Abstract
Opuntia spp. belong to the Cactaceae family and are native to Central America. The most economically important species is O. ficus indica, cultivated both for fruits and cladodes. The genus includes other important edible species (from diploid to octoploid) that occur worldwide as either wild or cultivated species in many arid or semiarid areas (e.g., the Mediterranean region). Several accessions are cultivated in different growing regions, but little is known about their ancestries and levels of genetic diversity. The aim of this study was to investigate the level of intraspecific genetic diversity among O. ficus indica cultivated varieties and some related species. Specifically, six highly polymorphic simple sequence repeats (SSR) and two expressed sequence tag (EST)-SSR loci were investigated in 62 wild and cultivated genotypes belonging to 16 Opuntia species. The clusters identified by the distance and model-based analyses clearly separated the wild opuntias from the cultivated ones. However, the O. ficus indica accessions did not cluster separately from other arborescent cactus pear species, such as O. amyclaea, O. megacantha, O. streptacantha, O. fusicaulis, and O. albicarpa, indicating that their current taxonomical classifications do not fit with their genetic variability. In general, the genotypes cultivated in Mexico showed high levels of diversity, whereas most of the spineless accessions collected in other countries had a very narrow genetic base. This study increases our knowledge of the variability among some of the most diffused Opuntia cultivated accessions. This study also points to the inconsistencies of previous taxonomical genotype assignments that were based solely on morphological characteristics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Opuntia belongs to the Cactaceae family (subfamily Opuntioideae) and includes several species that originated in North and South America, some of which were relatively recently distributed throughout the world. The number of species included in the genus is not known (Chávez-Moreno et al. 2009), but the number of species identified in the genus ranges from 160 (Gibson and Nobel 1986) to 250 (Britton and Rose 1963). This difference is mainly due to nomenclatural problems occurring not only in Opuntia, but also within other genera of the Opuntioideae family. The main reasons for such taxonomical chaos are the paucity of morphological characters, the high level of phenotypic plasticity within many opuntioid taxa, the recent diversification, the occurrence of polyploidy, and intra- and intergeneric hybridization (Wallace and Gibson 2002). Within the Opuntioideae family, ploidy levels range from diploid to octoploid (Felker et al. 2006), with the polyploid species representing more than 64% of the total (Pinkava et al. 1998).
Opuntias are often divided into cylindropuntias and platyopuntias. Cylindropuntias are shrubby species with cylindrical stems (or joints). Platyopuntias, which have flattened stems called cladodes (Gibson and Nobel 1986), include agronomically important species that are cultivated as both fruit and forage crops. Cultivated opuntias include O. megacantha, O. streptacantha, O. albicarpa, O. amyclaea, O. robusta, O. hyptiacantha, and O. cochenillifera (syn. Nopalea cochenillifera, primarily grown in Mexico as a forage crop) among others (Pimienta-Barrios 1994; Scheinvar 1995; Kiesling 1998; Mondragón-Jacobo 2001). The most diffused and economically important species is O. ficus indica. This species, commonly referred to as cactus pear, prickly pear, Indian fig, Barbary fig, etc., was probably domesticated about 9,000 years ago in central Mexico and diffused in several warm regions of the world by European travelers beginning in the late 15th century (Kiesling 1998; Griffith 2004).
The taxonomical concept of O. ficus indica is somewhat uncertain. This species is sometimes described as spineless and taxonomically distinct from other cultivated opuntias, such as O. megacantha, O. streptacantha, and O. amyclaea (Britton and Rose 1963; Scheinvar 1995; Reyes-Agüero et al. 2005). In other cases (Gibson and Nobel 1986; Pimienta-Barrios 1994; Felker et al. 2005), spined, cultivated genotypes have been classified as O. ficus indica. Actually, the presence of spines in the cladodes is an inadequate feature to discriminate O. ficus indica from other arborescent opuntias (Nieddu and Chessa 1997; Kiesling 1998; Felker et al. 2005). Within the genus, the growth habit, the presence of spines, the number of spines per areole, and the number of areoles may differ drastically in different growing regions (Rebman and Pinkava 2001).
Mexico and Italy are the most important cactus pear producers (Mondragón-Jacobo 2001). In Mexico, about 90% of the marketed fruits are produced from six cultivars, namely ‘Reyna’ (the most diffused cultivar), ‘Cristalina’, ‘Chapeada’, ‘Naranjona’, ‘Amarilla montesa’, and ‘Roja pelona’. Other varieties are marginally cultivated (Pimienta-Barrios 1994; Mondragón-Jacobo and Pérez-González 1996). Cactus pear production in Italy is exclusively based on three cultivars, ‘Bianca’ (white), ‘Gialla’ (yellow), and ‘Rossa’ (red), each with some variants. The cultivar names are exclusively based on fruit characteristics (peel and flesh color), both in Italy and in Mexico. Other varieties are also cultivated in North Africa, Israel (‘Ofer’), the United States (‘Andyboy,’ similar to the Italian ‘Rossa’) (Mondragón-Jacobo 2001), and South America (Felker et al. 2005). Other spineless accessions, the so-called Burbank varieties, are cultivated in South Africa. These genotypes were imported in the early 1900s from the United States (Brutsch and Zimmermann 1993; Potgieter and Mashope 2009).
Today, cactus pear commercial cultivars are octoploid (Felker et al. 2006), but their ancestry is unknown. Moreover, several authors reported that it was difficult to correctly assign cultivated genotypes to a defined taxon (Kiesling 1998; Felker et al. 2006; Mondragón-Jacobo 2001; Labra et al. 2003). The continuous morphological variation within the genus, the lack of clear descriptors for each species, and the relative ease of cross hybridization (with the presence of individuals with intermediate characteristics) has led to an erroneous species designation. As a result of incorrect assignments, the same varieties are often classified as belonging to different species, and in other cases they are considered to be hybrids among unknown parentals. To overcome these problems, molecular markers, which are reproducible and stable, might be useful tools to help unravel uncertainties in classification that are not addressed by morphological characterization.
In the past two decades, few studies have been performed to characterize existing germplasm collections using random molecular markers (Wang et al. 1998; Mondragón-Jacobo 2003; Zoghlami et al. 2007; Luna-Paez et al. 2007; García-Zambrano et al. 2009; Souto Alves et al. 2009). Two important studies (Labra et al. 2003; Griffith 2004) employed different molecular tools to elucidate taxonomical aspects of the genus, particularly the origin of O. ficus indica. Labra et al. (2003) used AFLP to verify the lack of genetic differentiation between O. ficus indica and O. megacantha populations and suggested that O. ficus indica should be considered to be a domesticated form of the spiny O. megacantha. On the other hand, Griffith (2004) considered the species to be a group of different clones, selected for their low number of spines and their fleshy fruits, that were derived from different parentals, most likely from other arborescent opuntias from central and southern Mexico. Most of these DNA-based analyses revealed discrepancies between molecular characterization and classical taxonomical classification.
Recently, other ambiguities in the taxonomic classification of Opuntia species emerged in a study that used microsatellite polymorphisms to try to discriminate between two morphologically distinct O. echios botanical varieties (echios and gigantea) native to the Galapagos Islands (Helsen et al. 2009). Once again, the authors highlighted that the current taxonomic differentiation between these taxa was not supported by molecular data.
Although these studies clarified some taxonomical aspects of the genus and were useful for cultivar fingerprinting, there is still a lack of knowledge regarding the level of genetic diversity among the most diffused cultivated genotypes throughout the world and the diversity of cultivars, wild genotypes, and species related to O. ficus indica.
Our aim was to elucidate the level of differentiation among cactus pear genotypes, including the most widespread cultivars, and their relation with wild accessions and related species. Our study also helps overcome some of the discrepancies that occur when Opuntia germplasm is classified solely on the basis of morphological features.
Materials and methods
Plant material
Sixty-two genotypes belonging to 16 Opuntia species (Table 1) were collected from several areas throughout the world and introduced into the experimental fields of Catania University. The analyzed genotypes consisted of cultivars, wild genotypes, and cactus pear–related species (Table 2).
Some of the accessions were sampled from different cultivated areas of Sicily. Another group of accessions (provided by Dr. Mondragón-Jacobo) consisted of the most diffused Mexican varieties, classified with different binomials or generically as Opuntia sp. (Pimienta-Barrios 1994; Mondragón-Jacobo and Pérez-González 1996; Fernandez-Montes et al. 2000), plus accessions of unknown origin. The third group of accessions (provided by Dr. Yaron Sitrit) consisted of cactus pear varieties collected in different countries (Kenya, South Africa, USA, and Mexico), artificial hybrids, and cactus pear–related species belonging to a germplasm collection from the Hebrew University of Jerusalem (Rehovot, Israel).
Microsatellite analysis
DNA was isolated from the outermost layer of mature cladodes using the ISOLATE Plant DNA Mini Kit (Bioline, London, UK). Opuntia-specific microsatellite primers were obtained from Helsen et al. (2007), who isolated 16 polymorphic SSRs from O. echios. Moreover, a set of 122 O. streptacantha–expressed sequence tags (accession no. from EX720493 to EX720614) found in the dbEST database was analyzed using Msatfinder software (Thurston and Field 2005) to search for sequences containing microsatellites of 20 or more bases. Three unigenes that contained SSRs (accession nos. EX720574, EX720594, and EX720605) were identified. EST-SSR primers were designed with Primer 3 software (Rozen and Skaletsky 2000). All primer pairs were synthesized by Eurofins MWG Operon (Ebersberg, Germany), and the forward primer from each set was tailed with M13F for labeling (Oetting et al. 1995).
The 3 EST-SSRs and the 16 SSRs were tested on six of our genotypes belonging to different species to search for polymorphisms. Amplifications were carried out separately for each primer pair. The PCR reaction included the two specific primers (0.3 μM) plus a labeled M13F primer (0.13 μM), approximately 50 ng of template DNA, 0.2 mM dNTPs, 1× PCR buffer II, 2 mM magnesium chloride, and 1 U of AmpliTaq Gold DNA polymerase (Applied Biosystems, Foster City, CA, USA). PCR was performed at 95°C for 12 min; followed by 35–40 cycles of 95°C for 1 min, 53–55°C for 1 min (the annealing temperature was specific for each primer pair), and 72°C for 1.5 min; and one final cycle of 72°C for 15 min. The PCR reactions were performed using GeneAmp 9700 and 2700 amplifiers (Applied Biosystems). An aliquot of 0.5–2 μl of PCR product (depending on the performance of amplification of each primer pair) was mixed with 10 μl of Formamide and 0.35 μl of LIZ-500 size standard and denatured at 95°C for 5 min. Up to three PCR products labeled with FAM, PET, or NED were pooled before separation in the ABI 310 Genetic Analyzer (Applied Biosystems) and analysis using Genemapper 4.0 software.
For more precise analysis of the allele patterns at each SSR locus, we repeated each amplification two or three times. Also, because SSR profiles of polyploids are influenced by the allele dosage at each peak as well as the PCR performance at each binding site, we tested each primer pair on progeny (10 individuals) of O. ficus indica ‘Bianca’ × ‘O. amyclaea 27′ to examine the segregation patterns at each locus.
Distance and model-based analyses
The analysis of microsatellite markers is not as straightforward in polyploids as it is in diploid individuals. An efficient method called MAC-PR (microsatellite DNA allele counting–peak ratios) has been developed for polyploid species with polysomic inheritance to determine the number of allele copies in microsatellite loci (Esselink et al. 2004).
For our statistical analyses, we considered the SSR peaks to be dominant markers. Although this strategy reduced the information generated by each primer pair, we believe that it was a useful way to deal with highly polyploid individuals belonging to different species (and likely with interspecific hybrids). The estimation of allele frequencies based on PCR dosage is subject to several types of errors, due in part to variations in the SSR flanking regions that could cause partial primer mismatches. Moreover, we didn’t observe fully heterozygous individuals in all SSR loci; such individuals are desirable for MAC-PR analysis to determine the ratios that are used as a baseline for calculations of allele quantification in partial homozygotes (Landergott et al. 2006). In addition to these aspects, due to the presence of stutter bands in some of the analyzed loci, and due to the high number of alleles per locus (Table 2), the application of MAC-PR might not be considered a robust method to estimate all the allele frequencies in our dataset.
To better understand the allelic patterns at each locus, we tested amplification of the eight SSRs on a small population of ten genotypes resulting from the hybridization of the Sicilian cultivar ‘Bianca’ with a spiny clone classified as O. amyclaea (O. amyclaea 27) (data not shown).
Each scored allele was treated as a dominant marker, and a binary matrix based on the presence or absence of each peak was then created. PowerMarker Version 3.25 (Liu and Muse 2005) was used to generate a similarity matrix and to construct a neighbor-joining (NJ) tree based on the log-transformed proportion of shared alleles (Bowcock et al. 1994). This technique had been used previously to assess the genetic diversity of nonnatural populations such as germplasm collections (Dangl et al. 2001; Barkley et al. 2006) and seemed to be the most appropriate for our analysis because it assumes that alleles are equally related, since the evolution of these alleles is unknown, and makes no assumptions about the structure of the population.
For the construction of the NJ tree, the species O. subulata, often referred to as Austrocylindropuntia subulata (Muehlenpfordt) Backeberg, was considered to be an outgroup. This species belongs to a basal lineage of the subfamily Opuntioideae (Wallace and Gibson 2002). It has very distinctive morphological features, such as cylindrical joints, which make it different from the other analyzed genotypes.
PowerMarker was also utilized to determine the average polymorphism information content (PIC value) for each primer pair. The NJ tree was visualized using Treeview (http://taxonomy.zoology.gla.ac.uk/rod/rod.html). Bootstrap analysis was performed with 1,000 replicates using PAUP* Version 4.0b10 (Swofford 1998).
Also, microsatellite data were used to perform a network analysis using the software SplitsTree version 4.11.3 (Huson and Bryant 2006). Specifically a NeighborNet network (Bryant and Moulton 2004) was computed based on the Dice distances.
In addition, a model-based Bayesian analysis was performed to evaluate the genetic structuring of the Opuntia germplasm and to identify admixed individuals using STRUCTURE software version 2.3.2 (Pritchard et al. 2000). This analysis allows one to probabilistically assign each individual to a population (K) or jointly to two or more populations if the individual’s genotype indicates admixture (Pritchard et al. 2000). STRUCTURE simulations can be performed without prior knowledge of the real population structure and without information regarding the origin of the genotypes. Due to differences in ploidy level (from 2X to 8X), each SSR marker was considered to be a dominant marker. All genotypes were then analyzed using the diploid dominant STRUCTURE data model. Because the analyzed genotypes were likely the result of natural and artificial hybridization, simulations were performed by setting K to between 1 and 14 using the “admixture ancestry” and the “correlated allele frequency” parameters. The burn-in was set to 30,000 followed by 70,000 Markov chain Monte Carlo (MCMC) iterations, and each run was replicated 15 times. The posterior probabilities of K [Pr (X|K)] were calculated as suggested by Pritchard et al. (2000) to infer the most appropriate value of K for our dataset.
Results and discussion
Microsatellite amplifications
Among the 19 SSR and EST-SSR primer pairs tested on six genotypes, 11 were discarded because they produced no amplification products in the majority of the analyzed genotypes or produced peak profiles that were not easy to interpret. These interpretation problems were mainly due to excess stutter bands or to the presence of more peaks than the ploidy level. The other eight primers (six O. echios SSRs and two O. streptacantha EST-SSRs) gave reliable and reproducible profiles over two or three replications (Fig. 1). The number of alleles per locus ranged from one to eight in the analyzed genotypes and 48 out of the 62 genotypes showed an average of more than four alleles per locus (Table 2).
The eight primers were then used to fingerprint 62 Opuntia genotypes previously classified as belonging to 16 putative species, including cultivated genotypes, artificial hybrids, and wild accessions. Of the eight SSR primer pairs, only one, OP3, failed to amplify in a small set of genotypes (O. quimilò, O. cochenillifera, O. streptacantha, ‘93042’, ‘R6’, ‘2.10.58’, ‘24’). This failure might be due to different mutation events at the level of the microsatellite and/or of the primer binding sites. The other primers successfully amplified fragments in all of the genotypes, showing a high degree of cross-transferability among the analyzed species.
Most of the analyzed genotypes have a documented ploidy level ranging from 2 to 8 (Table 1). The SSR primer pairs amplified a maximum of eight bands per genotype. Although we could not be sure if each SSR was single locus or multilocus, the number of peaks generated by each SSR was related to the known ploidy level of the taxa; the lowest number of amplicons was produced from the diploid species O. cochenillifera and O. quimilò and the highest number of amplicons was obtained from the octoploid cultivated varieties.
The SSRs loci exhibited a high level of polymorphism, with an average number of 16.9 alleles per locus. Considering the average PIC values for each primer pair, the most informative SSR loci were Ops24 (0.25), OP9 (0.23), Ops9 (0.22), and OP13 (0.21) (Table 3). The analysis generated enough polymorphic markers to discriminate among most of the genotypes, except for a few clones collected from various areas representing spineless fleshy-fruited cultivated genotypes. Specifically, five Sicilian clones (‘Rossa’, ‘Rossa trunzara 2’, ‘Gialla’, ‘Cuore’, ‘Linosa’) had the same profile as the South African ‘Gymno carpo’ and ‘Japie’ accessions and the ‘Kenya-I’ accession collected in Israel. Also, no marker distinguished between two clones of ‘Bianca trunzara’ versus ‘Gialla trunzara’ (which have different fruit colors), the Sicilian ‘Bianca sorba’ versus the accession ‘Kenya-II’, and the two different clones classified as O. amyclaea (O. amyclaea ‘23’ and ‘27’).
Distance-based analysis
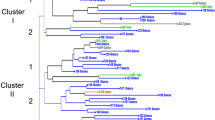
Distance-based analysis was performed using NJ. Also, to overcome the limits of the phylogenetic tree, where not all the branches were fully supported, we performed a network analysis based on the NeighborNet (NN) method (Bryant and Moulton 2004). This kind of analysis might be useful to better elucidate patterns of variability in case of reticulate evolution as in the case of Opuntia. In both the NJ and NN tree (Figs. 2, 3), most of the O. ficus indica–related species (O. robusta, O. elizondoana, O. spinulifera, O. vulgaris, O. quimilò, O. oligacantha, O. joconostle, O. cochenillifera, and O. subulata) are grouped together and are clearly separated from the cultivated varieties.
Neighbor-joining dendrogram of 62 Opuntia genotypes based on the log-transformed proportion of shared alleles. The numbers at the branch points indicate bootstrap support values >50% (1,000 replicates). Colors indicate different geographic origin (green Italy, red Mexico, blue Israel). Underlined names indicate cultivated varieties. The presence of spines in the cladodes is indicated by asterisks (* few; ** intermediate; *** many)
Some of the groups in the NJ tree were not strongly supported by bootstrap analysis, probably due to the presence of several hybrids. In fact, when the same distance-based analysis was performed without some putative hybrids (recognized by STRUCTURE analysis) higher bootstrap values were obtained (data not shown). However the groups of the NJ tree matched those obtained in NN.
The NN showed the presence of many parallel edges, especially among the octoploid opuntias (cultivated varieties and other cactus pear genotypes of unknown origin), indicating the presence of incompatible splits (appearing as boxes) among them. In this sense, network analyses can better explain the relationship among the analyzed genotypes, which are likely the results of complex evolution including polyploidization, hybridization, and recombination.
In NJ and NN analysis, the prickly pears classified as O. ficus indica, O. amyclaea, O. megacantha, O. streptacantha, O. fusicaulis, O. albicarpa, O. leucotricha, or generically as Opuntia sp., formed a major cluster divided into subgroups that fit poorly with their current taxonomical classification. Also, the clustering did not correspond to spinescence, which has been considered to be a distinctive characteristic for taxonomical classification purposes (Britton and Rose 1963; Scheinvar 1995; Reyes-Agüero et al. 2005). The Mexican varieties displayed a high level of diversification, whereas the spineless Sicilian varieties had a very narrow genetic base and were closely related to other spineless accessions coming from Israel (‘Jerico’), Kenya, South Africa (‘Gymno carpo’), and the United States (‘Texas’). This fact was particularly evident in NN analysis, where all the Sicilian varieties and the above-mentioned spineless accessions appeared strictly connected and clearly separated from the other accessions. Despite little or no divergence at all eight loci, some of these genotypes could be distinguished by their fruit color or fleshiness; it is likely that the phenotypic variation was the result of somatic mutations of a few clones that occurred in the cultivated region after the 16th century when opuntias started to become naturalized in the Mediterranean region and later in other warm regions of the world.
Other spineless genotypes included in the analysis, such as the Sicilian ‘Inerme’, the South African ‘Skinners court’ and ‘Fusicaulis’, and the accession ‘Castillo’, diverged from the main group of spineless cultivars. These results confirmed the hypothesis of Griffith (2004), who suggested that the spineless varieties might have originated from different ancestors.
Model based analysis
Natural and artificial cross-hybridization among Opuntia accessions has previously been reported (Wang et al. 1996; Griffith 2004; Reyes-Agüero et al. 2006). In our study, where natural and artificial hybrids are included, the construction of a phylogenetic tree alone did not fully reveal the relationships among genotypes. This is because hybrid individuals will often cluster with either one group or the other in the dataset, but not with both. Moreover, NN indicated the presence of several splits among the accessions. In such cases, a model-based strategy could help to overcome the limits of the distance-based method.
STRUCTURE software was used to probabilistically assign the analyzed genotypes to populations (K) and to search for admixture among individuals. Although it is not possible to know the true value of K, Pritchard et al. (2000) suggested calculating the posterior probability of K [Pr (X|K)] as a guideline for choosing the most appropriate K. In our estimate of Pr (X|K), the higher value (0.768525) was observed for K = 8. This value was then assumed to be the most appropriate estimate to describe the population structure. Besides Pr (X|K), other parameters should be taken into account to determine the presence of population structures such as the α value (Dirichlet parameter for the degree of admixture), which should be relatively constant during the run. At K = 8, α was 0.037, varying only by 0.020 during the course of the run. Moreover, the variability of ln Pr (X|K) across independent runs should be low, the proportion of the genotypes assigned to each cluster should not be symmetrical (~1/K in each population), and most individuals should be strongly assigned to a single population; these requirements were met at K = 8.
In the STRUCTURE bar plot (Fig. 4), the cactus pear–related species fell into three clusters that were clearly separated from the cultivated accessions. Specifically cluster number 6 included seven species (O. quimilò, O. cochenillifera, O. elizondoana, O. robusta, O. subulata, O. spinulifera, and O. vulgaris), cluster number 7 included O. leucotricha and cluster number 8 included O. joconostle and O. oligacantha. These prickly pear–related species showed different degrees of admixture, which may be due to the low number of genotypes included in the analysis (one or two genotypes for each species). However, it should be noted that most of these genotypes had unique alleles that clearly separated them from the cultivated genotypes (Table 2). The remaining five clusters included the O. ficus indica genotypes and the other arborescent cactus pear species O. amyclaea, O. megacantha, O. streptacantha, O. fusicaulis, and O. albicarpa (Fig. 4).
STRUCTURE bar plot assigning the 62 opuntias to eight clusters. The plot clearly distinguishes all of the cultivated genotypes, classified as Opuntia ficus indica, O. amyclaea, O. megacantha, O. streptacantha, O. fusicaulis, and O. albicarpa (clusters from 1 to 5) from cactus pear–related species (clusters from 6 to 8)
STRUCTURE evidenced different degrees of admixture among the analyzed genotypes, which might have been caused by natural and artificial hybridization. The analysis correctly identified hybrid genotypes, such as ‘hybrid’ (collected in Israel), and showed admixture in other genotypes labeled with codes (‘2.10.58’, ‘93034’), which were likely to come from breeding programs.
Concluding remarks
The analysis of eight highly polymorphic SSR loci allowed us to investigate the level of genetic diversity among cactus pear species, cultivars, and accessions from different regions of the world. SSRs, although scored as dominant markers, were more informative than random markers; they were able to produce useful information regarding the level of diversity among the most diffused cultivars, and may have revealed the level of hybridization between O. ficus indica and its related species. In our small sample of progeny resulting from a cross between O. ficus indica ‘Bianca’ and a clone of O. amyclaea, we observed a random combination of parental alleles, which is typical of autopolyploid species (Doyle and Egan 2010). Consequently, microsatellites could be used to analyze a greater number of individuals originating from controlled crosses with different parentals to investigate the molecular evolution of polyploidy in opuntias at a deeper level. Also, SSRs may serve as a quick and reliable tool to discriminate Opuntia apomictic seedlings from zygotic ones (Mondragón-Jacobo and Bordelon 2002; Reyes-Agüero et al. 2006).
In addition to distance-based analyses, which provided an overview of the genetic variation among cultivated genotypes and related species, Bayesian analysis revealed additional information regarding the diversification and the degree of admixture among different gene pools. In the NJ tree, the network analysis, and the Bayesian analysis, the clusters clearly diverged from current taxonomy, which classifies several Opuntia species on the basis of morphological parameters such as the presence/absence of spines and the joint shape. In particular, spinescence was considered to be a distinctive characteristic in the assignment of a genotype to a certain species in both early (Britton and Rose 1963) and later taxonomical classifications (Scheinvar 1995; Reyes-Agüero et al. 2005). However some phenotypic characters, including the presence/absence of spines, often considered for their agronomic value, show a great variability in progenies and can be very different from those of mother plants (Nieddu et al. 2006). According to Nieddu and Chessa (1997), this fact could be related to a different expression of the genes encoding these characters as well as to epigenetic and environmental factors (Labra et al. 2003).
Although most of the Opuntia wild species related to cactus pear are clustered in separate groups, the genotypes classified as O. amyclaea, O. megacantha, O. fusicaulis, O. streptacantha, and O. albicarpa are closely related to the O. ficus indica cultivated varieties. Our analysis, as well as previous work based on molecular variation (Wang et al. 1998; Labra et al. 2003; Griffith 2004), clearly supports the fact that the present classification of cultivated varieties and wild genotypes based on morphological parameters is misleading. Consequently, molecular tools are definitely the most appropriate tools for the assessment of the level of genetic diversity in Opuntia germplasm collections; such analysis should be a prerequisite for planning breeding programs that capture most of the existing variability among prickly pears. The use of these markers is strongly suggested to reclassify the cactus pear cultivated accessions, which exhibit a high level of variation regardless of the current taxonomical classification and probably should be classified as the same species, as suggested by Kiesling (1998).
Most of the spineless and fleshy-fruited genotypes included in this analysis showed a narrow genetic base, and some of them were not discriminated. Although they exhibited differences in fruit color, the SSR profiles of these genotypes were strongly similar not only in terms of presence/absence of the peaks, but also in terms of allele dosage observed at each peak, which should change if recombination has occurred. We may therefore hypothesize that these phenotypic differences were the result of somatic mutations that occurred in different growing regions. Other spineless cultivated genotypes or accessions appeared to be clearly separated from this main group (Fig. 2), indicating that the character of spinescence might have been developed multiple times during the evolution of the genus, and might have been selected from different populations. Indeed, the hypothesis of Griffith (2004), considering O. ficus indica as a group of multiple unrelated clones derived from different parental species and selected for common agronomical features, seems to be supported by our analyses.
References
Anderson EF (2001) The cactus family. Timber, Portland
Barkley NA, Roose ML, Krueger RR, Federici CT (2006) Assessing genetic diversity and population structure in a citrus germplasm collection utilizing simple sequence repeat markers (SSRs). Theor Appl Genet 112:1519–1531
Bowcock AM, Ruiz-Linares A, Tomfohrde J, Minch E, Kidd JR, Cavalli-Sforza LL (1994) High resolution of human evolutionary trees with polymorphic microsatellites. Nature 368:455–457
Britton NL, Rose JN (1963) The Cactaceae, vol 1. Dover, New York
Brutsch MO, Zimmermann HG (1993) The prickly pear (Opuntia ficus-indica) Cactaceae in South Africa: utilisation of the naturalised weed and of the cultivated plants. Econ Bot 47:154–156
Bryant D, Moulton V (2004) Neighbor-Net: an agglomerative method for the construction of phylogenetic networks. Mol Biol Evol 21:255–265
Chávez-Moreno CK, Tecante EA, Casas EA (2009) The Opuntia (Cactaceae) and Dactylopius (Hemiptera:Dactylopiidae) in Mexico: a historical perspective of use, interaction and distribution. Biodivers Conserv 18:3337–3355
Dangl GS, Mendum ML, Prins BH, Walker MA, Meredith CP, Simon CJ (2001) Simple sequence repeat analysis of a clonally propagated species: a tool for managing a grape germplasm collection. Genome 44:432–438
Doyle JJ, Egan AN (2010) Dating the origins of polyploidy events. New Phytol 186:73–85
Esselink D, Nybom H, Vosman B (2004) Assignment of allelic configuration in polyploids using the MAC-PR (microsatellite DNA allele counting–peak ratios) method. Theor Appl Genet 109:402–408
Fedorov A (1969) Chromosome numbers of flowering plants. Komarov Botanical Institute, Leningrad
Felker P, Rodriguez SC, Casoliba RM, Filippini R, Medina D, Zapata R (2005) Comparison of Opuntia ficus indica varieties of Mexican and Argentine origin for fruit yield and quality in Argentina. J Arid Environ 60:405–422
Felker P, Paterson A, Jenderek MM (2006) Forage potential of Opuntia clones maintained by the USDA National Plant Germplasm System (NPGS) collection. Crop Sci 46:2161–2168
Fernandez-Montes MR, Mondragon-Jacobo C, Luna-Vazquez J, Gutierrez-Acosta F, Saenz-Quintero LA, Zegbe-Dominguez JA, Mendez-Gallegos SDJ, Martinez-Gonzalez JC (2000) Principales cultivares mexicanos de nopal tunero. Publicación Técnica Núm. 1. INIFAP-CIRCE-Campo Experimental Norte de Guanajuato, Guanajuato, México
García-Zambrano EA, Zavala-García F, Gutiérrez-Diez A, Ojeda-Zacarías MC, Cerda-Hurtado I (2009) Estimation of the genetic diversity of Opuntia spp. using molecular markers AFLP. Phyton 78:117–120
Gibson AC, Nobel PS (1986) The cactus primer. Harvard University Press, Cambridge
Goldblatt P (1981) Index to plant chromosome numbers 1975–1978. Monogr Syst Bot Missouri Bot Gard 5:1–553
Goldblatt P, Johnson DE (1990) Index to plant chromosome numbers 1986–1987. Monogr Syst Bot Missouri Bot Gard 30:1–243
Goldblatt P, Johnson DE (2006) Index to plant chromosome numbers 2001–2003. Monogr Syst Bot Missouri Bot Gard 106:106–107
Griffith MP (2004) The origins of an important cactus crop, Opuntia ficus-indica (Cactaceae): new molecular evidence. Am J Bot 91:1915–1921
Helsen P, Verdyck P, Tye A, Desender K, Van Houtte N, Van Dongen S (2007) Isolation and characterization of polymorphic microsatellite markers in Galapagos prickly pear (Opuntia) cactus species. Mol Ecol Notes 7:454–456
Helsen P, Verdyck P, Tye A, Van Dongen S (2009) Low levels of genetic differentiation between Opuntia echios varieties on Santa Cruz (Galápagos). Plant Syst Evol 279:1–10
Huson DH, Bryant D (2006) Application of phylogenetic networks in evolutionary studies. Mol Biol Evol 23:254–267
Kiesling R (1998) Origen, domesticación y distribución de Opuntia ficus-indica. J Prof Assoc Cactus Dev 3:50–59
Labra M, Grassi F, Bardini M, Imazio S, Guiggi A, Citterio S, Banfi E, Sgorbati S (2003) Relationships in Opuntia Mill. genus (Cactaceae) detected by molecular marker. Plant Sci 165:1129–1136
Landergott U, Naciri Y, Schneller JJ, Holderegger R (2006) Allelic configuration and polysomic inheritance of highly variable microsatellites in tetraploid gynodioecious Thymus praecox agg. Theor Appl Genet 113:453–465
Liu K, Muse SV (2005) PowerMarker: an integrated analysis environment for genetic marker analysis. Bioinformatics 21:2128–2129
Luna-Paez A, Valadez-Moctezuma E, Barrientos-Priego AF, Gallegos-Vázquez C (2007) Characterization of Opuntia spp. by means of seed with RAPD and ISSR markers and its possible use for differentiation. J Prof Assoc Cactus Dev 9:43–59
Mondragón-Jacobo C (2001) Cactus pear breeding and domestication. Plant Breed Rev 20:135–166
Mondragón-Jacobo C (2003) Caracterización molecular mediante RAPDs de una colección de nopal de (Opuntia spp. Cactaceae) del centro de México, como base del mejoramiento genético. Revista Chapingo 9:97–114
Mondragón-Jacobo C, Bordelon BB (2002) Apomixis in crosses of mexican pear cactus, preliminary molecular identification. Rev Fitotec Mex 25:247–252
Mondragón-Jacobo C, Pérez-González S (1996) Native cultivars of cactus pear in Mexico. In: Janick J (ed) Progress in new crops. ASHS Press, Arlington, pp 446–450
Moore RJ (1977) Index to plant chromosome numbers 1967–1971. Regnum Veg 96:1–157
Nieddu G, Chessa I (1997) Distribution of phenotypic characters within a seedling population from Opuntia ficus-indica (cv. “Gialla”). Acta Hort 438:37–43
Nieddu G, Chessa I, Barberis A (2006) Characterization of seedlings obtained from open pollinated ‘Gialla’ cactus pear (Opuntia ficus-indica). Acta Hort 728:105–110
Oetting WS, Lee HK, Flanders DJ, Wiesner GL et al (1995) Linkage analysis with multiplexed short tandem repeat polymorphisms using infrared fluorescence and M13 tailed primers. Genomics 30:450–458
Pimienta-Barrios E (1994) Prickly pear (Opuntia spp.): a valuable fruit crop for the semi-arid lands of Mexico. J Arid Environ 28:1–12
Pinkava DJ, Rebman J, Baker M (1998) Chromosome numbers in some cacti of western North America—VII. Haseltonia 6:32–41
Potgieter JP, Mashope BK (2009) Cactus pear (Opuntia spp.) germplasm conservation in South Africa. Acta Hort 811:47–54
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Rebman JP, Pinkava DJ (2001) Opuntia cacti of North America—an overview. Fla Entomol 84:474–483
Reyes-Agüero JA, Aguirre JR, Hernández HM (2005) Systematic notes and a detailed description of Opuntia ficus-indica (L.) Mill. (Cactaceae). Agrociencia 39:395–408
Reyes-Agüero JA, Aguirre RJR, Valiente-Banuet A (2006) Reproductive biology of Opuntia: a review. J Arid Environ 64:549–585
Rozen S, Skaletsky H (2000) Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132:365–386
Scheinvar L (1995) Taxonomy of utilized opuntias. In: Barbera G, Inglese P, Pimienta-Barrios E (eds) Agroecology, cultivation and uses of cactus pear. FAO plant production and protection paper 132. FAO, Rome, pp 20–27
Segura S, Scheinvar L, Olalde G, Leblanc O, Filardo S, Muratalla A, Gallegos C, Flores C (2007) Genome sizes and ploidy levels in Mexican cactus pear species Opuntia (Tourn.) Mill. series Streptacanthae Britton et Rose, Leucotrichae DC., Heliabravoanae Scheinvar and Robustae Britton et Rose. Genet Resour Crop Evol 54:1033–1041
Souto Alves T, Vanusa Da Silva M, Alves De Almeida CM, Oliveira Jordão Do Amaral D, Cordeiro Dos Santos D, Farias I, Tenório Sabino Donato VM, Da Costa AF (2009) Genetic diversity in cactus clones using ISSR markers. Acta Hort 811:55–58
Swofford DL (1998) PAUP*: phylogenetic analysis using parsimony (* and other methods), version 4. Sinauer, Sunderland
Thurston MI, Field D (2005) Msatfinder: detection and characterisation of microsatellites. http://www.genomics.ceh.ac.uk/msatfinder/
Wallace RS, Gibson AC (2002) Evolution and systematics. In: Nobel PS (ed) Cacti: biology and uses. University of California Press, Berkeley, pp 1–22
Wang X, Felker P, Paterson AH, Mizrahi Y, Nerd A, Mondragon-Jacobo C (1996) Cross hybridization and seed germination in Opuntia species. J Prof Assoc Cactus Dev 1:49–60
Wang X, Felker P, Burrow MD, Paterson AH (1998) Comparison of RAPD marker patterns to morphological and physiological data in the classification of Opuntia accessions. J Prof Assoc Cactus Dev 3:3–14
Zoghlami N, Chrita I, Bouamama B, Gargouri M, Zemni H, Ghorbel A, Mliki A (2007) Molecular based assessment of genetic diversity within Barbary fig (Opuntia ficus indica (L.) Mill.) in Tunisia. Sci Hort 113:134–141
Acknowledgments
We thank Dr. Candelario Mondragón-Jacobo (Instituto Nacional de Investigaciones Forestales y Agropecuarias, México) and Dr. Yaron Sitrit (The Hebrew University of Jerusalem, Israel) for providing some of the accessions used for microsatellite analysis. Funding was provided by Ente Sviluppo Agricolo, Regione Siciliana (Project Miglioramento genetico del ficodindia).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Caruso, M., Currò, S., Las Casas, G. et al. Microsatellite markers help to assess genetic diversity among Opuntia ficus indica cultivated genotypes and their relation with related species. Plant Syst Evol 290, 85–97 (2010). https://doi.org/10.1007/s00606-010-0351-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-010-0351-9