganisms harboring THF/dioxane monooxygenases (Li et al. 2013a). Before microcosm 1-1S were terminated, the concentrations of thmA/dxmA genes reached 107.7±0.3 copies/g soil, which represents approximately 15 % of the total bacteria (assessed by total 16S-rRNA) in the microcosms. This is consistent with the high dioxane biodegradation activity observed in these microcosms. In contrast, no dioxane removal was detected in microcosm sets 1-4, 1-5 and 1-6 (background control), suggesting the absence or low population of dioxane degraders down gradient from the source zone. The absence of dioxane degradation activity in these microcosms was corroborated by the lack of detection of thmA/dxmA (<7,000 copies of thmA/dxmA genes/g soil) (Li et al. 2013a).
Although the contamination source is uncertain, dioxane was completely degraded by week 24 in both microcosms prepared with samples from Site 2. Similarly, significant dioxane degradation was observed in Site-3 microcosms prepared with samples from the source zone (i.e., 3-1 and 3-2) and diluted down-gradient area (i.e., 3-3 and 3-4), but not in up-gradient controls (i.e., 3-5). In addition, dioxane degradation rates greatly decreased with increasing distance between the sampling location and the source of Site 3 (Table 1). The highest dioxane degradation activity was observed in source-zone microcosms 3-1, with degradation rates as high as 326.5 ± 8.5 µg/L/week; this is more than one order of magnitude lower than the rates observed in microcosms 1-1S. Comparably, the abundance of dioxane degraders in microcosms 3-1 was 105.9±0.7 copies of thmA/dxmA genes/g soil, which is approximately 62 times lower than that in microcosms 1-1S (Li et al. 2013a).
Zero-order decay was observed in all natural attenuation microcosms experiencing dioxane biodegradation, with R2 > 0.80 (Table 1). The estimated degradation rates ranged widely from 0.3 ± 0.1 µg/L/week to 3,449 ± 459 µg/L/week. Previous microcosm studies using materials collected in north slope of Alaska exhibited a slow dioxane degradation rate of 9.8 ± 0.1 µg/L/week at a lower incubation temperature of 14 °C when the initial dioxane concentration was 500 µg/L (Li et al. 2010b). These degradation trends suggest saturated enzyme kinetics (low Monod Ks value) and little microbial growth (Alvarez and Illman 2006).
First-order degradation kinetics was observed in positive controls (inoculated with CB1190, which apparently exhibits higher Ks values (Mahendra and Alvarez-Cohen 2006)) with R2 > 0.95. The exponential decay rates were estimated as 0.53 ± 0.05 week−1 for Site 1, 0.35 ± 0.03 week−1 for Site 2, and 0.45 ± 0.04 week−1 for Site 3.
In order to verify the fast biodegradation observed in microcosm sets 1-1D and 3-1, these microcosms were respiked with dioxane after complete dioxane removal had occurred. The respiked dioxane was degraded even faster, and no dioxane was detected after 8 weeks (Fig. 4). The zero-order decay rates were 668 and 355 µg/L/week for the respiked microcosm 1-1D and 3-1, respectively. This suggests that the microcosms had become more acclimated to dioxane after the first stage (Table 1), possibly due to the proliferation of indigenous dioxane degraders.
The presence of indigenous dioxane degraders is further suggested by conversion to 14C-labeled bound residue
Sediment and biomass-associated 14C was recovered by analysis of the suspended solids (including bacteria) using a biological oxidizer with LSC at the end of the experiment. Since dioxane (log Kow = − 0.27; Schwarzenbach et al. 2003) and its main metabolites (e.g., 2-hydroxyethoxyacetic acid [HEAA], ethylene glycol, glycolate, and oxalate; (Mahendra et al. 2007)) are hydrophilic and have limited tendency to adsorb to aquifer materials (and thus would have been washed out during sample preparation), most of this 14C-labeled bound residue was likely associated with biomass growth (Table 2). This represents an additional line of evidence of the presence of indigenous dioxane degraders at these sites. Note that incorporation into bound residue was only observed in microcosms exhibiting significant dioxane biodegradation over 28 weeks, while no suspended-solids-associated 14C was detected in microcosms experiencing no dioxane degradation (Table 2). This corroborates limited adsorption and supports the hypothesis that most of the 14C recovered by BO-LS was associated with biomass. In source zone microcosms for Site 1, 2.8–7.9 % of the added 14C was converted into bound residue after 7 months’ incubation. Site 2 and Site 3 microcosms experienced a maximum of 6.9 % conversion (Table 2).
14C-Dioxane putatively assimilated by biomass was correlated with the observed biodegradation activity
A significant (p < 0.05) correlation was observed between the degradation rates and the extent of 14C accumulation in bound residue (Fig. 5a), further supporting the notion that dioxane was assimilated by the indigenous microorganisms. Student t test analysis indicated significant 14C accumulation in bound residue (and thus possible biomass growth) in microcosms that exhibited a degradation rate higher than 5 µg/L/week (Fig. 5a). Putative biomass assimilation was also significantly higher in microcosms prepared with samples from the source zone compared to those with leading-edge or background samples (Fig. 5a). For instance, microcosms 1-1, 1-2D, and 3-1, which contain source zone materials, experienced the greatest conversion to bound residue at 6.9–7.9 % of the total amount of the spiked 14C (Table 2). The apparent biomass yield found in this study is comparable with that reported in a previously study that found 5 % of radiolabeled dioxane was incorporated into CB1190 biomass (Mahendra et al. 2007). This suggests that the cell yield for these indigenous dioxane degrading consortia is relatively low (i.e., less than 0.1 mg protein/mg dioxane).
Mineralization activity correlated to degradation rates
To confirm the biodegradation of dioxane observed in uninoculated microcosms mimicking natural attenuation, accumulation of 14CO2 was also monitored during incubation. The overall mineralization observed in the biologically active microcosms (positive controls and degrading) is significantly greater (p < 0.05) than that of the inactive microcosms (negative controls and those exhibiting no degradation) (Table 3). For instance, mineralization extents of 44 ± 0.5, 38 ± 1.0, and 20 ± 0.4 % of the spiked 14C were observed for microcosms 1-1S, 1-1M, and 1-1D, respectively, while cumulative 14C trapped in all control (killed) microcosms was less than 5 % (Fig. 3). In addition, the cumulative mineralization as 14CO2 was significantly correlated (p < 0.05) with the zero-order dioxane decay rates observed in microcosms mimicking natural attenuation (Fig. 5b), corroborating the capability of indigenous microorganisms to metabolize dioxane. However, the total 14C recovery was relatively low with this experimental setup, especially for microcosms exhibiting fast dioxane degradation (e.g., 1-1S, and 3-1). In this microcosm design (Fig. 2a), the total volume of NaOH solution was merely 1 mL and the trap provided limited interfacial surface area for effective trapping of the generated 14CO2. Long incubation time and gas leakage during sampling may have also contributed to the low 14C recoveries observed in this experiment.
Therefore, a new microcosm design utilizing a larger mineralization trap and an improved (tighter) microcosm cap (Fig. 2b) was used to prepare a new set of 1-1S microcosms which ultimately yielded a more complete mass balance. In addition, freshly sampled groundwater from monitoring well 1-1 was used in this experiment to confirm the observed fast degradation rates. Within 12 weeks, dioxane removal reached 64 ± 0.2 % with 53 ± 0.2 % mineralization of the spiked 14C (Fig. 6). No dioxane loss and low 14CO2 recovery (<15 %) were observed in the 1-1S Replicate negative control. Note that some of the trapped 14C in the controls was likely associated with dioxane in condensed vapor rather than 14CO2 because the microcosms had been sterilized. Better mass balance recovery of the overall 14C was obtained with this new design, ranging from 92 to 98 % (Table 2). This suggests that insufficient interfacial surface area with the NaOH trap to capture the emitted CO2 in the original microcosm design (Fig. 2a) was one of the key reasons for low 14C recoveries.
Degradation rates were significantly higher in source-zone samples, possibly reflecting higher acclimation
With the exception of samples from well 3-3 and 3-4, the well samples demonstrating biodegradation are all within approximately 200 ft of the dioxane source for Site 1 and 3. Student t-tests indicated that dioxane biodegradation rates were significantly higher (p < 0.05) near the source-zone samples than down-gradient diluted area (i.e., more than 200 ft distance away from the source in this study). For example, microcosm sets 1-4 and 1-5 exhibited no degradation at the same site where the highest degradation, 1-1S (3,449 ± 459 µg/L/week), was observed in the source zone samples. It has been previously reported that long term exposure to high concentrations of xenobiotic compounds results in a higher acclimation and more rapid metabolic response over time (Leahy and Colwell 1990; Aelion et al. 1989). Such acclimation effects were also evident in other dioxane degradation studies using various lines of evidence. For instance, both functional microarray and denaturing gradient gel electrophoresis (DGGE) analysis indicated monooxygenase genes that are likely involved in initiating dioxane oxidation were significantly enriched in source-zone area at an Arctic site, suggesting selective pressure by dioxane (Li et al. 2013b). For another site in Arizona, compound specific isotope analysis (CSIA) demonstrated a significant amount of 13C from dioxane incorporated into biomass in the samples heavily impacted by dioxane, but not in the areas with no previous exposure history (Chiang et al. 2012). These previous studies concluded that MNA might be a feasible component of the remedial response.
Conclusions
This study is one of few conducted to assess the potential for natural attenuation/biodegradation of dioxane in groundwater microcosms. Three independent lines of evidence indicate dioxane biodegradation and utilization as a sole carbon source: (1) dioxane removal in biologically active microcosms but not in sterile controls; (2) 14C-labeled dioxane was oxidized to 14CO2 and a higher extent of mineralization was observed for (presumably more acclimated) source-zone samples exhibiting higher degradation activity; and (3) 14C was converted to bound residue (including likely incorporation into biomass) only in microcosms where dioxane was biodegraded. Both respiked and replicated microcosm sets from Site 1 confirmed relatively rapid dioxane degradation (i.e., 100 % removal by 20 weeks). It appears the remedial strategies for all three of these sites should consider MNA, which would involve long-term monitoring of groundwater quality and contamination levels.
References
Aelion CM, Dobbins DC, Pfaender FK (1989) Adaptation of aquifer microbial communities to the biodegradation of xenobiotic compounds—influence of substrate concentration and preexposure. Environ Toxicol Chem 8(1):75–86. doi:10.1897/1552-8618(1989)8
Alvarez PJ, Illman WA (2006) Bioremediation and natural attenuation: process fundamentals and mathematical models. Wiley, Hoboken
Anderson RH, Anderson JK, Bower PA (2012) Co-occurrence of 1,4-dioxane with trichloroethylene in chlorinated solvent groundwater plumes at US Air Force installations: fact or fiction. Integr Environ Assess Manag 8(4):731–737. doi:10.1002/ieam.1306
Andreozzi R, Caprio V, Insola A, Marotta R (1999) Advanced oxidation processes (AOP) for water purification and recovery. Catal Today 53(1):51–59. doi:10.1016/S0920-5861(99)00102-9
Beckles DM, Chen W, Hughes JB (2007) Bioavailability of polycyclic aromatic hydrocarbons sequestered in sediment: microbial study and model prediction. Environ Toxicol Chem 26(5):878–883. doi:10.1897/06-410R.1
Brode J, Fotouhi F, Kolon S (2005) Ultraviolet and hydrogen peroxide treatment removes 1,4-dioxane from multiple aquifers. Technology News and Trends. http://www.clu-in.org/products/newsltrs/tnandt/view.cfm?issue=0105.cfm. Accessed 27 Sept 2014
California EPA (2014) http://www.waterboards.ca.gov/drinking_water/certlic/drinkingwater/14-Dioxane.shtml. Accessed 27 Sept 2014
Chiang SYD, Mora R, Diguiseppi WH, Davis G, Sublette K, Gedalanga P, Mahendra S (2012) Characterizing the intrinsic bioremediation potential of 1,4-dioxane and trichloroethene using innovative environmental diagnostic tools. J Environ Monit 14(9):2317–2326. doi:10.1039/C2em30358b
Cronk G (2008) Case study comparison of multiple activation methods for sodium persulfate ISCO treatment. In: Sixth international conference on remediation of chlorinated and recalcitrant compounds, Monterey. Battelle Press, Columbus
DiGuiseppi W, Whitesides C (2007) Treatment options for remediation of 1,4-dioxane in groundwater. Environ Eng Appl Res Pract 2:1–7
Dombrowski PM, Weir BA, Kelly KM, Brown J (2010) Fast-track remedial design of full-scale ISCO application using pilot scale testing and field screening parameters. In: Proceedings of the annual international conference on soils, sediments, water and energy, 2010
Doucette WJ, Wheeler BR, Chard JK, Bugbee B, Naylor CG, Carbone JP, Sims RC (2005) Uptake of nonylphenol and nonylphenol ethoxylates by crested wheatgrass. Environ Toxicol Chem 24(11):2965–2972. doi:10.1897/05-171r.1
Epa US (2006) treatment technologies for 1,4-dioxane: fundamentals and field applications. EPA, Cincinnati
EPA US (2010) http://www.epa.gov/iris/subst/0326.htm. Accessed 27 Sept 2014
EPA US (2013) The third unregulated contaminant monitoring rule (UCMR 3): data summary. EPA, Washington DC
Houston KS, Horst J, Wroblewski G (2009) Focused in-situ chemical oxidation of chlorinated VOCs and 1,4-dioxane using sodium persulfate in fine-grained soils. Pollution Engineering. http://www.pollutionengineering.com/articles/88033. Accessed 27 Sept 2014
Huling SG, Pivetz BE (2006) In-situ chemical oxidation. Washington DC, EPA
IARC (1999) Monograph on 1,4-Dioxane. International Agency for Research on Cancer, Lyon
Kim YH, Engesser KH, Kim SJ (2007) Physiological, numerical and molecular characterization of alkyl ether-utilizing rhodococci. Environ Microbiol 9(6):1497–1510. doi:10.1111/j.1462-2920.2007.01269.x
Leahy JG, Colwell RR (1990) Microbial-degradation of hydrocarbons in the environment. Microbiol Rev 54(3):305–315
Li D, Fortner JD, Johnson DR, Chen C, Li QL, Alvarez PJJ (2010a) Bioaccumulation of C-14(60) by the earthworm Eisenia fetida. Environ Sci Technol 44(23):9170–9175. doi:10.1021/Es1024405
Li M, Fiorenza S, Chatham JR, Mahendra S, Alvarez PJJ (2010b) 1,4-Dioxane biodegradation at low temperatures in Arctic groundwater samples. Water Res 44(9):2894–2900. doi:10.1016/j.watres.2010.02.007
Li M, Conlon P, Fiorenza S, Vitale RJ, Alvarez PJJ (2011) Rapid analysis of 1,4-dioxane in groundwater by frozen micro-extraction with gas chromatography/mass spectrometry. Ground Water Monit R 31(4):70–76. doi:10.1111/j.1745-6592.2011.01350.x
Li M, Mathieu J, Liu Y, Van Orden ET, Yang Y, Fiorenza S, Alvarez PJJ (2013a) The abundance of tetrahydrofuran/dioxane monooxygenase genes (thmA/dxmA) and 1,4-dioxane degradation activity are significantly correlated at various impacted aquifers. Environ Sci Technol Lett 1(1):122–127. doi:10.1021/ez400176h
Li M, Mathieu J, Yang Y, Fiorenza S, Deng Y, He Z, Zhou J, Alvarez PJJ (2013b) Widespread distribution of soluble di-iron monooxygenase (SDIMO) genes in arctic groundwater impacted by 1,4-dioxane. Environ Sci Technol 47(17):9950–9958. doi:10.1021/es402228x
Macdonald JA (2000) Peer reviewed: evaluating natural attenuation for groundwater cleanup. Environ Sci Technol 34(15):346A–353A. doi:10.1021/es003359w
Mahendra S, Alvarez-Cohen L (2006) Kinetics of 1,4-dioxane biodegradation by monooxygenase-expressing bacteria. Environ Sci Technol 40(17):5435–5442. doi:10.1021/Es060714v
Mahendra S, Petzold CJ, Baidoo EE, Keasling JD, Alvarez-Cohen L (2007) Identification of the intermediates of in vivo oxidation of 1,4-dioxane by monooxygenase-containing bacteria. Environ Sci Technol 41(21):7330–7336. doi:10.1021/Es0705745
Miller CM, Valentine RL, Roehl ME, Alvarez PJJ (1996) Chemical and microbiological assessment of pendimethalin-contaminated soil after treatment with Fenton’s reagent. Water Res 30(11):2579–2586
Mohr T, Stickney J, DiGuiseppi W (2010) Environmental investigation and remediation: 1,4-dioxane and other solvent stabilizers. CRC Press, Boca Raton
Parales RE, Adamus JE, White N, May HD (1994) Degradation of 1,4-dioxane by an Actinomycete in pure culture. Appl Environ Microbiol 60(12):4527–4530
Sales CM, Grostern A, Parales JV, Parales RE, Alvarez-Cohen L (2013) Oxidation of the cyclic ethers 1,4-dioxane and tetrahydrofuran by a monooxygenase in two Pseudonocardia species. Appl Environ Microbiol 79(24):7702–7708. doi:10.1128/Aem.02418-13
Schwarzenbach R, Gschwend P, Imboden D (2003) Environmental organic chemistry. Wiley
Sei K, Kakinoki T, Inoue D, Soda S, Fujita M, Ike M (2010) Evaluation of the biodegradation potential of 1,4-dioxane in river, soil and activated sludge samples. Biodegradation 21(4):585–591. doi:10.1007/s10532-010-9326-3
Thiemer B, Andreesen JR, Schrader T (2003) Cloning and characterization of a gene cluster involved in tetrahydrofuran degradation in Pseudonocardia sp strain K1. Arch Microbiol 179(4):266–277
Vainberg S, McClay K, Masuda H, Root D, Condee C, Zylstra GJ, Steffan RJ (2006) Biodegradation of ether pollutants by Pseudonocardia sp strain ENV478. Appl Environ Microbiol 72(8):5218–5224
Wang HY, Ye QF, Yue L, Yu ZY, Han AL, Yang ZM, Lu L (2009) Kinetics of extractable residue, bound residue and mineralization of a novel herbicide, ZJ0273, in aerobic soils. Chemosphere 76(8):1036–1040. doi:10.1016/j.chemosphere.2009.04.051
Yang Y, Li M, Michels C, Moreira-Soares H, Alvarez PJ (2014) Differential sensitivity of nitrifying bacteria to silver nanoparticles in activated sludge. Environ Toxicol Chem. doi:10.1002/etc.2678
Acknowledgments
We thank Dr. Dong Li (Rice University) for her assistance on biological oxidizer.
Conflict of interest
The authors declare no competing financial interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, M., Van Orden, E.T., DeVries, D.J. et al. Bench-scale biodegradation tests to assess natural attenuation potential of 1,4-dioxane at three sites in California. Biodegradation 26, 39–50 (2015). https://doi.org/10.1007/s10532-014-9714-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-014-9714-1