Abstract
The high endemism, the natural habitat degradation, and the over-collection for ornamental purposes have led some species such as Melocactus paucispinus and Melocactus glaucescens to be threatened with extinction. The use of in vitro conservation techniques, such as slow growth storage, promotes the preservation of genetic diversity with integrity. The goal of this study was to establish a strategy for in vitro conservation of apical segments of the cladode of M. paucispinus and M. glaucescens and evaluate the genetic diversity of individuals from in vitro germinated plants. For such purpose, different concentrations of the plant regulator ancymidol and the osmotic agent sucrose on the inhibition of the in vitro growth were tested, and the genetic diversity of M. paucispinus and M. glaucescens individuals stored in vitro was evaluated. Sucrose showed higher efficiency in the reduction of growth than ancymidol for both species. However, due to the reduction in survival percentage, the use of sucrose over 75 g L−1 in the in vitro conservation of both species for 360 days is not recommended. In the genetic diversity analysis, 76.92% of polymorphic loci (P), expected heterozygosity (He) = 0.276 and Shannon index (S) = 0.414 were observed for M. paucispinus. For M. glaucescens, the observed values were P = 95.38%, He = 0.228 and S = 0.369. These values observed here were higher than those previously found for the natural populations of these species, which demonstrated that this in vitro collection showed genetic diversity and can be used in management and reintroduction programs of these species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cactaceae family is subject to intense extractive exploitation due to its great diversity and value, mainly in the ornamental trade, which is the reason why it is considered one the most endangered groups of plants (Goettsch et al. 2015; Pérez-Molphe-Balch et al. 2015). Brazil is one of the priority areas of Cactaceae family conservation because of its large number of endemic species (Goettsch et al. 2015).
Melocactus plants, popularly known as “cabeça-de-frade”, are significant components of the semi-arid regions’ flora and have high ecological importance in their ecosystems (Machado 2009). Despite that, a progressive reduction in the size of M. paucispinus and M. glaucescens populations has been observed, caused by frequent changes in their natural habitat as well as illegal harvesting for commercial and ornamental purposes (Lambert et al. 2006a, b). Due to these reasons, both species have been listed as endangered in the IUCN Red List of Threatened Species (IUCN 2020).
Under these circumstances, ex situ conservation techniques are important to preserve the genetic diversity from plant genetic resources with as much integrity as possible (Villalobos et al. 1991; Rao and Hodgkin 2002; Volis and Blecher 2010). In this context, the tissue culture technique represents an efficient method for ex situ genetic diversity conservation, allowing the reduction of space for the species maintenance and promoting high multiplication rates regardless of climatic conditions (Villalobos et al. 1991; Engelmann 2011). This technique is indicated for species such as M. paucispinus and M. glaucescens, whose seeds lose viability when stored for long periods (data not published).
Slow growth storage is an in vitro conservation method for one to two years of storage and is considered satisfactory for short- to medium-term conservation maintenance of plants under aseptic conditions by markedly reducing the frequency of periodic subculturing (Ozudogru et al. 2009; Carvalho et al. 2014); consequently, it promotes the reduction of contamination, error in material handling and equipment failures (Ozudogru et al. 2009; Carvalho et al. 2014). Then, slow growth storage reduces the costs of in vitro germplasm banks and manual labor (Ozudogru et al. 2009; Engelmann 2011; Pérez-Molphe-Balch et al. 2015).
Growth reduction is generally achieved by adding an inhibitory growth regulator that modifies plant growth and development (Thakur et al. 2015). Some retarders act by inhibiting the synthesis of gibberellin, such as ancymidol (Rademacher 2016).
Another strategy for reducing in vitro growth is the use of osmotic agents such as sucrose, which can promote slow growth due to their ability to reduce the water potential of the culture media, that limits the availability of water and nutrients to the cultures (Villalobos et al. 1991; Engelmann 2011; Pérez-Molphe-Balch et al. 2015).
In order to perform the correct management of the stored germplasm, it is necessary to identify an efficient method of in vitro conservation and validate the representativeness of the genetic diversity in relation to the natural populations (Nick et al. 2010; Engelmann 2011). One of the most efficient methods for this verification is the analysis of the genetic diversity of the collections through molecular markers (Rao and Hodgkin 2002).
Molecular characterization allows the identification of duplicates and reduction in the number of samples to be conserved, which is necessary to reduce the costs of in vitro conservation (Rao and Hodgkin 2002; Nick et al. 2010; Martín et al 2013). Genetic diversity analysis also provides information that may positively contribute to the use of germplasm successfully stored in future ecosystem restoration programs, including the reintroduction of species that have ex situ conservation (Rao and Hodgkin 2002; Volis and Blecher 2010; Pérez-Molphe-Balch et al. 2012). Among DNA molecular markers, Inter Simple Sequence Repeats (ISSR) have been frequently used for molecular characterization of plants from ex situ conservation (Luna-Paez et al. 2007; Oliveira et al. 2013), due to the low cost and little infrastructure demanded, high reproducibility and polymorphism, and good efficiency in distinguishing variability between individuals (Ganopoulos et al. 2015; Grover and Sharma 2016).
Then, considering all described above, the goal of this study was to establish the best strategy for in vitro conservation of M. paucispinus and M. glaucescens using apical segments of the cladodes from seed-derived plants germinated in vitro. For such purpose, different concentrations of the plant regulator ancymidol and the osmotic agent sucrose were used in the inhibition of growth of these species. In addition, the genetic diversity of individuals from in vitro germinated plants of these two species, stored in the in vitro collection of the Plant Tissue Culture Laboratory of the Federal University of Bahia (LCTV-UFBA), was evaluated. These data will contribute to management strategies of stored genetic resources, providing perspectives for the reintroduction of this germplasm into wild populations.
Material and methods
Plant material
Seeds of Melocactus paucispinus and M. glaucescens were collected in the city of Morro do Chapéu in the “Chapada Diamantina” (Bahia state, eastern Brazil), in localities popularly known as “Areia Branca” (11° 33′52′′ S; 41°10′37′′ W) (Fig. 1a) and “Lages” (11° 29′38.4′′ S; 41° 20′22.5′′ W) (Fig. 1b), respectively.
Melocactus paucispinus and Melocactus glaucescens in vitro conservation. a M. paucispinus in natural population. b M. glaucescens in natural population. c M. glaucescens in vitro conservation after 360 days of cultivation with 30 g L−1 sucrose (control), 3.90, 11.71, 19.51, 27.31, and 39.02 μM of ancymidol; arrow shows shoot formation. d M. glaucescens in vitro conservation after 360d of cultivation with 30 (control), 45, 60, 75, 90. and 105 g L−1 of sucrose. e M. paucispinus collection at in vitro germplasm bank of LCTV-UFBA
In vitro slow growth storage
For in vitro conservation, the apical segment of the cladodes (Torres-Silva et al. 2018), originated from M. paucispinus and M. glaucescens plants germinated in vitro for 300 days, were used. These plants were maintained for 70 days in 250-mL glass flask with 50 mL of half-strength Murashige and Skoog (MS/2) medium (Murashige and Skoog 1962), supplemented with 30 g L−1 sucrose (Synth), solidified with 6.5 g L−1 agar (Merk). The pH of the media was adjusted to 5.7 before chemical sterilization with 0.0003% of NaOCl, commercial bleach (2.5%) (commercial branch QBoa®) (Teixeira et al. 2006). In order to evaluate the effect of the inhibitory growth regulation on in vitro growth of apical segment of cladodes of M. paucispinus and M. glaucescens, two experiments were performed using ancymidol and the osmotic agent sucrose. Apical segments of cladodes with a height of 10–15 mm were inoculated in glass tubes (25 × 150 mm) containing 15 mL of full-strength MS medium, solidified with 6.5 g L−1 agar and supplemented with 30 g L−1 sucrose (control), and different concentrations of ancymidol (0, 3.90, 11.71, 19.51, 27.31, and 39.02 µM) or sucrose (45, 60, 75, 90, and 105 g L−1). The glass tubes were closed with two layers of polyvinylchloride film. The experimental design was completely randomized, with five replicates and five tubes per replicate (one apex per tube). At 360 days, survival (% S), length of the aerial portion (LAP) and diameter of the aerial portion (DAP) were evaluated. The LAP and DAP variables were evaluated using a digital caliper (150 mm).
Genetic diversity analyses
The seeds of M. paucispinus and M. glaucescens were collected in April 2003; February-May and July 2007, April 2008, August 2010, and April 2012. Each year of collection was considered as a sub-collection (Table 1). The cultures were maintained in MS/2, supplemented with 15 g L−1 of sucrose (Synth) and 6.5 g L−1 of agar (Merk), subcultured each six months. The samplings of M. paucispinus and M. glaucescens from the in vitro collection of the LCTV-UFBA were performed randomly and contemplating 10% of the plants originated from seeds collected between 2003 and 2012 (Table 1).
For the molecular analysis, a tissue fragment (5 mg) was taken from the donor plant base (under laminar flow hood conditions). After that, the remaining tissue (apical segment of cladode) was maintained in vitro for regeneration in MS/2, supplemented with 15 g L−1 of sucrose (Synth) and 6.5 g L−1 of agar (Merk). Then, the tissue samples obtained were submitted to DNA extraction performed following the CTAB method (Doyle and Doyle 1987) adapted to microtubes.
Amplification reactions were performed on a total final volume of 20 µL, containing reaction buffer 1 × (100 mM Tris–HCl pH 8.4; 500 mM KCl; 1% Triton X-100), MgCl2 (2.5 mM), dNTPs (0.2 mM), primer (0.5 mM), Taq DNA polymerase Invitrogen® (0.75 U), autoclaved ultrapure water, and 20 ng DNA. A negative control sample with the absence of DNA was included in each reaction to evaluate the absence of contamination.
In order to standardize the PCR conditions, 20 ISSR primers developed by Wolfe (2000) were tested. For such tests, amplification reactions with two samples from each species were performed. Then, considering higher number of loci detected and consistent amplification pattern as criteria, we chose four from the 20 primers tested: 899 [(CA)6-RG], 814 [(CT)8-TG], MAO [(CTC)4-RC], and MANNY [(CAC)4-RC], which were used in the amplifications of all samples.
All reactions were performed on the thermal cycler Veriti® (Applied Biosystems) with initial denaturation at 94 °C for 5 min, followed by 35 cycles composed of denaturation at 94 °C for 40 s, then annealing of primers at 45 °C (primers 899 and 814) and 46 °C (primers MANNY and MAO) for 40 s, and extension at 72 °C for 2 min. The reaction was finalized with one more extension at 72 °C for 7 min.
To compare electrophoretic profiles, the reaction products were separated by electrophoresis through agarose gel at 1.4% in 1 × TAE buffer (Tris-Acetate-EDTA) at 60 watts for four hours. To mark the weight of each fragment, 100 bp DNA molecular weight ladder (Kasvi®) was used in all electrophoresis runs. The gels were stained in a 0.5 µg mL−1 ethidium bromide solution for 30 min and then photodocumented using Wise Capture II version 1.0.0.0 software, with an ultraviolet light transilluminator. The band pattern obtained was converted into a presence (1) and absence (0) loci table.
In vitro culture conditions
All cultures were maintained at 25 ± 3 °C, under two fluorescent lights (Phillips 100 W) with photosynthetically active radiation level of 60 µmol m−2 s−1 and a 16/8-h light/dark photoperiod.
Statistical analyses
Slow growth storage data were submitted to regression analysis with 5% level of probability in package Exp.Des.pt 1.2.0 (Ferreira et al. 2018) available in RStudio v 1.3.1056 (R Core Team 2020).
The genetic diversity of M. paucispinus and M. glaucescens was measured from number of loci (N), number of unique loci (Nu), polymorphic loci (P), mean heterozygosity expected (He), and Shannon index (S) in GenAlex 6.5 (Peakall and Smouse 2012).
For M. paucispinus, the analyses were performed without discriminating between plants of different sub-collections, because the in vitro collection was established from just two sub-collections and one of them contributed with only 0.8% of the total samples. In contrast, for M. glaucescens, the molecular analyses were performed for each sub-collection, in order to evaluate the contribution of each one to the genetic diversity of the LCTV-UFBA in vitro collection.
Results
Slow growth storage
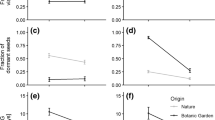
The ancymidol or sucrose effects promoted the survival of the apical segment of cladodes, 100 and 97%, respectively, of Melocactus paucispinus and M. glaucescens plants survived 360 days after inoculation (Fig. 1c, d). The samples submitted to ancymidol showed modification in color, with the stem becoming darker as the concentration of ancymidol in the medium increased, and at the highest concentration of ancymidol, the formation of shoots originating from the areola region was also observed (Fig. 1c). The highest concentration of sucrose was the most harmful one and led to 80–84% of survival (Fig. 1d).
For the diameter of the aerial portion (DAP) of both species, the quadratic reduction model was the most representative for ancymidol (Fig. 2a, b) and sucrose (Fig. 3a, b). In the treatment of ancymidol, the highest reduction percentage observed of DAP, compared to the control, was 14.69% for M. paucispinus (Fig. 2a) and 22.93% for M. glaucescens (Fig. 3a). In the treatment of sucrose, the highest reduction observed, compared to the control, was 26.86% for M. paucispinus (Fig. 2a) and 52.16% for M. glaucescens (Fig. 3a).
According to the regression model for the length of the aerial portion (LAP), a linear decrease was observed for both species as a function of the increase in ancymidol concentrations (Figs. 2c, 3c). The growth reduction, compared to the control, was 40.12% for M. paucispinus and 44.33% for M. glaucescens.
For the effect of sucrose on LAP growth reduction, the quadratic reduction model was the best fit for M. paucispinus (Fig. 2d), and the linear reduction model was the best fit for M. glaucescens (Fig. 3d). In addition, growth reduction of 39.85% for M. paucispinus and 54.76% for M. glaucescens (Figs. 2d, 3d) was observed when compared to the control.
Genetic diversity analyses
The ISSR primers were efficient to reveal genetic diversity between samples of plants originated from seeds of M. paucispinus and M. glaucescens from the in vitro collection of the LCTV-UFBA (Fig. 1e).
For M. paucispinus, the four primers used generated 39 loci. The primer MANNY generated the highest number of loci (12), followed by MAO (11) and 899 (10). The lowest number of fragments was produced by the primer 814 (7). The band sizes varied between 400 and 2500 base pairs (bp) approximately. The M. paucispinus collection showed 76.92% of polymorphic loci (P), mean of expected heterozygosity (He) = 0.276, and Shannon index (S) = 0.414 (Table 2).
For M. glaucescens, the four primers used generated 65 loci, of which 61 occurred in high frequency. The number of fragments analyzed per primer varied from 16 (primers 899, MANNY and MAO) to 17 (primer 814). Also, the band sizes varied between 300 and 2,000 bp approximately.
On the general analysis of the M. glaucescens sub-collections, values of P = 95.38%, S = 0.369 and He = 0.228 were observed (Table 2). The plants from the sub-collection of the year 2007 were the most polymorphic ones (P = 87.7%), showing 3 exclusive loci, followed by the plants from the sub-collection of the year 2012 (P = 72.3%) (Table 2). The lowest polymorphism indexes were observed in the sub-collections of 2010 (24.62%), 2003 (27.69%), and 2008 (29.23%) (Table 2).
The values of mean heterozygosity expected (He) and Shannon index (S) followed the same pattern as the P values observed (Table 2). Therefore, the highest values of diversity were observed in the sub-collections of the years 2007 (He = 0.205; S = 0.331) and 2012 (He = 0.198; S = 0.310). Despite that, the genetic diversity of the sub-collection of the year 2003 (He = 0.106; S = 0.157) was higher than that of the sub-collections of the years 2008 (He = 0.094; S = 0.142) and 2010 (He = 0.092; S = 0.136).
Discussion
Slow growth storage
The survival of the plants (%S) is one of the most relevant variables to evaluate the efficiency of the in vitro conservation. Therefore, the use of the apical segment of cladodes as an explant was efficient in both short- and medium-term conservation of Melocactus paucispinus and M. glaucescens. For Melocactus species, the use of the apical segment of the cladode, usually discarded in the production of explants for in vitro regeneration, is an efficient strategy for in vitro conservation (Torres-Silva et al. 2018). The apical segment of the cladode constitutes an excellent explant for these species, since in vitro cultivation without plant regulators allows the regeneration of whole plants, and then maintaining the same characteristics of the mother plant. Also, this approach reduces the collection of seeds from natural populations, consequently reducing the interference in the dynamics of these species in their natural habitat (Torres-Silva et al. 2018).
The %S observed in the present study were higher than those found for other species submitted to slow growth storage with osmotic agents. For example, Syngonanthus mucugensis species from Chapada Diamantina, cultivated in 45 and 60 g L−1 of sucrose for 180 days, showed low survival (< 35%) (Lima-Brito et al. 2011). Although, Silva et al. (2019) observed for Poincianella pyramidalis, typical of drought environment, values above 67% for 240 days cultivated in 30, 45, 60 and 75 g L−1 of sucrose.
The high %S under stress conditions observed for both species of cacti might be related to the morphophysiological mechanisms that M. paucispinus and M. glaucescens have in order to survive under stress conditions where their populations are exposed in their natural environment, characterized by long drought periods (Pérez-Molphe-Balch et al. 2012). Both species have a small genome that: (i) allows rapid cycles of cell division in the root cells when water is available; and (ii) allows up to four rounds of endoreduplication of the cortex cells, which are capable of major expansion to optimize water storage (Torres-Silva et al. 2020).
The increase of ancymidol and sucrose concentrations was efficient in reducing the growth of the plants of both species, since the values for DAP and LAP were lower, when compared to the control, in the presence of these substances. These results are in agreement with the results found by Lima-Brito et al. (2011), who observed growth reduction of LAP of Syngonanthus mucugensis cultures with increasing sucrose concentration.
Sarkar et al. (2001) observed growth reduction of Solanum tuberosum cultures supplemented with high concentrations of ancymidol. Similarly, the use of sucrose up to 80 g L−1 allowed the in vitro storage of Elettaria cardamomum plants for a longer period of time than the one observed for the control, without the necessity of subculture (Tyagi et al. 2009).
In general, sucrose showed a greater efficiency in the reduction of M. paucispinus and M. glaucescens growth, because the reduction of growth, comparing to the control, was 39 and 45% with ancymidol and 40 and 54% with sucrose, respectively.
For the maintenance of genetic stability, the use of osmotic agents for the inhibition of growth is the most appropriate method when compared to use of plant regulators, because these ones act directly on the metabolic routes and can modify plant development patterns (Rademacher 2016). This is not the case for osmotic agents, which have action on reduction of the water potential of the culture medium, inhibiting the absorption of water and nutrients by the explant and consequently reducing in vitro growth (Engelmann 1991; Caldas et al. 1998).
Sucrose is a carbon source, for this reason increasing its concentration up to 60 g L−1 in the culture media stimulated the development of DAP and LAP of M. paucispinus and LAP of M. glaucescens. However, with higher concentrations, sucrose started to act as a slow growth agent, making it difficult for the plant to absorb water and nutrients. The water deficit promoted by sucrose was lethal for 12.5% of M. paucispinus and 20.8% of M. glaucescens plants cultivated in 105 g L−1 of sucrose. As a similar result, high concentrations of sucrose were also lethal for long-term cultures of E. cardamomum (Tyagi et al. 2009).
Then, the %S reduction due to sucrose concentrations above 90 g L−1 indicates risks of viability loss of the cultures for storage period of 360 days, which is not recommended for long-term in vitro conservation of M. paucispinus and M. glaucescens. Therefore, the use of 75 to 90 g L−1 of sucrose allows in vitro conservation of these species without compromising the viability of the cultures after 360 days of storage. Thus, in order to reduce the costs of in vitro conservation, the lowest concentration is recommended.
Genetic diversity analyses
The use of the seeds for establishment of Melocactus paucispinus and M. glaucescens germplasm banks is efficient due to the high polymorphism (P) detected for M. paucispinus (76.9%) and M. glaucescens (95.4%). This result indicates that the in vitro collection of the LCTV-UFBA is diverse. The analysis of genetic diversity of this in vitro collection was essential for the characterization of the sub-collections that were stored, the management of the number of individuals in each sub-collection, and the validation of the representativeness of the entire collection, when comparing our results with the studies previously carried out by Lambert et al. (2006a, b) in the natural populations of Melocactus paucispinus and M. glaucescens.
The P values observed here are higher than the ones found in other genetic studies of Cactaceae germplasm stored in field, evaluated with ISSR and other dominant markers. For example, for Cereus jamacaru germplasm collection of Cactaceae from Embrapa Tropical Agroindustry (P = 49.2%; Oliveira et al. 2013), for accessions of species of the genus Opuntia in the Regional University Centre in Zacatecas-México (P = 41.9%; Luna-Paez et al. 2007), and for Opuntia ficus-indica in South Africa (P = 48.6%; Mashope 2007) and Tunisia (P = 53.2%; Zoghlami et al. 2007).
When comparing the two species analyzed here, the P observed for M. paucispinus was lower than that observed for M. glaucescens (Table 2). This result is probably related to the lower number of loci and lower number of individuals analyzed in the M. paucispinus collection. For M. glaucescens, the higher polymorphism observed can be related to the fact that the sub-collection of 2007 had more collecting events. For M. glaucescens, the P values observed are similar to the results found in a study of genetic characterization of the O. ficus-indica germplasm stored in field at the University of Catania-Italy using microsatellite markers (92.8%) (Caruso et al. 2010), and in a study of pitaya species (Hylocereus and Selenicereus) stored in field at Embrapa Cerrados-Brazil (95.06%) (Junqueira et al. 2010).
The values of mean heterozygosity expected (He) and Shannon index (S) observed for M. paucispinus were higher than those for M. glaucescens (Table 2). This difference observed here might be related to the larger geographical distribution as well as number of individuals in natural populations of M. paucispinus compared to the populations of M. glaucescens (Machado 2009; Fonseca et al. 2012).
The genetic diversity observed for M. paucispinus and M. glaucescens differs from what was previously reported by Lambert et al. (2006a, b). In that study, the authors analyzed morphological characteristics and 12 alloenzymatic loci of ten natural populations of M. paucispinus and four of M. glaucescens, finding 9.98% and 25% of P and 0.031 and 0.062 of He, respectively.
DNA molecular markers, such as ISSR markers, are more efficient in detecting polymorphisms, since they amplify both coding and non-coding regions (Mondini et al. 2009). Isoenzymatic markers, on the other hand, are expressed in coding regions of the genome and, therefore, are more subject to the selection pressure. This could explain the difference between the values of diversity found in the present study and the previous ones for these species. Other studies carried out with molecular markers and isoenzymes have also shown that the molecular marker was able to detect a greater gene flow than isoenzymes in the same population (Rao and Hodgkin 2002).
In addition, the present study also differs from the studies of Lambert et al. (2006a, b) with regard to the life cycle stage and number of individuals analyzed. In contrast to the present work, in studies of natural populations, such as those performed by Lambert et al. (2006a, b), samples of tissue are collected from adult individuals. Then, the adult population that was sampled went through stochastic events and selective pressures of the environment in the period of germination and establishment of these individuals (Godínez-Álvarez et al. 2003).
In this context, water availability, substrate preference, association with nursery plants, herbivory and competition are factors that make germination and the initial establishment of these plants naturally difficult (Godínez-Álvarez et al. 2003; Machado 2009; Barrios et al. 2020). Consequently, only a part of the seeds present in the soil would germinate. Therefore, the process of germination and establishment in the natural environment seems to function as a "bottleneck", which restricts alleles present in adult individuals from natural populations (Godínez-Álvarez et al. 2003), which does not occur in the in vitro environment, because the conditions are ideal for successful germination. Then, in vitro germination favors the maintenance of a larger set of alleles.
Thus, the genetic diversity observed in the individuals stored in the in vitro collection of the LCTV-UFBA probably represents the genetic diversity of these species in a broader way, considering alleles present in in situ seed banks, juvenile individuals of the natural populations, and the genetic variability of adult individuals.
The genetic diversity found in M. paucispinus and M. glaucescens on in vitro collection of the LCTV-UFBA suggests that factors that promote variability, such as recombination, mutation and gene flow (Nick et al. 2010), might be acting in the natural populations from which these individuals have come. In this context, further in situ studies with markers that detect a higher level of polymorphism might provide information for a better understanding of populational structure of these species.
Finally, the polymorphism observed in this study indicates that the collections of M. paucispinus and M. glaucescens can be considered representative of the variability of the natural populations of these species. In vitro germination can be considered a viable strategy to ex situ conservation programs, as recommended by Nascimento et al. (2018) when seedlings could be produced in a laboratory setting, thereby contributing to the conservation of the species in the field and eliminating the threat of extinction. In addition, the number of individuals of M. paucispinus and M. glaucescens stored in the in vitro collection of the LCTV-UFBA is higher than the ones observed in the natural population of these species, as reported by Fonseca et al. (2012), who found 250 individuals of M. paucispinus and 58 of M. glaucescens.
Conclusions
The use of the apical segment of cladode of seed-derived plants germinated in vitro as explant, and 75 g L−1 of sucrose is efficient in the in vitro conservation of Melocactus paucispinus and M. glaucescens for 360 days.
The creation of in vitro collections from in vitro seed germination is an efficient method of storing the genetic diversity of M. paucispinus and M. glaucescens.
The genetic diversity and representativeness information obtained in this study allow us to consider this in vitro collection as a germplasm bank of M. paucispinus and M. glaucescens, and unique to promote the in vitro conservation of these species.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Barrios D, Sánchez JA, Flores J, Jurado E (2020) Seed traits and germination in the Cactaceae family: a review across the Americas. Bot Sci 98(3):417–440. https://doi.org/10.17129/botsci.2501
Caldas LS, Haridasan P, Ferreira ME (1998) Meios nutritivos. In: Torres AC, Caldas LS, Buso JA (eds) Cultura de tecidos e transformação genética de plantas, vol 1. Embrapa SPI/Embrapa-CNPH, Brasília, pp 87–132
Caruso M, Currò S, Las Casas G et al (2010) Microsatellite markers help to assess genetic diversity among Opuntia ficus-indica cultivated genotypes and their relation with related species. Plant Syst Evol 290:85–97
Carvalho V, Santos DS, Nievola CC (2014) In vitro storage under slow growth and ex vitro acclimatization of the ornamental bromeliad Acanthostachys strobilacea. S Afr J Bot 92:39–43. https://doi.org/10.1016/j.sajb.2014.01.011
Doyle JJ, Doyle JL (1987) A rapid DNA isolation method for small quantities of fresh tissues. Phytochem Bull 19:11–15
Engelmann F (1991) In vitro conservation of tropical plant germplasm—a review. Euphytica 57:227–243
Engelmann F (2011) Use of biotechnologies for the conservation of plant biodiversity. Vitro Cell Dev Biol-Plant 47:5–16. https://doi.org/10.1007/s11627-010-9327-2
Ferreira EB, Cavalvanti PP, Nogueira DA (2018) ExpDes.pt: pacote experimental designs (Portuguese). R package version 1.2.0. https://CRAN.R-project.org/package=ExpDes.pt
Fonseca RBS, Funch LS, Borba EL (2012) Dispersão de sementes de Melocactus glaucescens e M. paucispinus (Cactaceae), no Município de Morro do Chapéu, Chapada Diamantina—BA. Acta Bot Bras 26(2):481–492
Ganopoulos I, Kalivas A, Kavroulakis N, Xanthopoulou A, Mastrogianni A, Koubouris G, Madesis P (2015) Genetic diversity of Barbary fig (Opuntia ficus-indica) collection in Greece with ISSR molecular markers. Plant Gene 2:29–33. https://doi.org/10.1016/j.plgene.2015.04.001
Godínez-Álvarez H, Valverde T, Ortega-Baes P (2003) Demographic trends in the Cactaceae. Bot Rev 69(2):173–203
Goettsch B, Taylor CH, Piñón GC et al (2015) High proportion of cactus species threatened with extinction. Nat Plants. https://doi.org/10.1038/NPLANTS.2015.142
Grover A, Sharma PC (2016) Development and use of molecular markers: past and present. Crit Rev Biotechnol 36(2):290–302
IUCN (2020) IUCN Red list of threatened species. version 2020.1. https://www.iucnredlist.org/. Accessed 22 Apr 2020
Junqueira KP, Faleiro FG, Junqueira NTV et al (2010) Diversidade genética de pitayas nativas do Cerrado com base em marcadores RAPD. Rev Bras Frutic 32(3):819–824
Lambert SM, Borba EL, Machado MC et al (2006a) Allozyme diversity and morphometrics of Melocactus paucispinus (Cactaceae) and evidence for hybridization with M. concinnus in the Chapada Diamantina, North-eastern, Brazil. Ann Bot 97:389–403
Lambert S, Borba MEL, Machado MC (2006b) Allozyme diversity and morphometrics of the endangered Melocactus glaucescens (Cactaceae), and investigation of the putative hybrid origin of Melocactus x albicephalus (Melocactus ernestii x M. glaucescens) in north-eastern Brazil. Plant Species Biol 21:93–108
Lima-Brito A, Albuquerque MM, Alvim BFM et al (2011) Agentes osmóticos e temperatura na conservação in vitro de sempre-viva. Ciên RURAL 41:1354–1361
Luna-Paez A, Valadez-Moctezuma E, Barrientos-Priego AF et al (2007) Caracterización de Opuntia spp. mediante semilla con marcadores RAPD e ISSR y su posible uso para diferenciación. J Prof Assoc Cactus Dev 9: 43–59
Machado MC (2009) The genus Melocactus in eastern Brazil: part I—an introduction to Melocactus. Brit Cact Succ J 27:1–16
Martín C, Senula A, González I, Acosta A, Keller RJ, González-Benito ME (2013) Genetic identity of three mint accessions stored by different conservation procedures: field collection, in vitro and cryopreservation. Genet Resour Crop Evol 60:243–249. https://doi.org/10.1007/s10722-012-9830-x
Mashope BK (2007) Characterization of cactus pear germplasm in South Africa. Thesis of Philosophiae. Doctor, University of the Free State
Mondini L, Noorani A, Pagnotta MA (2009) Assessing plant genetic diversity by molecular tools. Diversity 1:19–35
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nascimento JPB, Meiado MV, Siqueira-Filho JA (2018) Seed germination of three endangered subspecies of Discocactus Pfeiff. (Cactaceae) in response to environmental factors. J Seed Sci 40(3):253–262. https://doi.org/10.1590/2317-1545v40n3183036
Nick C, Silva DJH, Mattedi AP et al (2010) Conservação ex situ dos recursos fitogenéticos. In: Pereira TNS (ed) Germoplasma: conservação, manejo e uso no melhoramento de plantas. Arca, Viçosa, pp 59–88
Oliveira FIC, Bordallo PN, Castro ACR et al (2013) Genetic diversity of spineless Cereus jamacaru accessions using morphological and molecular markers. Genet Mol Res 12(4):4586–4594
Ozudogru E, Ozden-Tokatli Y, Gumusel F, Benelli C, Lambardi M (2009) Development of a cryopreservation procedure for peanut (Arachis hypogaea L.) embryonic axes and its application to local Turkish germplasm. Adv Hortic Sci 23(1):41–48
Peakall R, Smouse PE (2012) GenAlex 6.5: genetic in Excel. Population genetic software for teching and research-an update. Bioinformatics 29(19):2537–2639
Pérez-Molphe-Balch E, Pérez-Reyes ME, De La Rosa-Carrillo ML (2012) In vitro conservation of Turbinicarpus (Cactaceae) under slow growth conditions. Haseltonia 17:51–57
Pérez-Molphe-Balch E, Santos-Díaz MS, Ramírez-Malagón R et al (2015) Tissue culture of ornamental cacti. Sci Agric 72(6):540–561
Rademacher W (2016) Chemical regulators of gibberellin status and their application in plant production. Annu Plant Rev 49:359–403
R Core Team (2020) A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed 10 July 2020
Rao RV, Hodgkin T (2002) Genetic diversity and conservation and utilization of plant genetic resources. Plant Cell Tissue Organ Cult 68:1–19. https://doi.org/10.1023/A:1013359015812
Sarkar D, Chakrabarti SK, Naik PS (2001) Slow-growth conservation of potato microplants: efficacy of ancymidol for long-term storage in vitro. Euphytica 117:133–142
Silva TS, Nepomuceno CF, Soares TL et al (2019) In vitro conservation of Poincianella pyramidalis (Tul.) L.P. Queiroz under minimal growth conditions. Ciênc Agrotec 43:e014519
Teixeira SL, Ribeiro JM, Teixeira MT (2006) Influence of NaClO on nutrient medium sterilization and on pineapple (Ananas comosus cv Smooth cayenne) behavior. Plant Cell Tissue Organ Cult 86:375–378
Thakur S, Tiwari KL, Jadhav SK (2015) In vitro approaches for conservation of Asparagus racemosus Willd. Vitro Cell Dev Biol-Plant 51:619–625
Torres-Silva G, Resende SV, Lima-Brito A et al (2018) In vitro shoot production, morphological alterations and genetic instability of Melocactus glaucescens (Cactaceae), an endangered species endemic to eastern Brazil. S Afr J Bot 115:100–107
Torres-Silva G, Matos EM, Correia LF, Fortini EA, Soares WS, Batista DS, Otoni CG, Azevedo AA, Viccini LF, Koehler AD, Resende SV, Specht CD (2020) Anatomy, flow cytometry, and x-ray tomography reveal tissue organization and ploidy distribution in long-term in vitro cultures of Melocactus species. Front Plant Sci 11:1314. https://doi.org/10.3389/fpls.2020.01314
Tyagi RK, Goswami TR, Sanayaima R et al (2009) Micropropagation and slow growth conservation of cardamom (Elettaria cardamomum Maton). Vitro Cell Dev Biol-Plant 45:721–729
Volis S, Blecher M (2010) Quasi in situ: a bridge between ex situ and in situ conservation of plants. Biodivers Conserv 19:2441–2454. https://doi.org/10.1007/s10531-010-9849-2
Villalobos VM, Ferreira P, Mora A (1991) The use of biotechnology in the conservation of tropical germplasm. Biotechnol Adv 9:197–215
Wolfe AD (2000) ISSR protocols. http://www.biosci.ohio-state.edu/~awolfe/ISSR/protocols.ISSR.html. Accessed 20 March 2014
Zoghlami N, Chrita I, Bouamama B et al (2007) Molecular based assessment of genetic diversity within Barbary fig (Opuntia ficus indica (L.) Mill.) in Tunisia. Sci Hortic 113:134–141
Acknowledgements
We thank Delmar Lopes Alvim for the help during field work.
Funding
This work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES); the Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB) (Grant No. PNE0020/2011); and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)—SiB-Br (Grand No. 504208/2012–8).
Author information
Authors and Affiliations
Contributions
Conceptualization: SVR, GTS, and ALB; Methodology: SVR, GTS, and ASS; Formal analysis and investigation: GTS, HBB and SVR; Writing—original draft preparation: GTS and SVR; Writing—review and editing: GTS, ASS, HBB, ALB, and SVR; Funding acquisition: SVR and ASS; Resources: SVR and ASS; Supervision: SVR and ASS.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Communicated by Daniel Sanchez Mata.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the Topical Collection: Ex-situ conservation.
Rights and permissions
About this article
Cite this article
Torres-Silva, G., Schnadelbach, A.S., Bezerra, H.B. et al. In vitro conservation and genetic diversity of threatened species of Melocactus (Cactaceae). Biodivers Conserv 30, 1067–1080 (2021). https://doi.org/10.1007/s10531-021-02132-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-021-02132-8