Abstract
Asparagus racemosus is a commercially important medicinal plant, traditionally used for combating gynecological problems in India. The majority of plants used by the pharmaceutical industry come from wild sources, endangering the natural population of the species. The plants are being overharvested, so this species faces a real danger of becoming vulnerable in its natural habitat. Ex situ conservation using in vitro tools is a possible solution to this problem. Ex situ conservation of plants involving in vitro tools has been initiated through axillary branching using nodal explants. Studies on in vitro storage under slow-growth conditions were carried out to develop an efficient protocol for conservation of A. racemosus germplasm. In vitro shoot cultures generally require a 4-wk subculture onto fresh medium when grown at 25 ± 2°C under a 16-h photoperiod. In this research, the use of mannitol or sorbitol as an osmoticum and reduction of sucrose to 1.5% (w/v) in half-strength MS medium led to maintenance of the cultures for 6 mo at 25 ± 2°C with no subculture. Surviving shoots from the slow-growth cultures could be regenerated with 100% efficiency, indicating that the subculture interval was successfully extended by this method. Temperature and medium modification both had significant effects on the growth of stored shoots, and the two factors showed significant interaction. In experiments designed to test encapsulation as a storage method, micropropagated shoot clusters encapsulated in calcium alginate beads were successfully stored up to 75 d at 25 ± 2°C under a 16-h photoperiod. Stored shoots from both storage methods were subsequently recovered and multiplied on MS medium with 3% sucrose and 1.11 μM benzylaminopurine at 25 ± 2°C. Well-developed shoots were rooted and acclimatized successfully.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Asparagus is a member of the Asparagaceae family and contains approximately 200 species distributed throughout the world (Dahlgren et al. 1985). This genus consists of herbs, shrubs, and vines that are known all over the world for their medicinal importance in addition to their use as food. Members of this genus are characterized as herbaceous perennials, tender woody shrubs, and photosynthetic stems (cladodes) (Obermeyer 1983). Asparagus racemosus is an important medicinal plant and is found in tropical and subtropical regions of India. It is a climbing undershrub with leaves reduced to minute scales and spines. Its roots are tuberous, smooth, and tapering at both ends.
The plant is used for its phytoestrogenic properties in combating menopausal symptoms and increasing lactation (Mitra et al. 1999). Steroidal saponins are the major phytoconstituents in A. racemosus that impart its immunomodulant, galactagogue, adaptogen, antitussive, anticarcinogenic, antioxidant, and antidiarrheal properties (Pise et al. 2012). The plant contains saponins called Shatavarin I–X, which support the body’s own production of estrogen. Due to its multiple uses, the demand for this medicinal plant is continuously escalating and has led to destructive harvesting practices, thus endangering natural populations. This plant is being overharvested for its roots, which contain its core properties, and it is thus regarded as vulnerable in its natural habitat (Warrier et al. 2001). The extensive use of A. racemosus has led to its inclusion in a list of 32 medicinal plants prioritized for conservation and development by the National Medicinal Plants Board (2002). Micropropagation protocols for A. racemosus have been standardized (Kar and Sen 1985; Bopana and Saxena 2008; Pant and Joshi 2009). Further, in vitro tools involving slow-growth techniques have paved the way to conserve species ex situ and have been employed in many species (Withers 1980; Dodds and Roberts 1995), including other species of Asparagus (Fletcher 1994; Bekheet 2000). These slow-growth techniques are widely used due to their reliability, as genotypes can be effectively conserved without the loss of viability in the form of disease-free stocks in a controlled environment. Slow-growth techniques are based primarily on conditions that allow minimal growth of cells, tissues, or organs by reducing temperature or adding osmotic regulators and growth retardants to the medium. In general, the most widely applied procedure for minimizing culture growth is temperature reduction, which can be combined with decreased light intensity or storage in the dark (Engelmann 1997). Based on this general observation, Pandey and Sinha (2013) successfully stored A. racemosus cultures at 4°C for 3 mo under dark conditions. A storage period of 3 mo is quite short because cultures would need to be subcultured four times in a year when maintained for germplasm preservation. Further, the low temperature used in the protocol requires cold-storage facilities, which increases the conservation costs and makes the technique uneconomical. Thus, it is necessary to develop more efficient methods for germplasm conservation of this species, with longer subculture intervals.
The aim of the investigation was to devise a conservation technique that is easy to establish, is cost-effective, and provides the maximum regeneration rate for stored cultures. The most ideal conditions for slow-growth storage of plants were determined. We worked to design a conservation technique that utilizes a normal culture-room temperature to store shoot cultures, thereby providing an extended storage period as compared to previous reports on this plant. In addition to slow-growth storage, we also tested an alginate encapsulation technique that could serve as an alternative to cryopreservation. To the best of our knowledge, this is the first time that an alginate encapsulation method has been employed in conserving this plant.
Materials and Methods
Plant material.
Single nodal segments were used for establishment of in vitro cultures and were collected from plants of A. racemosus maintained in the greenhouse of the School of Studies in Biotechnology, Pt. Ravishankar Shukla University, Raipur, India. Disinfected explants (0.1% (w/v) HgCl2 for 10 min) were inoculated into Murashige and Skoog medium, (MS; Murashige and Skoog 1962) with 3% (w/v) sucrose, 0.75% (w/v) agar (Himedia, Mumbai, India), and 2.22 μM 6-benzylaminopurine (BA; Sigma-Aldrich, Saint Louis, MO). The pH of medium was adjusted to 5.8 before autoclaving at 121°C and 15 PSI for 20 min. All cultures were maintained at 25 ± 2°C, under a 16-h photoperiod with a light intensity of approximately 2000 lx provided by cool white fluorescent tubes.
In vitro slow-growth conservation.
The semisolid basal medium used for the study consisted of half-strength MS salts with MS vitamins. All culture media were prepared from stock solutions of macro- and micronutrients according to Chawla (2002) and were solidified with 0.75% (w/v) agar. Sucrose was used as the carbon source at 1.5 or 3% (w/v). Mannitol and sorbitol were added individually to the medium at 2 or 4% (w/v) for slow-growth storage. The vitamins, sucrose, osmotic agents, and agar used in culture media composition were from Himedia. The pH of each medium was adjusted to 5.8, and the molten media were dispensed into 250-ml glass flasks (60 ml per flask) and autoclaved for 15 min at 121°C. To determine the effect of temperature, cultures were maintained at 15 ± 2 or 25 ± 2°C under a 16/8-h light/dark photoperiod provided by daylight fluorescent lamps.
Shoots clusters containing 3–4 shoots were isolated from the proliferating cultures and placed in the slow-growth storage medium described above. The experimental design was fully randomized in a 2 × 7 factorial arrangement, consisting of two temperatures each with seven osmotic treatments (T1–T7; Table 1). Each treatment consisted of six replicates, where each replicate was a flask with three shoot clusters (n = 18 clusters per treatment).
Encapsulation of shoot clusters and culture incubation.
The gelling medium was prepared with liquid MS basal medium containing 3% (w/v) sodium alginate (Himedia). The pH of each medium was adjusted to 5.8. Media were autoclaved for 15 min at 121°C. Shoot clusters containing 2–3 shoots with a length of 0.4–1.0 cm were excised from proliferating cultures, coated with medium-viscosity gelling medium, and placed into a sterile 100 mM CaCl2 solution for 15–25 min. The encapsulated shoot clusters were then washed with deionized water three times and placed in culture bottles containing sterile cotton soaked with sterile distilled water to maintain the relative humidity inside the bottle. To evaluate the temperature and light requirements for successful encapsulation, the encapsulated shoots were stored at 8 ± 2 and 25 ± 2°C and placed under both light (16-h photoperiod) and dark conditions.
Regrowth of stored cultures and data collection.
The medium for growth recovery consisted of semisolid MS basal medium containing 1.11 μM BA and 3% sucrose. All in vitro cultures stored at 15 ± 2°C were regenerated after 4 mo of storage. Cultures stored in T1–T5 treatments at 25 ± 2°C were recovered after 4 mo and the cultures stored in T6 and T7 medium at this temperature were regenerated after 6 mo. For slow-growth storage, observations were recorded every 2 mo under storage conditions; for encapsulated cultures, observations were recorded every 15 d. Plant development was evaluated by measuring survival, number of shoots, and plant height after 4 wk of recovery on MS + 1.11 μM BA and 3% sucrose.
Rooting and acclimatization.
Recovered shoots from the slow-growth storage and encapsulation treatments were rooted and acclimatized according to Bopana and Saxena (2008). Shoot clusters containing 3–4 shoots were inoculated into half-strength MS medium containing 1.61 μM naphthalene acetic acid (NAA), 0.46 μM kinetin (Kin), 98.91 μM adenine sulfate, 500 mg/l malt extract, 198.25 μM phloroglucinol (PG), and 3% sucrose. After 40 d in rooting medium, rooted plants were hardened by transplanting them into pots containing cocopeat for 15 d and eventually into a mixture of soil and sand (2:1).
Statistical analysis.
One- or two-way analysis of variance (ANOVA) appropriate for the design was carried out to detect the significance of differences among the treatment means. Means were compared using Duncan’s multiple range test at a 5% probability level using software SPSS 16.0.
Results and Discussion
In vitro slow-growth conservation.
The cultures kept at 15 ± 2°C grew slowly but survived for only 4 mo. Effective storage for 6 mo was observed at 25 ± 2°C when the carbon source was used at 1.5% in half-strength MS medium with 2% of an osmotic regulator (Table 1).
A two-way ANOVA (Table 2) revealed that the two storage temperatures and seven treatments used in the study had significant effects on the growth of stored shoots. Moreover, these two factors showed significant interaction effects for both shoot number and shoot length under storage conditions.
Effect of temperature.
Of the two storage temperatures tested, 25 ± 2°C was significantly more effective in maintaining culture viability under storage conditions in terms of survival percentage, shoot length, and shoot number (Tables 1 and 3). The survival percentage after 2 mo of storage was much higher in cultures stored at 25 ± 2°C than those stored at 15 ± 2°C (Table 1). Low temperature reduced shoot growth but at the same time exerted a deleterious effect on shoot survival and quality regardless of the osmotic treatment applied. The most effective treatment in terms of shoot survival at 15 ± 2°C was provided by the addition of 2% sorbitol and reduction of the sucrose concentration. Under these conditions, shoots were able to survive for 4 mo. Slow-growth conservation in A. racemosus has also been practiced by Pandey and Sinha (2013), who achieved 3 mo of storage at 4°C in the dark. Although their protocol slowed shoot growth, the storage period was short and the regeneration efficiency of stored shoots was not evaluated. The authors also reported drying and whitening of shoots at 25°C, which may be due to storage of cultures in the dark. Through our experiments, an extended storage period was achieved in a standard culture room. Although low temperatures may be more effective for slowing the growth of some in vitro cultures (Fletcher 1994; Akdemir et al. 2010), Tyagi et al. (2009) were able to conserve cardamom effectively at 25 ± 2°C, consistent with our findings. Thus, the temperature requirements appear to vary from species to species and may depend on the agro-climatic conditions in which a particular species is found. Tropical and subtropical plant species are generally cold sensitive and undergo chilling injury at low temperature (Engelmann 1991).
Effect of osmoticum and carbon source concentration.
In order to restrict the growth of A. racemosus, storage medium was modified by addition of mannitol or sorbitol at 2 or 4% and reduction of sucrose to lower levels (from 3 to 1.5%). These modifications induced slow growth relative to the control, and in the best treatments 75.00% (T7) and 77.78% (T6) of the cultures could be maintained for a period of 6 mo at 25 ± 2°C (Table 1). Out of seven treatments tested, only two, i.e., half-strength MS medium with 1.5% sucrose and 2% mannitol or sorbitol, allowed the cultures to be maintained for over 6 mo. Shoot number and shoot length were both significantly greater during storage in these two media than in most other treatments (Table 4). The plantlets maintained in these media showed maximum survival with 100% regeneration of surviving (green) shoots.
Short- and medium-term storage of plant tissues under in vitro culture conditions leads to increased oxidative stress and senescence. Mannitol acts as a scavenger of hydroxyl radicals and protects plant tissues against oxidative stress damage (Shen et al. 1997; Abebe et al. 2003). Previous trials (Pandey and Sinha 2013) in this plant suggested that 1% mannitol was beneficial for the in vitro storage of A. racemosus. Also, in other species such as potato (Solanum tuberosum: Sarkar and Naik 1998) and enset (Enset ventricosum: Negash et al. 2001), the addition of mannitol to the storage medium resulted in improved survival of stored cultures. The sugar-alcohol sorbitol has an osmotic potential similar to that of mannitol in solutions at equivalent molarity and has been employed as an osmoticum in many species (Cordeiro et al. 2014). Fletcher (1994) incorporated 4% sorbitol in the conservation medium for storage of Asparagus officinalis L. up to 16 mo. Moreover, the effect of these osmotic agents depends on the temperature, genotype, and culture conditions, as suggested by Marino et al. (2010). Mannitol has often been found to have deleterious effects on the quality of stored cultures (Conner and Falloon 1993; Cordeiro et al. 2014), in contrast to our findings. In our experiments, effective storage was achieved by incorporation of mannitol at low concentrations into the medium, whereas higher concentrations of osmoticum were deleterious to in vitro-stored cultures of A. racemosus. A positive effect of mannitol was reported by Marino et al. (2010) and Kovalchuk et al. (2009), who used gas-permeable culture vessels. We also used cotton plugs to close the mouths of the flasks, i.e., they were not air-tight or sealed. This could explain the positive effect of mannitol and sorbitol on in vitro-stored cultures.
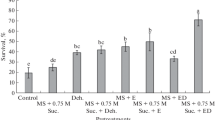
The efficacy of each technique used for slow-growth storage was measured by the regeneration percentage and the quality of shoots regenerated after fixed periods of storage. Cultures stored at 15 ± 2°C were recovered after 4 mo of storage, and cultures at 25 ± 2°C were recovered after 4 mo (T1–T5) or 6 mo (T6–T7) in MS medium with 1.11 μM BA (Fig. 1). The regeneration percentage was found to be 100% irrespective of the storage medium and temperature used. All of the cultures that survived under storage conditions regenerated fully and had a normal multiplication rate. The shoots stored on media with 2% osmoticum and 1.5% sucrose (treatments T6 and T7) had good proliferation rates when regenerated. The cultures stored on medium with 2% sorbitol with 3% sucrose (T4) also displayed a good proliferation rate, which again demonstrated that a lower osmoticum level is best suited for storage of shoots of this plant. This technique thus substantially increased the interval between subcultures, allowing in vitro cultures of A. racemosus to be maintained for several years. Figure 2a–c shows examples of stored cultures and their regrowth after storage. In the present study, a storage period of more than 6 mo was difficult to achieve due to desiccation of the medium. Other strategies such as the use of growth retardants might help and would need to be standardized.
Mean values of (A) number and (B) length of shoots regenerated after 4 mo (T1–T5) or 6 mo (T6–T7) from slow-growth-conserved cultures of A. racemosus stored at 25 ± 2°C. Parameters recorded 4 wk after transfer to MS + 1.11 μM BA. Within each figure part, values marked with different letters differ significantly at 5% as analyzed by Duncan’s multiple range test.
Slow-growth storage, encapsulation, and regeneration of Asparagus racemosus shoot cultures. (a) Stored shoots at 25 ± 2°C on half-strength MS + 2% mannitol + 1.5% sucrose. (b) Stored shoots at 25 ± 2°C on half-strength MS + 2% sorbitol + 1.5% sucrose. (c) Regenerating shoots 10 d after transfer into MS + 1.11 μM BA stored up to 6 mo. (d) Small shoot clusters encapsulated in sodium alginate beads. (e) Shoots emerging from encapsulated beads when transferred to MS + 1.11 μM BA for regrowth. (f) Regenerating plantlet from encapsulated beads 10 d after transfer to MS + 1.11 μM BA. (g) Well-developed shoots after regeneration from slow-growth stored shoots. (h) Rooted plantlet. (i) Hardening of plantlets in cocopeat.
Encapsulation of shoot clusters.
Encapsulation technology has been worked out by many researchers in recent years for different plant species. Vegetative propagules of uninodal cuttings have been used for encapsulation of woody plants such as mulberry (Morus indica and Morus alba: Bapat and Rao 1990; Pattnaik et al. 1995; Pattnaik and Chand 2000), olive (Micheli et al. 1998), and the medicinal plant Withania sp. (Singh et al. 2006) using cold storage.
The most important feature of the encapsulated propagules is their capability to retain viability in terms of regrowth abilities after encapsulation and even after storage (Standardi and Piccioni 1998). In this study, the encapsulated shoot cultures could be effectively stored at 25 ± 2°C for 75 d when 16/8-h light/dark conditions were provided (Table 5). Figure 2d–f shows encapsulated shoots and their regeneration. There was significant interaction observed between light and storage temperature that affected the survival of encapsulated shoots. Low temperature was not suitable for storage of encapsulated shoots as they lost viability within 30 d irrespective of the light/dark conditions. On the other hand, the viability of encapsulated shoots stored at 25 ± 2°C was markedly influenced by light: after 30 d of storage greater viability was observed when illumination was provided than in continuous darkness. Temperature and light requirements have been studied in different species (Micheli et al. 2007; Sujatha and Ranjitha Kumari 2008), and a number of studies report that normal growth-room temperature and low illumination is beneficial for encapsulation of plant parts (Janeiro et al. 1995; Divakaran et al. 2006). The viability and regeneration capacity was checked every 30 d by inoculating the shoots into MS medium containing 1.11 μM BA. All the encapsulated microcuttings stored at 25 ± 2°C showed viability immediately after encapsulation and after 1 mo of storage. After that, the regeneration percentage started declining and reached an average of 60% regeneration after 75 d of storage at 25 ± 2°C (Table 6). The shoot number and shoot length of the cultures regenerated from encapsulated shoots are given in Table 6. The stored cultures were fully regenerated, rooted, and acclimatized as shown in Fig. 2g–i . After acclimatization, 90% survival of regenerated plants was obtained.
The present study describes an efficient and cost-effective protocol for short- and medium-term conservation of A. racemosus. Through the slow-growth storage procedure, shoot cultures could be maintained without subculturing for 6 mo at 25 ± 2°C, which is much more economical than cryopreservation that requires deep freezing or vitrification. Moreover, encapsulation of shoot cultures into alginate beads could provide a method for its short-term storage and easy renewal of cultures when required. This is the first report of in vitro slow-growth storage of A. racemosus for up to 6 mo and utilization of encapsulation technology in conservation of this plant.
References
Abebe T, Guenzi AC, Martin B, Cushman JC (2003) Tolerance of mannitol-accumulating transgenic wheat to water stress and salinity. Plant Physiol 131:1748–1755
Akdemir H, Kaya E, Ozden Y (2010) In vitro proliferation and minimum growth storage of Fraser photinia: influences of different medium, sugar combinations and culture vessels. Sci Hortic 126:268–275
Bapat VA, Rao PS (1990) In vivo growth of encapsulated axillary buds of mulberry (Morus indica L.). Plant Cell Tissue Organ Cult 20:67–70
Bekheet SA (2000) In vitro preservation of Asparagus officinalis. Biol Plant 43:179–183
Bopana N, Saxena S (2008) In vitro propagation of a high value medicinal plant: Asparagus racemosus Willd. In Vitro Cell Dev Biol Plant 44:525–532
Chawla HS (2002) Introduction to plant biotechnology. Science Publishers Inc., Enfield
Conner AJ, Falloon PG (1993) Osmotic versus nutritional effects when rooting in vitro asparagus minicrowns on high sucrose media. Plant Sci 89:101–106
Cordeiro SZ, Simas NK, Henriques AB, Sato A (2014) In vitro conservation of Mandevilla moricandiana (Apocynaceae): short-term storage and encapsulation–dehydration of nodal segments. In Vitro Cell Dev Biol Plant 50:326–336
Dahlgren RMT, Clifford HT, Yeo PF (1985) The families of the monocotyledons. Springer, Heidelberg
Divakaran M, Nirmal Babu K, Peter KV (2006) Conservation of Vanilla species, in vitro. Sci Hortic 110:175–180
Dodds JH, Roberts LW (1995) Experiments in plant tissue culture. Cambridge University Press, New York
Engelmann F (1991) In vitro conservation of tropical plant germplasm—a review. Euphytica 57:227–243
Engelmann F (1997) In vitro conservation methods. In: Callow JA, Ford-Lloyd BV, Newbury HJ (eds) Biotechnology and plant genetic resources. CAB International, Wallingford, pp 119–161
Fletcher PJ (1994) In vitro long‐term storage of Asparagus. N Z J Crop Hortic 22:351–359
Janeiro LV, Vieitez AM, Ballester A (1995) Cold storage of in vitro cultures of wild cherry, chestnut and oak. Ann Sci 52:287–293
Kar DK, Sen S (1985) Micropropagation of Asparagus racemosus. Plant Cell Tissue Organ Cult 5:89–95
Kovalchuk I, Lyudvikova Y, Volgina M, Reed BM (2009) Medium, container and genotype all influence in vitro cold storage of apple germplasm. Plant Cell Tissue Organ Cult 96:127–136
Marino G, Negri P, Cellini A, Masia A (2010) Effect of carbohydrates on in vitro low-temperature storage of shoot cultures of apricot. Sci Hortic 126:434–440
Micheli M, Mencuccini M, Standardi A (1998) Encapsulation of in vitro proliferated buds of olive. Adv Hortic Sci 12:163–168
Micheli M, Hafiz IA, Standardi A (2007) Encapsulation of in vitro-derived explants of olive (Olea europaea L. cv. Moraiolo) II. Effects of storage on capsule and derived shoots performance. Sci Hortic 113:286–292
Mitra SK, Gopumadhavan S, Venkataranganna MV, Sarma DNK, Anturlikar SD (1999) Uterine tonic activity of U-3107, a herbal preparation, in rats. Indian J Pharm 31:200–203
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
National Medicinal Plants Board (2002) http://www.nmpb.nic.in. Accessed Jun 2014
Negash A, Krens F, Schaart J, Visser B (2001) In vitro conservation of enset under slow-growth conditions. Plant Cell Tissue Organ Cult 66:107–111
Obermeyer AA (1983) Protasparagus Oberm. nom. nov.: new combinations. S Afr J Bot 2:243–244
Pandey A, Sinha A (2013) Effect of mannitol, sorbitol and sucrose on growth inhibition and in vitro conservation of germplasm of Asparagus racemosus—an important medicinal plant. Med Plants Int J Phytomed Relat Industries 5:71–74
Pant KK, Joshi SD (2009) In vitro multiplication of wild Nepalese Asparagus racemosus through shoots and shoot induced callus cultures. Bot Res Int 2:88–93
Pattnaik SK, Chand PK (2000) Morphogenic response of the alginate encapsulated axillary buds from in vitro shoot cultures of six mulberries. Plant Cell Tissue Organ Cult 60:177–185
Pattnaik SK, Sahoo Y, Chand PK (1995) Efficient plant retrieval from alginate-encapsulated vegetative buds of mature mulberry trees. Sci Hortic 61:227–239
Pise M, Rudra J, Bundale S, Begde D, Nashikkar N, Upadhyay A (2012) Asparagus racemosus cell cultures: a source for enhanced production of shatavarins and sarsapogenin. In Vitro Cell Dev Biol Plant 48:85–91
Sarkar D, Naik PS (1998) Factors affecting minimal growth conservation of potato microplants in vitro. Euphytica 102:275–280
Shen B, Jensen RG, Bohnert HJ (1997) Mannitol protects against oxidation by hydroxyl radicals. Plant Physiol 115:527–532
Singh AK, Varshney R, Sharma M, Agarwal SS, Bansal KC (2006) Regeneration of plants from alginate-encapsulated shoot tips of Withania somnifera (L.) Dunal, a medicinally important plant species. J Plant Physiol 163:220–223
Standardi A, Piccioni E (1998) Recent perspectives on the synthetic seed technology using non-embryogenic in vitro-derived explants. Int J Plant Sci 159:968–978
Sujatha G, Ranjitha Kumari BD (2008) Micropropagation, encapsulation and growth of Artemisia vulgaris node explants for germplasm preservation. S Afr J Bot 74:93–100
Tyagi RK, Goswami R, Sanayaima R, Singh R, Tandon R, Agrawal A (2009) Micropropagation and slow growth conservation of cardamom (Elettaria cardamomum Maton). In Vitro Cell Dev Biol Plant 45:721–729
Warrier PK, Nambiar VPK, Ganapathy PM (2001) Some important medicinal plants of the western ghats, India: a profile. International Development Research Centre, Artstock, p 15
Withers LA (1980) Tissue culture storage for genetic conservation. IBPGR technical report. International Board for Plant Genetic Resources, FAO/ United Nations, Rome
Acknowledgment
We acknowledge the School of Studies in Biotechnology, Pt. Ravishankar Shukla University, Raipur, India, for providing the necessary lab facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Barbara Reed
Rights and permissions
About this article
Cite this article
Thakur, S., Tiwari, K.L. & Jadhav, S.K. In vitro approaches for conservation of Asparagus racemosus Willd.. In Vitro Cell.Dev.Biol.-Plant 51, 619–625 (2015). https://doi.org/10.1007/s11627-015-9706-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-015-9706-9