Abstract
The aim of the present study was to determine the optimum parameters for high regrowth following cryostorage (− 196 °C) of seven endemic and endangered Dianthus species. A cryopreservation approach based on a droplet-vitrification protocol was successfully applied using explants (shoot apices and axillary buds) from a collection of in vitro grown Dianthus species. The plants were micropropagated for two years prior using them as explant donors. Osmoprotection in different sucrose concentrations and various dehydration durations in the plant vitrification solution (PVS2) were tested to assess survival and regrowth following cryostorage. The regrowth rates after cryostorage ranged between 63% (D. glacialis ssp. gelidus) and 73% (D. nardiformis) and were achieved after osmoprotection in 0.5 M sucrose and 30 min dehydration in PVS2 for D. glacialis ssp. gelidus and osmoprotection in 0.25 M sucrose and 30 min dehydration in PVS2 for D. nardiformis. The morphogenetic response to liquid nitrogen storage was direct multiple shoot formation for both non-cryopreserved and cryopreserved explants for all species. This biotechnological approach can be efficiently applied for the ex situ conservation of endemic and endangered Dianthus species to ensure the long-term conservation of biodiversity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Climate changes, habitat destruction and loss, are significant threats to global biodiversity and ecosystem integrity (Urban 2015) which have already affected plant diversity worldwide (Nunez et al. 2019). Moreover, endemic plant species are usually more vulnerable to anthropogenic threats (Coelho et al. 2020). In Europe, as region with long land use history, the European Union was determined to set new policy goals to preserve biodiversity (De Meester et al. 2011). Arguments for the importance of biodiversity conservation including a multi-national exploration of stakeholder’s views, have been comprehensively revised and analyzed (Berry et al. 2018), demonstrating the importance of a constructive dialogue for a better understanding of the value of biodiversity to decision makers (Tinch et al. 2018). International organizations, such as the International Union for Conservation of Nature (IUCN) and others are making constant efforts to safeguard as many species as possible (IUCN 2014). Plant conservation strategies are crucial also due to excessive human impact on natural landscapes which in some cases led to loss endemic species or habitats (Hunter 2007). Since these species are restricted to particular areas the susceptibility to getting extinct is higher (Işik 2011). It is assumed that, approximately one fifth of the world's plants are at risk of extinction (Wade et al. 2016) thus, preventing their extinction should be a priority of the scientific community.
Currently, there are two common complementary strategies for the conservation of plant genetic resources, in situ conservation where plant species are maintained in their natural habitat, and ex situ conservation where plant species are conserved outside their natural habitats, in botanical gardens and gene banks, ranging from seed genebanks to in vitro conservation facilities, including cryopreservation genebanks and DNA libraries (Engelmann 2012; GSPC 2012; Peres 2016; O’Donnell and Sharrock 2018). In the selection of the most appropriate conservation approach for a specific plant gene pool, various criteria should be considered, including both the storage characteristics of the species and the reliability of the chosen methods (Cruz-Cruz et al. 2013). The Millennium Seed Bank conserves seed collections originating from 189 countries and 35 biodiversity hotspots of which 74% of taxa represents endemic, endangered or species with economic, ecological or scientific value (Liu et al. 2018). In vitro propagation techniques have been successfully applied for the short to medium-term conservation of certain plant species that might be at risk or are grown in threatened habitats (Cristea 2010; Laslo et al. 2011; Hammond et al. 2019; Kulus 2019). Likewise, cryopreservation (− 196 °C) is a feasible alternative for both orthodox seeded species, to complement other conservation strategies and for plant species that do not produce viable seeds or the seeds are ‘recalcitrant’ being desiccation sensitive (Izgü and Mendi 2017). Currently, cryopreservation is the only available technique for the safe long-term storage of clonally propagated plant species (Kulus and Zalewska 2014; Umesha 2019).
The South-Eastern Carpathians have a high distribution of endemic taxa including numerous Dianthus species (Mráz and Ronikier 2016). Dianthus genus (Family Caryophyllaceae) has importance due to the large number of endemic and/or threatened species some of them with economic value due to its potential ornamental use (Jarda et al. 2011). The Romanian flora comprises approximately 21% endemic taxa of this genus (Ciocârlan 2009) of which 30% are on the Red List (Dihoru and Negrean 2009). Studies related to micropropagation and medium-term preservation of some endangered Dianthus species have been reported (Cristea 2010; Cristea et al. 2006, 2010, 2013, 2014; Holobiuc et al. 2009a, b; Jarda et al. 2011; Marković et al. 2013). Cryopreservation studies have been reported mainly for different ornamental Dianthus species and cultivars and only for one endemic taxa (Dianthus giganteus ssp. banaticus) using different techniques including: two-step cooling (Fukai et al. 1991), encapsulation-dehydration and encapsulation-vitrification (Tannoury et al. 1995; Tannoury and Vintejoux 1997; Halmagyi and Lambardi 2006; Halmagyi and Deliu 2007), PVS2-droplet freezing, DMSO-droplet freezing (Halmagyi and Lambardi 2006), and aluminium cryo-plate vitrification (Sekizawa et al. 2011) and vitrification-based methods (Jarda et al. 2011). However, there are numerous cryopreservation protocols developed for different endemic species worldwide (Turner et al. 2001; Coste et al. 2012; Kaczmarczyk et al. 2013).
Considering biodiversity decrease the high extinction threats and the potential loss of these endemic species, conservation measure must be undertaken to support their long-term storage. Based on this significant prerequisite, the major aim of our study was to optimize genotype specific cryopreservation protocols with high regrowth frequencies following cryostorage for seven endemic and endangered Dianthus taxa (D. callizonus; D. glacialis ssp. gelidus; D. henteri; D. nardiformis; D. spiculifolius; D. tenuifolius; D. trifasciculatus ssp. parviflorus) from a two years active in vitro collection. Priority for cryopreservation should be given to plant species with an increased risk of extinction. This is the first study on cryopreservation of shoot apices and axillary buds of these endemic and endangered Dianthus species.
Material and methods
Plant material and in vitro propagation of cultures
The endemic and endangered Dianthus species selected to be cryopreserved in this study were collected from Natura 2000 sites in Romania (Fig. 1, Table 1). Prior to cryopreservation, the plant material was maintained for 2 years as an active in vitro collection. Micropropagation protocols were previously published. The specific culture media for the active in vitro culture collections were as follows: D. callizonus on Murashige and Skoog (1962) (MS) culture medium supplemented with B5 vitamins (Gamborg et al. 1968), 2 mg L−1 6-Benzylaminopurine (BAP), 2 mg L−1 Kinetin (K), 0.2 mg L−1 1-naphtaleneacetic acid (NAA), 30 g L−1 sucrose and 8 g L−1 agar (Holobiuc et al. 2004–2005); D. glacialis ssp. gelidus on MS culture medium including vitamins with 1 mg L−1 BAP, 0.1 mg L−1 (NAA), 20 g L−1 sucrose and 7 g L−1 agar (Cristea et al. 2006); D. henteri on MS culture medium including vitamins with 1 mg L−1 BAP, 0.1 mg L−1 NAA, 30 g L−1 sucrose and 8 g L−1 agar (Cristea et al. 2010); D. nardiformis on MS culture medium with B5 vitamins, 1 mg L−1 BAP, 1 mg L−1 K, 0.2 mg L−1 2,4-d, 30 g L−1 sucrose and 8 g L−1 agar (Holobiuc et al. 2009a); D. spiculifolius on MS culture medium including vitamins, 1 mg L−1 N6-(2-isopentenyl)adenine (2-iP), 0.1 mg L−1 NAA, 20 g L−1 sucrose and 8 g L−1 agar (Butiuc-Keul et al. 2001); D. tenuifolius on MS culture medium with B5 vitamins, 1 mg L−1 BAP, 1 mg L−1 K, 0.25 mg L−1 NAA, 0.5 mg L−1 gibberellic acid (GA3), 30 g L−1 sucrose and 8 g L−1 agar (Holobiuc et al. 2004–2005); Dianthus trifasciculatus ssp. parviflorus on MS culture medium with B5 vitamins, 2 mg L−1 Zeatine (Z), 0.2 mg L−1 NAA and 30 g L−1 sucrose and 8 g L−1 agar (Holobiuc et al. 2013). All culture medium components were provided by Duchefa. The pH-value was adjusted to 5.8 before autoclaving (20 min at 121 °C). The plants were cultured in glass vessels (6 cm diameter and 12 cm height) sealed with metal lids having a hole (0.5 cm diameter) in the middle covered with leucopore tape (Duchefa), on 50 ml culture medium (Fig. 2a). The density was four explants per jar. The plants were grown at 23 ± 1 °C with a 16 h photoperiod and a light intensity of 40 µmol m−2 s−1 photosynthetic active radiation (PAR) provided by cool white fluorescent tubes (Philips). The cultures were transferred to fresh medium depending on the protocol established for each species at 30 to 40 days for two years (Fig. 2a).
Cryopreservation by droplet-vitrification and morphogenetic response of explants. a In vitro Dianthus species; b shoot apices (inset) in a droplet (6 µl) of PVS2 vitrification solution; c D. callizonus (non-cryopreserved); d D. callizonus (cryopreserved); e D. glacialis ssp. gelidus non-cryopreserved shoot apices (left side) and after cryopreservation (right side); f D. henteri (cryopreserved); g D. nardiformis (cryopreserved); h D. spiculifolius (non-cryopreserved); i D. spiculifolius (cryopreserved); j D. tenuifolius; k D. trifasciculatus ssp. parviflorus (non-cryopreserved); l D. trifasciculatus ssp. parviflorus (cryopreserved). Circles with dashed lines represent dead shoot apices having a light brown to white color; bar 1 cm
Osmoprotection, dehydration and cooling
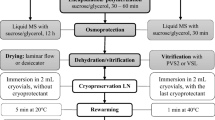
For cryopreservation studies the Dianthus species micropropagated for two years were used as explant donors. For all species shoot tips were used for cryostorage, except for D. callizonus, where axillary buds were used (Fig. 2b, inset). After excision of shoot tips (apical dome with 2–4 leaf primordia, approximately 2–3 mm in length) and axillary buds (2–3 mm in length) under a stereo microscope in aseptic conditions the explants were cultured for osmotic dehydration in sucrose (0.1, 0.25, 0.5, 0.75, 1.0 M) enriched liquid MS medium (without growth regulators). The explants were placed directly in the mentioned sucrose solutions, each for 24 h in the same environmental conditions as mentioned for plant micropropagation. The sucrose concentrations providing the highest regrowth rates per species were further used for the investigation of other exposure times to the vitrification solution (PVS2). For further dehydration, the explants were incubated in the plant vitrification solution 2 (PVS2) (Sakai et al. 1990) for various durations (0, 10, 20, 30, 40 or 50 min) at 23 ± 1 °C. For cooling the explants individually placed in a drop (6 μl) of PVS2 on previously sterilized (4 h at 180 °C) aluminium foil strips (0.5 cm × 2.0 cm) were transferred to 1.8 ml cryovials (two foils per cryovial with five drops per foil) (Fig. 2b) and immersed in a 25 l Dewar flask filled with liquid nitrogen (− 196 °C) where the samples remained for 24 h. To get accurate information about the effects of sucrose and the potential toxicity of PVS2 on tissues, two controls were used: control 0 = osmoprotected (in sucrose 0.1, 0.25, 0.5, 0.75, 1.0 M enriched liquid MS medium for 24 h) explants, and control 1 = osmoprotected (in sucrose 0.1, 0.25, 0.5, 0.75, 1.0 M enriched liquid MS medium for 24 h) and dehydrated (20 min in PVS2) explants. The 20 min PVS2 treatment duration was selected based on the good results obtained with other species of the Dianthus genus.
Survival and regrowth
After 24 h storage in liquid nitrogen, rewarming of samples was performed by transfer of the aluminium strips to liquid MS culture medium with 30 g L−1 sucrose (without growth regulators) at 23 ± 1 °C. By gentle shaking of the aluminium strips in the medium the drops melted instantly. Shortly after rewarming, the explants were transferred to MS medium with 30 g L−1 sucrose (without growth regulators) with 6 g L−1 agar in Petri dishes (5 cm in diameter) under standard illumination conditions (described above) to regenerate shoots.
Assessment of recovery and statistical analyses
Each cryopreservation related treatment was performed using three replicates, each of ten explants. Two main parameters were assessed: (a) the survival rate evaluated four weeks after cryopreservation by counting the number of shoots that remained green after liquid nitrogen storage in early stage of shoot formation (approximately < 0.5 cm in length), and (b) regrowth,defined as further development of shoot apices into shoots with leaf emergence (> 1 cm in length) from the original explants, six weeks after rewarming. Brown or white shoot apices were considered dead. Survival and regrowth percentages of explants (shoot apices and axillary buds) were calculated according to the formulas:
In addition, the number of directly formed shoots per explant was assessed after 60 days.
The Pearson’s correlation coefficient was determined between sucrose concentration and shoot regrowth (from control 0 and control 1 explants) using the Excel spreadsheet software (v16.0 Microsoft). A − 1 value represent a perfect negative correlation, 0 represent no correlation and + 1 represent a perfect positive correlation.
The regression analysis was based on the value of the coefficient of determination (r2) and was conducted between survival and regrowth frequencies of explants following liquid nitrogen storage using the Excel spreadsheet software (v16.0 Microsoft). Reliability decreases with a decrease of the r2 value (r2 = 1 means completely reliable and r2 = 0 means completely unreliable).
The statistical significance of data was determined by one-way analysis of variance (ANOVA) followed by Tukey’s honestly significant difference (HSD) test (PB ≤ 0.05) using SPSS program ver. 17.0 (SPSS Inc., Chicago, USA).
Results
Sensitivity of explants to osmoprotection and dehydration treatments
For non-cryopreserved shoot apices, a strong negative correlation (Pearson’s correlation coefficient − 0.93 to − 0.99) between the sucrose concentration and the regrowth of shoots from control 0 explants was observed in all Dianthus species, whereas weak to moderate negative correlation (Pearson’s correlation coefficient ranged between − 0.07 and − 0.50) between the sucrose concentration and shoot development from control 1 shoot apices was noted (Fig. 3a–g). The mean regrowth rates for all species for the same sucrose concentration were higher for control 0, although the differences were not always significant (Fig. 3a–g). For control 1 shoot apices the highest shoot regrowth was obtained after osmoprotection in 0.25 M sucrose (75% D. henteri, 80% D. nardiformis, 72% D. spiculifolius, and 68% D. trifasciculatus ssp. parviflorus) or in 0.5 M sucrose (74% D. callizonus, 76% D. glacialis ssp. gelidus, 70% D. tenuifolius) (Fig. 3a–g). It was found that low (0.1 M) and high (1.0 M) sucrose concentrations dropped significantly the shoot regrowth for control 1 shoots (Fig. 3a–g).
Survival and shoot regrowth following cryostorage
Regression analysis (P < 0.05) showed a significant strong positive linear correlation between survival and regrowth frequencies of explants following liquid nitrogen storage (r2 = 0.93 D callizonus, r2 = 0.97 D. glacialis gelidus, r2 = 0.87 D. henteri, r2 = 0.94 D. nardiformis, r2 = 0.91 D. spiculifolius, r2 = 0.93 D. tenuifolius, r2 = 0.97 D. trifasciculatus ssp. parviflorus (Fig. 4a, b). Although, the highest survival rate for cryopreserved shoot tips was 78% (D. spiculifolius) recorded after osmoprotection in 0.25 M sucrose, the regrowth percentage was 61% for the same sucrose concentration (Fig. 4a, b). The lowest (0.1 M) and the highest (1.0 M) sucrose concentrations used did not have a beneficial effect in osmoprotection of explants leading to a mean regrowth rate of 22% for 0.1 M sucrose and 10% for 1.0 M sucrose (Fig. 4a, b). After testing various exposure times it was ascertained that the survival and regrowth percentages were significantly influenced by the dehydration durations (Table 2). The post-cryopreservation regrowth frequencies increased with increasing exposure time and reached the highest values after 30 min exposure to PVS2 for all species (Table 2). The species with the highest survival rate did not necessarily exhibit increased regrowth rate. For instance, a 30 min dehydration duration lead to the highest survival for D. henteri (83%) whereas, the highest regrowth for the same dehydration time was recorded for D. nardiformis (73%) (Table 2). A 50 min PVS2 exposure time lead to significantly lower regrowth percentages (up to 21%) for all species, while without PVS2 treatment no survival was observed (Table 2). The morphogenetic response to liquid nitrogen storage was direct multiple shoot formation without any intermediate callus phase for both non-cryopreserved and cryopreserved explants for all species (Table 3, Fig. 1). The mean number of shoots per explant, following liquid nitrogen storage, ranged between 3 (D. glacialis ssp. gelidus and D. henteri) and 5.6 (D. nardiformis), while for axillary buds the mean number of shoots was 4.6 (D. callizonus). The number of shoots after cryopreservation was not significantly different for all species compared to the number of regrowing shoots from non-cryopreserved explants (Table 3).
Discussion
In the present study, a cryopreservation protocol by droplet-vitrification for seven endemic and endangered Dianthus species is reported for the first time. In vitro conservation methods become increasingly important especially for the conservation of endemic and endangered species or for species for which traditional seed banking does not apply (Sarasan et al. 2006; Kaczmarczyk et al. 2011). The short to medium-term in vitro conservation methods allows the storage of germplasm in a protected environment. Whereas in vitro micropropagation for a long time is known as potential inducer of somaclonal variations (Larkin and Scowcroft 1981), cryopreservation is considered a more adequate and efficient method for the secure long-term storage of endangered plant species (Kaczmarczyk et al. 2013; Kulus 2016). Vitrification based cryopreservation protocols eliminate the potential damage due to intracellular water crystallization by using highly concentrated vitrification solutions (Sakai et al. 1990). In the last decade, cryostorage by droplet-vitrification was successfully applied for shoot apices of various endemic or endangered plant species (Coste et al. 2012; Kaczmarczyk et al. 2013; Coelho et al. 2014; Whiteley et al. 2016; Cristea et al. 2019). In the present study, vitrification did not affect the morphogenetic response of shoot tips and axillary buds leading to direct shoot regrowth. The main advantage of this method is the achievement of high cooling and warming rates due to the small volume (microliters) of cryoprotective medium in which the explants are treated, and the presence of aluminium foil (Sakai and Engelmann 2007). Although, there are no previous reports on cryostorage of the studied Dianthus endemic species, the results can be analyzed and compared to those obtained for other species of the same genus. For Dianthus species or carnation cultivars various cryopreservation protocols have been applied, i.e. two-step cooling (Fukai et al. 1991); encapsulation-dehydration and encapsulation-vitrification (Tannoury et al. 1995; Tannoury and Vintejoux 1997), PVS2-droplet freezing, DMSO-droplet freezing and encapsulation-dehydration procedures (Halmagyi and Lambardi 2006). Subsequently, Halmagyi and Deliu (2007) used an encapsulation-vitrification approach for D. caryophyllus and obtained up to 73% regrowth, while Sekizawa et al. (2011) using the aluminium cryo-plate vitrification obtained 95% shoot formation from cryostored shoot tips for the same species. The regrowth percentages of Dianthus species and cultivars after cryopreservation were reported to vary depending on many factors (pretreatment, cryoprotection, cooling, tissue type, etc.). For example, using a two-step cooling procedure for shoot tips, Fukai et al. (1991) obtained 100% regrowth for eight D. hybridus cultivars and five Dianthus species. On the other hand, applying an encapsulation-dehydration procedure (4 h dehydration and 20% water content of alginate beads), Tannoury et al (1995) obtained up to 90% regrowth of D. caryophyllus shoot apices. Lower regrowth percentages (up to 65%) were obtained for shoot apices of the same species after PVS2-droplet freezing (Halmagyi and Lambardi 2006). In the present study, the best regrowth rate following cryopreservation was registered for D. nardiformis (73%), after osmoprotection in 0.25 M sucrose and 30 min dehydration in PVS2. A correlation between the extent of PVS2 penetration into the shoot tips and the exposure time has been demonstrated before (Volk and Walters 2006). Confirming previous studies, our results underline the importance of osmoprotection in sucrose prior dehydration treatments with PVS2 (Halmagyi and Deliu 2007). This may be attributed to an increased cell osmolarity that minimizes the potential damages of the vitrification solutions (Reed 2008). Osmoprotection in sucrose proved to be essential for a successful cryopreservation for other plant species as well (Wang et al. 2014; Yin et al. 2014; Kulus 2018, 2020). Sugars form H-bonds with water contributing to the increment of cell viscosity (Benson 2008), while PVS2 reduces or avoids water crystallization, protects the membranes and prevents further damage during cooling and rewarming (Volk and Walters 2006). Although, it is known that each type of explant or species has specific requirements regarding the dehydration level compatible with survival after cryopreservation, a certain control of dehydration is critical for prevention of tissue damage by chemical toxicity during PVS2 treatment (Sakai et al. 2008). Plant species and genotypes respond differently to the stress factors associated with cryopreservation (Folgado et al. 2015; Kulus 2015). In the present study, the highest regrowth following cryostorage was achieved after 30 min dehydration in PVS2 and ranged between 65% (D. callizonus) and 73% (D. nardiformis). In the first case, osmoprotection was performed in 0.5 M sucrose, while for D. nardiformis the sucrose concentration was 0.25 M. Applying a droplet-vitrification protocol to in vitro grown buds of the critically endangered species Rubus humulifolius, regrowth percentages up to 52% were achieved (Edesi et al. 2020), while applying an encapsulation-dehydration method, González-Benito et al. (1997) obtained 70% survival of cryopreserved nodal explants from the endangered Centaurium rigualii species. In vitro plant regeneration via direct multiple shoot induction, without a callus phase, eliminates the problem of somaclonal variation (Larkin and Scowcroft 1981), and has been also reported for plant species following cryopreservation (Kulus et al. 2018). In Dianthus species and carnation cultivars, multiple shoot formation was reported for shoot tips and nodal buds even on culture medium with low cytokinins concentrations (Ali et al. 2008; Khatun et al. 2013; Ziv et al. 2005). Multiple shoot formation (6.4/explant) was also reported for cryopreserved Buxus hyrcana (endangered ornamental shrub) by using a culture medium with 1.0 mg L−1 BAP (Kaviani and Negahdar 2017) as well as for Chrysanthemum shoot tips (41% multiple shoot formation) (Kulus et al. 2018). In our study, multiple shoot proliferation was achieved without any specific treatment of explants, and moreover it was ascertained for both non-cryopreserved and cryopreserved shoot apices.
Conclusion
Cryopreservation has the potential to become an important tool for the long-term storage of endangered plant genetic resources. This study showed that a successful cryopreservation in terms of high regrowth rates directly from the cryostored explants is related to parameters like sucrose concentrations and exposure time to the vitrification solution. This study proves that, the vitrification based cryopreservation protocol for shoot tips and axillary buds offers a valuable option for the long-term storage of the endangered Dianthus germplasm. Moreover, we consider that our findings may contribute to future habitat restoration actions of these species in the wild.
Availability of data and material
All experimental data and pictures are available at the first author.
References
Ali A, Afrasiab H, Naz H, Rauf M, Iqbal J (2008) An efficient protocol for in vitro propagation of carnation (Dianthus caryophyllus). Pak J Bot 40:111–121
Benson EE (2008) Cryopreservation theory. In: Reed BM (ed) Plant cryopreservation: a practical guide. Springer, New York, pp 15–32
Berry PM, Fabók V, Blicharska M, Bredin YK, Llorente MG, Kovács E, Geamana N, Stanciu A, Termansen M, Jääskeläinen T, Haslett JP, Harrison PA (2018) Why conserve biodiversity? A multi-national exploration of stakeholders’ views on the arguments for biodiversity conservation. Biodivers Conserv 27:1741–1762
Butiuc-Keul A, Suteu A, Muntean-Deliu C, Deliu C (2001) Study on the in vitro preservation of Dianthus spiculifolius Schur. Contrib Bot 36:137–143
Ciocârlan V (2009) Flora ilustrată a României. Pteridophyta et Spermatophyta. Ceres, Bucureşti
Coelho N, González-Benito ME, Romano A (2014) Cryopreservation of shoot tips from the endangered endemic species Tuberaria major. Acta Physiol Plant 36:333–336
Coelho N, Gonçalves S, Romano A (2020) Endemic plant species conservation: biotechnological approaches. Plants 9:345
Coste A, Halmagyi A, Butiuc-Keul AL, Deliu C, Coldea G, Hurdu B (2012) In vitro propagation and cryopreservation of Romanian endemic and rare Hypericum species. Plant Cell Tiss Org Cult 110:213–226
Cristea V (2010) Culturi in vitro fotoautotrofe la specii de Dianthus endemice şi periclitate din România. Todesco, Cluj-Napoca
Cristea V, Puşcaş M, Miclăuş M, Deliu C (2006) Conservative micropropagation of some endemic or rare species from the Dianthus genus. Acta Hort 725:357–364
Cristea V, Brummer AT, Jarda L, Miclăuş M (2010) In vitro culture initiation and phytohormonal influence on Dianthus henteri - a Romanian endemic species. Rom Biotechnol Lett 15:25–33
Cristea V, Jarda L, Holobiuc I (2013) Ex situ conservation of three endemic and/or endangered Dianthus species. Not Bot Hort Agro 41:73–78
Cristea V, Crăciunaş C, Marcu D, Palada M, Butiuc-Keul A (2014) Genetic stability during in vitro propagation of Dianthus spiculifolius Schur. Prop Ornam Plants 14:26–31
Cristea V, Besenyei E, Jarda L, Farkas A, Marcu D, Clapa D, Halmagyi A, Butiuc-Keul A (2019) In situ genetic variability and micropropagation of Cerastium banaticum (Rochel) Heuff. (Caryophyllaceae)—a rare and endemic species from Romania. Acta Biol Crac Bot 61:65–74
Cruz-Cruz CA, González-Arnao MT, Engelmann F (2013) Biotechnology and conservation of plant biodiversity. Resources 2:73–95
De Meester L, van Tienderen P, Werger M, Hector A, Wörheide G, Niemelä J, Aguilar A, Smets E, Godfray C, Sutherland W, Bauhus J, Courchamp F, Gandini G, Koch M, Le Maho Y, Manuel M, Pawlowski J, Quéinnec E, Owens J, Keustermans L (2011) Challenges for biodiversity research in Europe. Proc Social and Behav Sci 13:83–100
Dihoru G, Negren G (2009) Cartea roşie a plantelor vasculare din România. Editura Academiei Române, Bucuresti
Edesi J, Tolonen J, Ruotsalainen AL, Aspi J, Häggman H (2020) Cryopreservation enables long-term conservation of critically endangered species Rubus humilifolius. Biodivers Conserv 29:303–314
Engelmann F (2012) Germplasm collection, storage, and conservation. In: Altman A, Hasegawa PM (eds) Plant biotechnology and agriculture. Elsevier, Amsterdam, pp 255–267
Folgado R, Panis B, Sergeant K, Renaut J, Swennen R, Hausman JF (2015) Unravelling the effect of sucrose and cold pretreatment on cryopreservation of potato through sugar analysis and proteomics. Cryobiology 71:432–441
Fukai S, Goi M, Tanaka M (1991) Cryopreservation of shoot tips of Caryophyllaceae ornamentals. Euphytica 56:149–153
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
González-Benito ME, Pérez C, Viviani AB (1997) Cryopreservation of nodal explants of an endangered plant species (Centaurium rigualii Esteve) using the encapsulation-dehydration method. Biodivers Conserv 6:583–590
GSPC (2012) Global strategy for plant conservation. Botanic Gardens Conservation International, Richmond
Halmagyi A, Deliu C (2007) Cryopreservation of carnation (Dianthus caryophyllus L.) shoot tips by encapsulation-vitrification. Sci Hortic 113:300–306
Halmagyi A, Lambardi M (2006) Cryopreservation of carnation. In: Teixeira JA (ed) Floriculture, ornamental and plant biotechnology. Advances and topical issues. Global Science Books, UK, Ikenobe, pp 415–423
Hammond HSD, Viehmannova I, Zamecnik J, Panis B, Cepkova PH (2019) Efficient slow-growth conservation and assessment of clonal fidelity of Ullucus tuberosus Caldas microshoots. Plant Cell Tissue Org Cult 138:559–570
Holobiuc I, Păunescu A, Blîndu R (2004–2005) Ex situ conservation using in vitro methods in some Caryophyllaceae plant species from Red List of vascular plants in Romania. Rom J Biol Plant Biol 49–50:3–16
Holobiuc I, Blîndu R, Cristea V (2009a) Researches concerning in vitro conservation of the rare plant species Dianthus nardiformis Janka. Biotechnol Biotechnol Equip 23:221–224
Holobiuc M, Blîndu R, Mitoi M, Heleciuc F, Cristea V (2009b) The establishment of an in vitro gene bank in Dianthus spiculifolius Schur and D. glacialis ssp. gelidus (Schott Nym. et Kotschy) Tutin: I. The initiation of a tissue collection and the characterization of the cultures in minimal growth conditions. Ann Forest Res 52:117–128
Holobiuc I, Catană R, Voichiţă C, Helepciuc F (2013) In vitro introduction of Dianthus trifasciculatus Kit ssp. parviflorus as ex situ preservation method. Muzeul Olteniei Craiova. Studii şi Comunicări Ştiinţele Naturii Tom 29:93–100
Hunter P (2007) The human impact on biological diversity. EMBO Rep 8:316–318
Işik K (2011) Rare and endemic species: why are they prone to extinction? Turk J Bot 35:411–417
IUCN (1997) Globally threatened plants in Europe, a subset from the 1997 IUCN Red List of Threatened Plants. World Conservation Monitoring Centre, Cambridge, pp 23–24
IUCN SSC (2014) Guidelines on the use of ex situ management for species conservation. Version 2.0. IUCN Species Survival Commission, Gland
Izgü T, Mendi YY (2017) The usage of cryopreservation and synthetic seeds on preservation for plant genetic resources. Intl J Cell Sci Mol Biol 2:555583
Jarda L, Cristea V, Halmagyi A, Palada M (2011) In vitro culture initiation and cryopreservation of endemic taxa (Dianthus giganteus ssp. banaticus). Acta Hortic 918:153–159
Kaczmarczyk A, Turner SR, Bunn E, Mancera RL, Dixon KW (2011) Cryopreservation of threatened native Australian species—what have we learned and where to from here? In Vitro Cell Dev Biol Plant 47:17–25
Kaczmarczyk A, Funnekotter B, Turner SR, Bunn E, Bryant G, Hunt TE, Mancera RL (2013) Development of cryopreservation for Loxocarya cinerea—an endemic Australian plant species important for post-mining restoration. Cryoletters 34:508–519
Kaviani B, Negahdar N (2017) Propagation, micropropagation and cryopreservation of Buxus hyrcana Pojark., an endangered ornamental shrub. S Afr J Bot 111:326–335
Khatun MM, Rahman MM, Roy PK (2013) In vitro regeneration and field evaluation of carnation (Dianthus caryophyllus L.) through shoot tip and node culture. J Appl Sci Technol 9:93–99
Kulus D (2015) Application of cryopreservation for chrysanthemum genetic resources conservation. Acta Hortic 1087:225–232
Kulus D (2016) Application of cryogenic technologies and somatic embryogenesis in the storage and protection of valuable genetic resources of ornamental plants. In: Mujib A (ed) Somatic embryogenesis in ornamentals and its applications. Springer, New Delhi, pp 1–25
Kulus D (2018) Effects of various preculture, pretreatment, and recovery conditions on the morphogenetic response of cryopreserved Lady Orange chrysanthemum shoot tips. Turk J Biol 42:76–86
Kulus D (2019) Application of synthetic seeds in propagation, storage, and preservation of Asteraceae plant species. In: Faisal M, Alatar A (eds) Synthetic seeds. Springer, Cham, pp 155–179
Kulus D (2020) Cryopreservation of bleeding heart (Lamprocapnos Spectabilis (L.) Fukuhara) shoot tips using encapsulation-dehydration. Cryoletters 41:75–85
Kulus D, Zalewska M (2014) Cryopreservation as a tool used in long-term storage of ornamental species—a review. Sci Hortic 168:88–107
Kulus D, Abratowska A, Mikuła A (2018) Morphogenetic response of shoot tips to cryopreservation by encapsulation-dehydration in a solid mutant and periclinal chimeras of Chrysanthemum × grandiflorum /Ramat./Kitam. Acta Physiol Plant 40:18–31
Larkin P, Scowcroft W (1981) Somaclonal variation - a novel source of variability from cell cultures for plant improvement. Theor Appl Genet 60:197–214
Laslo V, Zăpârţan M, Agud E (2011) In vitro conservation of certain endangered and rare species of Romanian spontaneous flora, Analele Univ Oradea. Fasc Protecţia Mediului 16:252–261
Liu U, Breman E, Cossu TA, Kenney S (2018) The conservation value of germplasm stored at the Millenium Seed Bank, Royal Botanic Gardens, Kew, UK. Biodivers Conserv 27:1347–1386
Marković M, Grbić M, Djukić M (2013) Micropropagation of the endangered and decorative specie Dianthus serotinus Waldst. et Kit. Not Bot Horti Agrobot 41:370–377
Mráz P, Ronikier M (2016) Biogeography of the Carpathians: evolutionary and spatial facets of biodiversity. Biol J Linn Soc 119:528–559
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco cell cultures. Physiol Plant 15:473–497
Nunez S, Arets E, Alkemade R, Verwer C, Leemans R (2019) Assessing the impacts of climate change on biodiversity: is below 2°C enough? Clim Change 154:351–365
O’Donnell K, Sharrock S (2018) Botanic gardens complement agricultural gene bank in collecting and conserving plant genetic diversity. Biopreserv Biobank 16:384–390
Oprea A (2005) Lista Critică a Plantelor Vasculare din România. Editura Universităţii „Alexandru Ioan Cuza”, Iaşi
Peres S (2016) Saving the gene pool for the future: seed banks as archives. Stud Hist Philos Biol Biomed Sci 55:96–104
Reed BM (2008) Plant cryopreservation: a practical guide. USDAARS National Clonal Germplasm Repository, Corvallis, pp 3–11
Sakai A, Engelmann F (2007) Vitrification, encapsulation-vitrification and droplet-vitrification: a review. CryoLett 28:151–172
Sakai A, Kobayashi S, Oiyama I (1990) Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Rep 9:30–33
Sakai A, Hirai D, Niino T (2008) Development of PVS-based vitrification and encapsulation-vitrification protocols. In: Reed BM (ed) Plant cryopreservation: a practical guide. Springer, New York, pp 421–426
Sarasan V, Cripps R, Ramsay M, Atherton C, McMichen M, Prendergast G, Rowntree J (2006) Conservation in vitro of threatened plants—progress in the past decade. Vitro Cell Dev Biol Plant 42:206–214
Sârbu I, Ştefan N, Oprea A (2013) Plante Vasculare din România. Editura Victor B Victor, Bucureşti
Sekizawa K, Yamamoto S, Rafique T, Fukui K, Niino T (2011) Cryopreservation of in vitro-grown shoot tips of carnation (Dianthus caryophyllus L.) by vitrification method using aluminium cryo-plates. Plant Biotechnol 28:401–405
Tannoury M, Vintejoux C (1997) Cytological studies of Dianthus caryophyllus apex after cryopreservation. Acta Bot Gallica 144:107–118
Tannoury M, Vintéjoux C, Dereuddre J (1995) Cryoconservation par encapsulation et déshydratation d'apex d'œillet (Dianthus caryophyllus L.) cultivés in vitro. Acta Bot Gallica 142:415–424
Tinch R, Bugter R, Blicharska M, Harrison P, Haslett J, Jokinen P, Mathieu L, Primmer E (2018) Arguments for biodiversity conservation: factors influencing their observed effectiveness in European case studies. Biodivers Conserv 27:1763–1788
Turner SR, Senaratna T, Bunn E, Tan B, Dixon KW, Touchell DH (2001) Cryopreservation of shoot tips from six endangered Australian species using a modified vitrification protocol. Ann Bot 87:371–378
Umesha S (2019) Plant biotechnology. CRC Press/Taylor & Francis Group, New York
Urban MC (2015) Accelerating extinction risk from climate change. Science 348:571–574
Volk GM, Walters C (2006) Plant vitrification solution 2 lowers water content and alters freezing behavior in shoot tips during cryoprotection. Cryobiology 52:48–61
Wade EM, Nadarajan J, Yang X, Ballesteros D, Sun W, Pritchard HW (2016) Plant species with extremely small populations (PSESP) in China: a seed and spore biology perspective. Plant Divers 38:209–220
Wang RR, Gao XX, Chen L et al (2014) Shoot recovery and genetic integrity of Chrysanthemum morifolium shoot tips following cryopreservation by droplet-vitrification. Sci Horticult 176:330–339
Whiteley SE, Bunn E, Menon A, Turner SR (2016) Ex situ conservation of the endangered species Androcalva perlaria (Malvaceae) by micropropagation and cryopreservation. Plant Cell Tissue Org Cult 125:341–352
Yin ZF, Bi WL, Chen L et al (2014) An efficient, widely applicable cryopreservation of Lilium shoot tips by droplet vitrification. Acta Phys Plant 36:1683–1692
Ziv M (2005) Simple bioreactors for mass propagation of plants. In: Hvoslef-Eide A, Preil W (eds) Liquid culture systems for in vitro plant propagation. Springer, Dordrecht, pp 79–94
Acknowledgements
Partial funding from the Ministry of Education and Research through the Projects 31-008/2007 (Partnerships in priority areas PN II), BIOSERV 25N/2019 (Core Program PN2019-2022 BIODIVERS 3), and 22PFE/2018. The authors thank to the colleagues Gheorghe Coldea, Mihai Puşcaş, Adrian Oprea, Dan Cogălniceanu and Liviu Filipaş for collecting the Dianthus species mentioned above.
Funding
Partial funding from the Ministry of Education and Research through the Projects 31-008/2007 (Partnerships in priority areas PN II), BIOSERV 25N/2019 (core program PN2019-2022 BIODIVERS 3), and 22PFE/2018.
Author information
Authors and Affiliations
Contributions
Adela Halmagyi designed and performed the cryopreservation experiments and wrote the paper. Ana Coste contributed to the cryopreservation experiments, in vitro micropropagation and performed the statistical analysis of the data. Victoria Cristea and Liliana Jarda performed the initiation of in vitro cultures and multiplication for two years of Dianthus species and provided in vitro plant material. Anca Butiuc-Keul provided critical revision of the article, Irina Holobiuc contributed to the collection of plant material from natural habitats and to the in vitro micropropagation of Dianthus species. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Additional information
Communicated by Daniel Sanchez Mata.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the Topical Collection: Ex-situ conservation.
Rights and permissions
About this article
Cite this article
Halmagyi, A., Coste, A., Jarda, L. et al. A safeguard measure of endemic and endangered plant species: cryostorage of Dianthus taxa. Biodivers Conserv 29, 3445–3460 (2020). https://doi.org/10.1007/s10531-020-02032-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-020-02032-3