Abstract
The objective of the present study was the cryopreservation of monotypic endemic Hladnikia pastinacifolia Rchb. shoot tips from an in vitro culture, via encapsulation-dehydration (ED) or encapsulation-vitrification (EV). For all tested genotypes, the highest rates of shoot regrowth and multiplication were obtained after overnight preculture in 0.4 M sucrose, encapsulation in Murashige and Skoog (MS) medium with 0.4 M sucrose and 1 M glycerol, followed by polymerization in 3% (w/v) Na-alginate in MS with 0.4 M sucrose. Optimal osmoprotection was achieved for ED with 0.4 M sucrose plus 1 M glycerol and for EV with 0.4 M sucrose plus 2 M glycerol. The best dehydration time for ED was 150 min in a desiccation chamber with silica gel, and the best vitrification time for EV was 85 min in plant vitrification solution 2 (PVS2). For ED, dehydration for 150 min resulted in explant water content of 22%. When the encapsulation method was combined with ED, 53% regrowth was achieved, and when it was combined with EV, 64% regrowth was achieved. Both methods could become applicable for the long-term cryopreservation of H. pastinacifolia germplasm, although EV was faster and resulted in better final regrowth success. Genetic stability analysis of cryopreserved plant samples was carried out for two genotypes, using random amplified polymorphic DNA (RAPD) markers to compare the two different cryopreservation protocols. Significant genetic differences between the genotypes were detected and a low level of genomic variation was observed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hladnikia pastinacifolia Rchb. (Apiaceae) is a monotypic endemic genus, with an extremely narrow distribution area of 4 km2 within the Trnovo Forest Plateau (Trnovski gozd), Slovenia, where it occurs in one larger and three smaller isolated populations. Its rarity and its limited habitats make this species vulnerable and protected by legislation (Čušin 2004). To insure its long-term conservation, both in situ and ex situ strategies for nature conservation have been established: in situ protection of natural habitats and ex situ protection of seeds and whole plants at the Botanical Garden, University of Ljubljana (Šajna et al. 2012; Ambrožič-Dolinšek et al. 2016). Both methods are traditional and represent the most efficient form of conservation for a majority of plant species (Pence 2013, 2014). However, these methods have several distinct disadvantages for the unique species H. pastinacifolia. One reason lies in the short-lived seeds with their complicated dimorphic dormancy, a difficulty that renders preservation in the form of seeds useless (Šajna et al. 2014). The plant’s monocarpic life cycle represents another problem: it flowers and produces seeds only once, usually several years after germination. All these issues have called for conservation measures in addition to ex situ plant collection and seed storage in seed banks. Biotechnological techniques, such as plant tissue culture, could be such measures, as they can provide an alternative method for propagation and long-term preservation of protected species (Coelho et al. 2012; Pence 2013). Research on alternative conservation measures for H. pastinacifolia was initiated in 2006 with the development of a micropropagation procedure (Ambrožič-Dolinšek et al. 2016).
The use of cryopreservation for the safekeeping of rare and endangered plants is another technology for addressing such conservation challenges. However, cryopreservation techniques are mostly known from an agricultural perspective. The majority of cryopreservation techniques have been used for agronomically important plant species, which are artificially selected and domesticated, and thus have well-known cultivation conditions. However, for wild plants, especially where key biological features of the species are still poorly understood, cryopreservation represents a completely different challenge, and thus is far from a routine procedure. In contrast to easily grown domesticated crop varieties, certain wild species of plants have resisted cultivation and domestication efforts, often due to an underlying lack of knowledge about their basic biology. This is especially true for H. pastinacifolia, which bears special biological traits, has problematic sexual reproduction, and requires specific habitat conditions, as explained in more detail by Šajna et al. (2012) and Ambrožič-Dolinšek et al. (2016). The present study therefore focused on cryopreservation of a very particular species, a genetically depauperated in situ survivor of the last glaciation with extremely narrow distribution, being one of the four endemic monotypic genera of the Alps.
Cryopreservation is an ideal means for long-term storage of biological materials (Wang et al. 2012; Benelli et al. 2013). Many cryopreservation techniques have been developed (Wang et al. 2014) and are successfully used with a range of cells, tissues, and organs of a multitude of plant species (Engelmann et al. 2008). Encapsulation-dehydration (ED) and encapsulation-vitrification (EV) are relatively simple cryopreservation procedures, which do not require special equipment. The methods are inexpensive and safe to implement for both plants and humans. Encapsulation means that small explants, such as seeds, shoots, or embryos, are enclosed in a protective, non-toxic capsule, also called a bead. The encapsulation technology used with artificial seeds, so-called synthetic seed (or synseed) technology, continues to be a promising technique for clonal propagation and conservation, despite being at least two decades old. Both ED and EV include encapsulation of plant material in calcium alginate beads and require treatment in a medium containing high levels of sucrose (Shatnawi et al. 1999) and/or glycerol (Lipavska and Vreugdenhil 1996). The advantages of ED are that simple dehydration methods are combined with commonly available chemicals, namely sucrose and glycerol; therefore, potential toxicity concerns caused by cryoprotectants, such as dimethyl sulfoxide (DMSO), are avoided (Lipavska and Vreugdenhil 1996). Compared to ED, the vitrification method involves additional steps (Charoensub et al. 2004). In the case of EV, the alginate beads are vitrified in cryoprotectant solutions composed of DMSO, ethylene glycol (EG), or the like, in addition to sucrose and glycerol (Sakai et al. 1990).
In the case of long-term storage of plant-germplasm, a major concern is the genetic integrity and stability of plants regenerated from cryopreserved material (Benson 2008). The random amplification of polymorphic DNA (RAPD) technique is a fast, simple, and efficient method for evaluating the genetic stability of material regenerated after the completion of a cryopreservation experiment (Hirai and Sakai 2000); also, it has been successfully used to study the population genetics of H. pastinacifolia (Šajna et al. 2012).
With the goal of long-term conservation of this rare and endangered plant, the objective of the present study was to establish and compare two efficient protocols for cryopreservation of H. pastinacifolia, based on the encapsulation of shoot tips: encapsulation-dehydration and encapsulation-vitrification. Additionally, the genetic stability of plant material regenerated after cryo-storage was evaluated using RAPD analysis.
Materials and methods
Plant material
The in vitro-grown shoot tips of H. pastinacifolia selected for cryopreservation in this study, were obtained from the micropropagation collection maintained at the Faculty of Natural Sciences and Mathematics, University of Maribor, Slovenia. Shoot cultures of several genetically different lines were maintained on solid MS medium (Murashige and Skoog 1962), with or without 5 μM 6-benzylaminopurine (BAP; Sigma-Aldrich®, St. Louis, MO) and 3 μM indole-3-butyric acid (IBA; Sigma-Aldrich®), 0.8% (w/v) Difco®-Bacto® (Becton, Dickinson and Company, Franklin Lakes, NJ) agar, 3% (w/v) sucrose (Duchefa Biochemie, Haarlem, Netherlands) with the pH adjusted to 5.7–5.8 using 1 M HCl or 1 M NaOH, all before autoclaving for 15 min at 121°C (Ambrožič-Dolinšek et al. 2016). Growth regulators were prepared as 1 mM stock water solutions, dissolved first with 150–200 μL of 1 M NaOH. The shoots were cultured in 200 mL jars (Sigma-Aldrich®) with 20 mL of solid MS medium, closed with Magenta™ B-caps (Sigma-Aldrich®), and transferred to fresh medium every 4–6 wk. The cultures were maintained in a growth chamber at 23 ± 2°C, with Osram L 36 W/77 FLUORA fluorescent lights (Kambič d.o.o., Semič, Slovenia), under a 16-h photoperiod and with 37–50 μmol m−2 s−1 light intensity. All the cryopreservation experiments were carried out with material from three different genotypes (L0, L3, and L9).
Cryopreservation
Figure 1 shows a flowchart schematic of the successive stages of H. pastinacifolia cryopreservation, described in detailed below:
Schematic representation of successive stages of encapsulation-dehydration (ED) and encapsulation-vitrification (EV) cryopreservation of Hladnikia pastinacifolia shoot tips. MS Murashige and Skoog medium; PVS2 plant vitrification solution 2; VSL vitrification solution L; LN liquid nitrogen; BAP 6-benylaminopurine; IBA indole-3-butyric acid.
Preconditioning of plant material from culture collection
The in vitro-micropropagated shoot culture was cold hardened in culture vessels at 4°C in the dark for 7–14 d.
Pre-cultivation of explants
Shoot tips (2.5–3.5 mm), individually excised under a stereomicroscope from the preconditioned culture collection, were pre-cultivated in a liquid MS medium with 0.1, 0.25, or 0.4 M sucrose 12–14 h at 25 ± 1°C.
Encapsulation
The excised pre-cultivated shoot tips were incubated on a rotary shaker in 3% (w/v) Na-alginate with different concentrations of sucrose, or combinations of sucrose and glycerol, in liquid MS media without CaCl2 for 30–60 min at 25 ± 1°C. From among the different concentrations of sucrose (0.25–0.75 M) or combination of sucrose (0.25–0.75 M) with glycerol (1 or 2 M) tested (Table S1), Table 1 presents only the most successful encapsulation solutions. Each shoot tip in a drop of alginate medium was transferred into fresh liquid MS medium with 100 mM CaCl2 (for solidification of alginate), and supplemented with different concentrations of sucrose, or sucrose plus glycerol (Table 1), for 30–60 min at 25 ± 1°C (Engelmann et al. 2008). This resulted in encapsulated shoots, each trapped in the middle of a polymerized alginate bead.
Osmoprotection and dehydration for encapsulation-dehydration
The shoots, encapsulated in alginate beads, were osmoprotected in a MS medium containing either sucrose or sucrose plus glycerol (Table 1) for 10–12 h, on a rotary shaker (130 rpm), at 25 ± 1°C. The osmoprotected encapsulated shoots were dried under laminar air flow for 8 h, or in a desiccation chamber with silica gel for 150–180 min, to lower the water content (WC) to 28–32%, and then frozen by direct immersion in liquid nitrogen (LN; Engelmann et al. 2008).
The WC of the beads was determined during dehydration, which was carried out prior to cooling. The beads were weighed every 30 min for preparation of the desiccation curves for each osmoticum. The WC of the beads was calculated using the following equation:
Osmoprotection and dehydration for encapsulation-vitrification
The encapsulated shoots were osmoprotected in a MS medium containing sucrose, or sucrose plus glycerol, as for ED, for 30 min. The osmoprotected encapsulated shoots were then dehydrated with different vitrification solutions (Tables 2 and 3) on a rotary shaker (130 rpm) at 0ºC, for different durations (70, 85, or 100 min) (Sakai and Engelmann 2007; Jeon et al. 2015). Two vitrification solutions were tested: plant vitrification solution 2 (PVS2), which contained 30% (v/v) glycerol (Sigma-Aldrich®), 15% (v/v) ethylene glycol (Sigma-Aldrich®), 15% (v/v) DMSO (Sigma-Aldrich®), in MS medium with 0.4 M sucrose (Duchefa Biochemie) (Sakai et al. 1990), or vitrification solution L (VSL), containing 20% (v/v) glycerol, 30% (v/v) ethylene glycol, 10% (v/v) DMSO, 5% (w/v) sucrose in MS medium with 0.4 M sucrose (Duchefa Biochemie) (Suzuki et al. 2008). After the PVS2 or VSL treatment, at least 20 beads from each treatment, at a maximum of ten beads per 2-mL cryo-vial, were placed in the last-used cryoprotectant medium filled to the top of beads.
Cryopreservation
The ED or EV shoots in the cryo-vials were directly immersed into LN (− 196°C) contained in a 10-L Dewar flask. The samples remained in the liquid nitrogen for 1 to 7 d.
Regrowth after cryopreservation
The cryo-vials with cooled ED shoot tips were removed from the LN and were allowed to rewarm for 5 min at 25 ± 1°C. This was followed by 15 min of rehydration in the 2-mL cryo-vials filed to the top with the last-used cryoprotection solution. The cryo-vials with cooled EV shoot tips were removed from the LN and rapidly warmed in a water bath at 40°C for 1 min, followed by rehydration by triple washing in liquid MS medium with 1.2 M sucrose (Sakai and Engelmann 2007). The encapsulated shoots from ED and EV were transferred directly to 200 mL vessels (V8630; Sigma-Aldrich®), always 5–7 shoots per vessel, with 20 mL of solid MS medium without growth regulators, and were kept in the growth chamber for 1–2 wk. The surviving shoots were then transferred to vessels (V8630; Sigma-Aldrich®), with solid MS medium supplemented with 2 μM BAP to induce regrowth, and 2 wk later to vessels (V8630; Sigma-Aldrich®), with solid MS medium with 5 μM BAP and 3 μM IBA (Ambrožič-Dolinšek et al. 2016). Survival was evaluated in the first 1–2 wk after cryopreservation by counting the number of green shoots showing signs of viability, expressed as the ratio of surviving shoots counted per treated shoots. In the preliminary pre-cultivation and WC experiments, only the survival was evaluated. For later cryopreservation experiments, only regrowth was evaluated and confirmed by development of new organs, such as shoots from meristems. Regrowth was evaluated after 4–6 wk of culture by counting the number of shoots showing signs of growth. Multiplication per treated shoots was calculated.
Comparison of ED and EV
The effect of ED or EV (with PVS2) on regrowth before (−LN) and after cryopreservation (LN) was investigated for three H. pastinacifolia genotypes (L0, L3, and L9).
Random amplification of polymorphic DNA analysis
Genetic analysis was carried out in two different genotypes (L3 and L9). For each genotype, three samples from the non-cryopreserved control plants and three samples from each cryopreservation treatment (ED or EV) were considered. Total genomic DNA was extracted from the leaves of the samples, according to a modified cetyl trimethylammonium bromide (CTAB) protocol (Šuštar-Vozlič and Javornik 1999; Šajna et al. 2012). Polymerase chain reaction (PCR) of RAPD amplifications was performed in a 25-μL reaction volume with a PCR system (T Professional Standard Gradient Thermocycler 070–851; Biometra (Biomedizinische Analytik GmbH, Goettingen, Germany). Each reaction contained 5 × My Taq Reaction Buffer (Bioline GmbH, Luckenwalde, Germany): 5 mM dNTPs, 15 mM MgCl2, 20 μM of each primer (Table 4), 0.5 U My Taq DNA polymerase (Bioline GmbH), and 20 ng template DNA. The PCR conditions consisted of initial denaturation at 95°C for 1.5 min; followed by 35 cycles of strand denaturation at 95°C for 15 s, primer annealing at 37°C for 15 s, DNA extension at 72°C for 10 s; with a final extension at 72°C for 10 min. Sixteen primers (Operon Technologies Ltd., Alameda, CA; and Jena Bioscience GmbH, Jena, Germany) were tested with the H. pastinacifolia samples in order to find the most informative and reproducible primers for RAPD analysis (Šuštar-Vozlič and Javornik 1999; Šajna et al. 2012). Ten primers were selected for further analysis.
Polymerase chain reaction products were separated electrophoretically in ethidium bromide-stained 2% (w/v) agarose-TBE (Tris-borate-ethylenediaminetetraacetic acid) gels. The sizes of the PCR products were compared to a molecular size standard (Gene Ruler DNA Ladder Mix #SM0332; Thermo Fisher Scientific®, Waltham, MA). A negative control without DNA was included to check for contamination. Reproducible RAPD bands were scored as binary presence/absence data.
Statistical analysis
The statistical software package SPSS® 24.0 (SPSS Inc., Chicago, IL) was used for data analysis. The chi-squared (χ2) test of the analysis of variance (ANOVA) using the post hoc Duncan test was used for evaluating the levels of statistical significance (P) between the control samples and the cryopreserved ones. The significant differences were indicated by distinct letters in tables and figures. The symbols used in the figures are as follows: NS denotes not significant, * denotes P < 0.05, ** denotes P < 0.01, *** denotes P < 0.001.
All experiments were repeated at least twice. In each repetition, at least three replicates, with no fewer than 20 explants per replicate, were used.
The amplified fragments from the RAPD analyses of the control and cryopreserved samples were scored as present (1) or absent (0). Genetic similarities were calculated using the Jaccard similarity coefficient. The resultant matrix was subjected to cluster analysis by the unweighted pair-group method analysis (UPGMA), and a dendrogram was constructed from the clustering of the results with the help of the TREE program, using the software package NTSYS-PC version 1.8 (Rohlf 1992).
The statistical analysis for the RAPD analysis was done with GENALEX 6.501 (Peakall and Smouse 2012). Analysis of the RAPD pattern was performed by an analysis of molecular variance (AMOVA) with the computer program GenAlex 6.501 (Peakall and Smouse 2012). Three samples from the non-cryopreserved control plants and three samples from each of the two genotypes (L3, L9) of plants exposed to each cryopreservation treatment were included in the statistical analyses to estimate variation due to genotype differences and experimental treatments.
Results
Cryopreservation by ED
The effect of several different combinations and concentrations of the osmoprotectants sucrose and glycerol on survival into green shoot tips during encapsulation/polymerization and osmoprotection was tested on the encapsulated shoots before (−LN) and after (LN) cryopreservation in liquid nitrogen (Table S1). Only the data from treatments resulting in regrowth into new growing shoots are shown in Table 1. The best combination and concentrations of osmoprotectants for encapsulation proved to be 0.4 M sucrose with 1 M glycerol, while for polymerization the conditions were 3% (w/v) Na-alginate in MS with 0.4 M sucrose, and for the subsequent osmoprotection, 0.4 M sucrose plus 1 M glycerol was optimal. During that procedure, between 35 and 53% of encapsulated shoots survived cryopreservation, and exhibited further regrowth.
For further optimization of ED, it was investigated whether overnight preculture of shoots in liquid MS with 0.1, 0.25, or 0.4 M sucrose concentrations immediately after excision influenced their survival (Table S2). It was found that, among the three concentrations tested, the best survival of 33% was achieved after preculture in the 0.4 M concentration of sucrose. The survival of shoots precultured overnight at 0.25 M sucrose was lower (23%), while shoots, precultured overnight in the usual MS medium with 0.1 M sucrose, did not survive cryopreservation (Table S2).
The effect of two dehydration methods and exposure time on WC and survival of ED shoots
The effect of exposure time on the WC of the encapsulated and osmoprotected shoots after the application of the two physical dehydration methods was evaluated in a separate experiment without cryopreservation of plant material in LN. The encapsulated shoots that entered the experiment were prepared with the previously established most successful concentrations of osmoprotectants: 0.4 M sucrose with 1 M glycerol or 0.4 M sucrose with 2 M glycerol. The two different dehydration methods took place inside a laminar flow hood or a desiccation chamber with silica gel. The effectiveness of both dehydration methods on the desiccation of the encapsulated shoots was evaluated every 30 min for a total duration of 180 min.
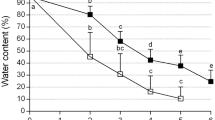
Drying was faster in the desiccation chamber and resulted in encapsulated shoots with lower WC than found in those prepared by drying under laminar airflow (Fig. 2A). The WC of encapsulated shoots dried in the desiccation chamber for 120 min reduced to values between 28 and 33%; thereafter, further decreases occurred gradually. However, the WC of the encapsulated shoots dried for 120 min in the laminar flow consistently reached significantly higher values (Fig. 2A). After 150 min, the differences between both drying methods diminished and were not significant anymore. The WC of the encapsulated beads after drying in the laminar flow for 180 min equaled the WC of the encapsulated beads they had dried for 120 min in the desiccation chamber (Fig. 2A). There were significant differences between each osmoprotection treatment exposed to different drying methods and no statistically significant differences within either osmoprotection treatment tested for a particular drying method during the first 120 min of drying (Fig. 2A). Both dehydration procedures could be used for drying encapsulated shoot tips, although the method employing the desiccation chamber with silica gel was faster, especially in the first 2 h of drying, and ultimately yielded a lower final WC.
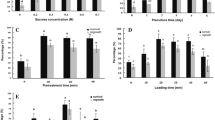
Effect of osmoprotection, duration of dehydration, and drying method [laminar flow (LF) or desiccation chamber (DC)] on (A) the water content of Hladnikia pastinacifolia encapsulated shoots after osmoprotection in 1—osmo (0.4 M sucrose with 1 M glycerol); or, 2—osmo (0.4 M sucrose with 2 M glycerol). (B) Effect of two dehydration methods [laminar flow (LF) or desiccation chamber (DC)] on survival [%] of encapsulated shoots, evaluated after 0, 150, or 180 min, before liquid nitrogen (−LN) and after (LN) cryopreservation. Values followed by the same letter do not differ significantly (Duncan, P ≤ 0.05; n = 20). Effect of cryopreservation method [encapsulation-dehydration (ED) or encapsulation-vitrification (EV)] on regrowth of three different genotypes (L0, L3, L9) of H. pastinacifolia after 4–6 wk. on regrowth medium, (C) before (−LN) and (D) after cryopreservation (LN). Statistical differences were calculated between ED and EV for the same line, treatment, and observed parameters (χ2 test; NS not significant; *P < 0.05, **P ≤ 0.01, ***P < 0.001; n = 20). Effect of cryopreservation by ED or EV on development of (E) new shoots (± SD) and (F) new shoots with callus formation. (E) Average numbers of developed shoots not treated by cryopreservation (C control) were compared with ED and EV cryopreserved shoots. Values followed by the same letter do not differ significantly (Duncan, P ≤ 0.05; n = 20). (F) Percentages of new shoots with callus formation not treated by cryopreservation (C control) were compared with ED and EV cryopreserved shoots. Values followed by the same letter do not significantly differ (χ2 test, P < 0.05; n = 20).
The effect of the two different dehydration methods after two exposure times on the WC and the survival of the encapsulated shoots was evaluated before (−LN) and after (LN) cryopreservation (Fig. 2B). The encapsulated shoots used for this experiment were again prepared with the most successful concentrations of osmoprotectants during osmoprotection. Both desiccation methods were tested at the beginning, after 150, or 180 min of dehydration. The survival of encapsulated shoots after 150 min in the desiccation chamber with silica gel reached 49%, whereas for shoots dried in the laminar flow it was only 24% (Fig. 2B). The survival rate of encapsulated shoots after 180 min reached 45% in the desiccation chamber and 53% in the laminar flow method. The survival levels of encapsulated shoots exposed to 180 min of drying by either of the two dehydration methods did not significantly differ (Fig. 2B). For this period, the encapsulated shoots that were dried in the desiccation chamber were determined to have 24% WC, while those dried in the laminar flow showed 36% WC.
Cryopreservation by EV
Treatments before dehydration included encapsulation, polymerization, and the two osmoprotection treatments developed for ED, followed by the vitrification part of EV with solutions PVS2 or VSL for 85 min (Table 2). The highest regrowth was obtained through vitrification with PVS2, which consisted of a combination of osmoprotectants, 0.4 M sucrose, and 2 M glycerol. With 60% of the explants regenerating, this method proved better than other methods tested and results were comparable for all germplasm lines that were tested (Table 2).
The effect of exposure time and vitrification treatment on regrowth of EV shoots
The effect of exposure time and vitrification solutions on the regrowth of encapsulation shoots tested before (−LN) and after (LN) liquid nitrogen preservation was evaluated for both best EV treatment regimens. The greatest regrowth, with 64% regenerated explants after cryopreservation, was obtained after 85 min of vitrification with PVS2 (Table 3); this duration was better than the others tested, and comparable results were obtained for all tested lines (data not shown).
Comparison of the two cryopreservation procedures
The effects of ED and EV (using PVS2) on regrowth before (−LN) and after cryopreservation (LN) on three H. pastinacifolia genotypes L0, L3, and L9 were compared (Fig. 2C, D). All three genotypes yielded consistently better regrowth with EV, before and after cryopreservation, than with ED, although only one genotype, L0, regenerated at significantly better rates after EV than after ED treatment (Fig. 2C, D).
Further development of ED and EV cryopreserved shoots
Regrowth, exhibited as the development of new shoots, was determined after four transfers of shoots to fresh multiplication medium after treatment with ED or EV, before or after cryopreservation in LN (Fig. 2E). Both the control non-cryopreserved shoots and the cryopreserved shoots showed the same growth and developmental patterns and grew into normal shoots with the same proliferation capacity. On average, 3.3 to 5.9 new shoots developed in all treatments, irrespective of whether or not they had been treated by ED or EV, or whether they had been cryopreserved (Fig. 2E). In general, shoot development during the first transfer after EV was significantly lower and during the next transfers slightly (but not significantly) higher, compared to the control or ED (Fig. 2E).
Through the first few transfers, regrowth after cryopreservation was accompanied by callus formation. This was the only morphological deviation observed when compared to shoots that were not cryopreserved. The callus formed at the base of newly developed shoots regenerated from shoot tips cryopreserved by ED or EV. The cryopreserved shoots exhibited significant amounts of callus formation for the first three to four transfers (Fig. 2F), but after that, callus formation ceased. At the first transfer, between 86 and 94% of new shoots derived from cryopreserved explants were accompanied by callus formation, but this percentage decreased over the next transfers. By the third transfer, between 20 and 43% of shoot regrowth was accompanied by callus formation. On the fourth transfer there was no callus on the shoots cryopreserved by ED, while in the EV cryopreserved shoots, 15% of shoots still exhibited callus formation at the base (Fig. 2F). On subsequent transfers (data not shown), there was no callus formation. Subsequent behavior of the shoots was comparable to that of non-cryopreserved shoots, from which they were indistinguishable. The majority of new shoots readily developed roots, and the overall root development rates resulted in between 85 and 98% of shoots having functional roots.
Assessment of genetic stability
The ten primers used yielded 63 scorable bands, which were selected for further analysis (Table 4). The average number of bands was 6 per primer, with a minimum of 5 and a maximum of 10 bands per primer. Fragment size ranged from 130 to 1500 bp (Table 4; Fig. 3).
Example of random amplification of polymorphic DNA (RAPD) analysis obtained from control and cryopreserved samples of genotypes L3 and L9 of Hladnikia pastinacifolia using the primers A11, E19, OPQ-14. C non-cryopreserved control; ED encapsulation-dehydration; EV encapsulation-vitrification; M molecular weight scale (DNA ladder).
Thirty-eight markers out of 63 were monomorphic; therefore, 31.5% were polymorphic. However, the percentage of polymorphism decreased to 16 and 9.7% when genotypes L3 and L9, respectively, were considered separately. When comparing the two different genotypes, the AMOVA indicated significant differences (P value = 0.045) among them. Thirty percent of the total diversity detected was attributed to differences between genotypes. However, when the three different treatments (C non-cryopreserved control, ED encapsulation-dehydration, and EV encapsulation-vitrification) were analyzed, no significant differences among treatments were detected.
These results agreed with the distribution given in the dendrogram (Fig. 4). The two genotypes formed two distinct clusters with a 79% of similarity. Diversity within each cluster was low (both genotypes showed around 95% similarity among all their samples), and some samples even showed identical RAPD profiles (100% similarity), as was the case with samples 3C.3–3V.1 and 3D.2–3V.3 in genotype L3, and samples 9C.2–9D.1 in genotype L9 (Fig. 4).
Dendrogram generated by the unweighted pair-group method analysis (UPGMA) method using Jaccard’s similarity coefficient, based on random amplification of polymorphic DNA markers from Hladnikia pastinacifolia shoot tips of genotypes L3 and L9. C non-cryopreserved control; D encapsulation-dehydration; V encapsulation-vitrification.
The AMOVA indicated that the majority of the variation (99%) could be attributed to differences between samples from different genotypes, and only 1% of the variation might be accounted for by the differences in treatments. Two variable bands were observed. One of them had a 1200-bp-sized fragment with sample L9 cryopreserved by ED and amplified with OPO-15 oligonucleotide, and the other had a fragment of 1500 bp with the sample L9 cryopreserved after EV with oligonucleotide E19 (Fig. 3).
Discussion
Cryopreservation of encapsulated germplasm is now used as a conservation tool, which, once adjusted to minimize the adverse effects of cryoprotectants and post-preservation damage (Sharma et al. 2013), facilitates further manipulation of the encapsulated material. In the present case two encapsulation protocols (encapsulation-dehydration and encapsulation-vitrification) were evaluated for long-term storage of H. pastinacifolia shoot tips.
The minimum dehydration and vitrification time for ED and EV was identified. The optimal time to obtain a sufficient level of dehydration, allowing for shoot formation after cryopreservation and at the same time avoiding the toxic effects of the vitrification solution, was for EV shorter than in the case of ED. For H. pastinacifolia, the exposure time to PVS2 yielding the best regrowth rate was found to be 85 min. For other species, for example Dianthus caryophyllus L., the duration of exposure to PVS2 was considerably longer and could last up to 200 min (Halmagyi and Deliu 2007).
Encapsulation-dehydration is a good choice when plant material is sensitive to the toxicity of the cryoprotective solution used in vitrification-based procedures (Benson 2008). In the present study this did not appear to be the case, since one of the cryoprotective solutions used, namely PVS2, resulted in superior cryopreservation of H. pastinacifolia shoots, with regrowth rates above 60%.
Often, one of the limitations encountered with cryopreservation is a strong genotype-dependent response. Here, both cryopreservation methods were tested on at least three different genotypes (lines) of H. pastinacifolia, and it was demonstrated that both ED and EV were suitable for the long-term conservation of these genotypes. Thus, the success of and the tolerance to cryopreservation might have been to some extent genotype-dependent.
Conservation methods are only useful if the stored plant material remains genetically stable. The aim of conservation of plant genetic resources is not only to store germplasm, but also to minimize or eliminate genetic variations during conservation. The first consideration in checking that culture responses remain unchanged after cryopreservation treatment was to analyze growth and development characteristics. In the present study, cryopreserved and non-cryopreserved shoots exhibited similar growth and development patterns. Cryopreserved shoots regenerated at the same rate as the non-cryopreserved controls and displayed the same shoot and root regrowth capacity. However, after cryopreservation of shoot tips using the ED or EV techniques, callus formed on the regenerating shoots was observed for the first three (ED) or four (EV) transfers to fresh medium, which subsequently ceased in the later transfers. Callus formation accompanying shoot regrowth during those early transfers may have occurred due to several factors, such as damage caused by ultra-low temperatures, and/or the dehydration rate in the case of ED, or the toxicity of DMSO found in PVS2, in the case of EV (Sakai and Engelmann 2007).
When, during cryopreservation procedures, tissues are exposed to physical, chemical, and physiological stresses and certain chemicals like DMSO (Panis and Lambardi 2005), genetic (Harding 2004; Martin and Gonzalez-Benito 2005) or epigenetic (Nuc et al. 2016) changes may occur. For this reason, the general practice has been the assessment of genetic stability during the introduction of ED (Castillo et al. 2010; Martin et al. 2011) or EV (Wang et al. 2014; Li et al. 2015) protocols. Genetic stability is assessed at the end of the cryopreservation procedure, or after each step in the cryopreservation procedure (Martin and Gonzalez-Benito 2005; Martin et al. 2011), or even later (Castillo et al. 2010). In the present investigation, genetic stability was assessed after successful regrowth of the cryopreserved material. A genetic stability analysis of cryopreserved plant samples was carried out for two genotypes, comparing two different cryopreservation protocols, ED and EV, using RAPDs markers. This analysis revealed significant differences between the two tested genotypes. Although previous works observed high homogeneity among populations of H. pastinacifolia (Šajna et al. 2012), in the present study a slight difference between different genotypes was identified. This difference between genotypes, however, was larger than the differences between cryopreservation treatments. Only genotype L3 seemed to exhibit a certain level of differentiation between non-cryopreserved (control) and cryopreserved samples, since the three samples corresponding to the control were grouped in the same sub-cluster (Fig. 4). However, some differences were observed even among the control samples. Therefore, this variation could have been the result of the previous tissue culture process, as the shoot tips were derived from in vitro cultures. In any event, differences were lower than 5% and should not represent a serious objection to the cryopreservation of H. pastinacifolia. A similar percentage of variation was detected by Martín et al. (2015) in some genotypes of Mentha × piperita L., cryopreserved through the encapsulation-dehydration protocol.
Conclusion
Encapsulation-based cryopreservation techniques, as presented in this study, are relatively easy to perform, inexpensive, and there is no need for sophisticated equipment. In addition, they have several other technical advantages: the size of the alginate bead-encapsulated explant is relatively large, which makes them easier to manipulate, and dehydration or vitrification are relatively straightforward. While these techniques are not as fast as some other techniques, when encapsulation is followed by vitrification, the time required for dehydration can be greatly reduced (Sakai and Engelmann 2007).
In the current study, several steps of ED and EV protocols were refined and optimized, before the conditions for regrowth of cryopreserved H. pastinacifolia were improved. To start with, overnight preculture of excised shoots of H. pastinacifolia in liquid MS with 0.4 M sucrose was found to be the most effective conditioning. The use of high sucrose concentrations was also effective for shoot treatment immediately after cryopreservation and during rehydration of H. pastinacifolia, as was noted for other species (Hirai and Sakai 1999; Halmagyi and Deliu 2007).
Cryopreservation is becoming an important tool for long-term storage of plant germplasm, requiring minimum space and maintenance. Besides its agricultural importance, it also offers the potential for long-term preservation of endangered species like H. pastinacifolia (Sakai and Engelmann 2007). This current report indicated that both encapsulation-based techniques of cryopreservation were efficient in producing high rates of plant regrowth from cryopreserved shoot tips and further, that the protocols worked successfully with several different genotypes of H. pastinacifolia.
References
Ambrožič-Dolinšek J, Ciringer T, Kaligarič M (2016) Micropropagation of the narrow endemic Hladnikia pastinacifolia (Apiaceae). Acta Bot Croat 75:244–252
Benelli C, de Carlo A, Engelmann F (2013) Recent advances in the cryopreservation of shoot-derived germplasm of economically important fruit trees of Actinidia, Diospyros, Malus, Olea, Prunus, Pyrus and Vitis. Biotechnol Adv 31:175–185
Benson EE (2008) Cryopreservation of phytodiversity: a critical appraisal of theory & practice. Crit Rev Plant Sci 27:141–219
Castillo NRF, Bassil NV, Wada S, Reed BM (2010) Genetic stability of cryopreserved shoot tips of Rubus germplasm. In Vitro Cell Dev Biol Plant 46:246–256
Charoensub R, Hirai D, Sakai A (2004) Cryopreservation of in vitro-grown shoot tips of cassava by encapsulation-vitrification method. CryoLetters 25:51–58
Coelho N, Gonçalves S, González-Benito ME, Romano A (2012) Establishment of an in vitro propagation protocol for Thymus lotocephalus, a rare aromatic species of the Algarve (Portugal). Plant Growth Regul 66:69–74
Čušin B (2004) Hladnikia pastinacifolia Rchb. – rebrinčevolistna hladnikija, hladnikovka. In: Čušin B, Babij V, Bačič T, Dakskobler I, Frajman B, Jogan N, Kaligarič M, Praprotnik N, Seliškar A, Skoberne P, Surina B, Škornik S, Vreš B. (eds) Natura 2000 v Sloveniji, Rastline. Založba ZRC, ZRC SAZU, Ljubljana (ISBN 961-6500-66-X), pp 107–113 (Slovenian language)
Engelmann F, Gonzalez Arnao MT, Wu Y, Escobar R (2008) Development of encapsulation in dehydration. In: Reed BM (ed) Plant cryopreservation: a practical guide. Springer, New York, pp 59–75
Halmagyi A, Deliu C (2007) Cryopreservation of carnation (Dianthus caryophyllus L.) shoot tips by encapsulation-vitrification. Sci Hortic 113:300–306
Harding K (2004) Genetic integrity of cryopreserved plant cells: a review. CryoLetters 25:3–22
Hirai D, Sakai A (1999) Cryopreservation of in vitro grown axillary shoot-tip meristems of mint (Mentha spicata) by encapsulation-vitrification. Plant Cell Rep 19:150–155
Hirai D, Sakai A (2000) Cryopreservation techniques. Cryopreservation of in vitro grown meristems of potato (Solanum tuberosum L.) by encapsulation–vitrification. JIRCAS Int Agric Ser 8:205–211
Jeon SM, Arun M, Lee SY, Kim CK (2015) Application of encapsulation-vitrification in combination with air dehydration enhances cryotolerance of Chrysanthemum morifolium shoots tips. Sci Hortic 194:91–99
Li BQ, Feng CH, Wang MR, Hu LY, Volk G, Wang QC (2015) Recovery patterns histological observations and genetic integrity in Malus shoot tips cryopreserved using droplet-vitrification and encapsulation-dehydration procedures. J Biotechnol 214:182–191
Lipavska H, Vreugdenhil D (1996) Uptake of mannitol from the media by in vitro grown plants. Plant Cell Tissue Organ Cult 45:103–107
Martin C, Gonzalez-Benito ME (2005) Survival and genetic stability of Dendranthema grandiflora Tzvelev shoot apices after cryopreservation by vitrification and encapsulation-dehydration. Cryobiology 51:281–289
Martin C, Cervera MT, Gonzalez-Benito ME (2011) Genetic stability analysis of chrysanthemum (Chrysanthemum x morifolium Ramat) after different stages of an encapsulation-dehydration cryopreservation protocol. J Plant Physiol 168:158–166
Martín C, Kremer C, González I, González-Benito ME (2015) Influence of the cryopreservation technique, recovery medium and genotype on genetic stability of mint cryopreserved shoot tips. Plant Cell Tissue Organ Cult 122:185–195
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Nuc K, Marszałek M, Pukacki PM (2016) Cryopreservation changes the DNA methylation of embryonic axes of Quercus robur and Fagus sylvatica seeds during in vitro culture. Trees 30:1831–1841
Panis B, Lambardi M (2005) Status of cryopreservation technologies in plants (crops and forest trees). The role of biotechnology, Villa Gualino, Turin, Italy – 5-7 March pp.43–54
Peakall R, Smouse P (2012) GenAlEx 6.501: genetic analysis in excel. Population genetic software for teaching and research—an update. Bioinformatics 28:2537–2539
Pence VC (2013) In vitro methods and the challenge of exceptional species for target 8 of the global strategy for plant conservation. Ann Mo Bot Gard 99:214–220
Pence VC (2014) Tissue cryopreservation for plant conservation: potential and challenges. Int J Plant Sci 175:40–45
Rohlf FJ (1992) NTSYS-PC: numerical taxonomy and multivariate analysis system. Exeter Software, New York
Šajna N, Kavar T, Šuštar-Vozlič J, Kaligarič M (2012) Population genetics of the narrow endemic Hladnikia pastinacifolia Rchb. (Apiaceae) indicates survival in situ during the Pleistocene. Acta Biol Cracov Ser Bot 54:1–13
Šajna N, Šuštar-Vozlič J, Kaligarič M (2014) New insights into the anatomy of an endemic Hladnikia pastinacifolia Rchb. Acta Bot Croat 73:375–384
Sakai A, Engelmann F (2007) Vitrification encapsulation-vitrification and droplet-vitrification: a review. CryoLetters 28:151–172
Sakai A, Kobayashi S, Oiyama I (1990) Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Rep 9:30–33
Sharma S, Shahzad A, Teixeira da Silva JA (2013) Synseed technology—a complete synthesis. Biotechnol Adv 31:186–207
Shatnawi M, Engelmann F, Frattarelli A, Damiano C, Withers LA (1999) Cryopreservation of apices from in vitro plantlets of almond (Prunus dulcis). CryoLetters 20:13–20
Šuštar-Vozlič J, Javornik B (1999) Genetic relationships in cultivars of hops, Humulus lupulus L., determined by RAPD analysis. Plant Breed 118:175–181
Suzuki M, Tandon P, Ishikawa M, Toyomasu T (2008) Development of a new vitrification solution, VSL, and its application to the cryopreservation of gentian axillary buds. Plant Biotech Rep 2:123–131
Wang B, Zhang Z, Yin Z, Feng C, Wang Q (2012) Novel and potential application of cryopreservation to plant genetic transformation. Biotechnol Adv 30:604–612
Wang B, Li JW, Zhang ZB, Wang RR, Ma YL, Blystad DR, Keller ERJ, Wang QC (2014) Three vitrification-based cryopreservation procedures cause different cryo-injuries to potato shoot tips while all maintain genetic integrity in regenerants. J Biotechnol 84:47–55
Funding
The Slovene Ministry of Higher Education, Science, and Technology supported this research within the program “Research to Ensure Food Safety and Health” with the Grant No. P1-0164, led by D. Škorjanc.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Barbara Reed
Electronic supplementary material
ESM 1
(DOCX 21 kb)
Rights and permissions
About this article
Cite this article
Ciringer, T., Martín, C., Šajna, N. et al. Cryopreservation of an endangered Hladnikia pastinacifolia Rchb. by shoot tip encapsulation-dehydration and encapsulation-vitrification. In Vitro Cell.Dev.Biol.-Plant 54, 565–575 (2018). https://doi.org/10.1007/s11627-018-9917-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-018-9917-y