Abstract
We report the establishment of the first in situ collection of beneficial symbiotic microorganisms (arbuscular mycorrhizal fungi) in the world, located in an integrally protected area of coastal sand dunes, within the UNESCO Biosphere Reserve “Selva Pisana”, in Tuscany, Italy. In this collection the genus Scutellospora, which has been reported to be threatened by anthropogenic disturbance, was a regular component of Ammophila arenaria and Helichrysum stoechas rhizospheres, and was represented by two species: Scutellospora fulgida and Scutellospora persica. Such species were morphologically identified and molecularly characterised by SSU and ITS sequence analyses. The establishment of an in situ collection of Scutellospora species in a protected area, where anthropogenic impact is under control of national and international authorities, is important for the conservation of rare and endangered microorganisms, representing a precious resource for future generations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Human impacts on the environment have altered the distribution and abundance of different species throughout the world, leading many native species to extinction (Pimm and Raven 2000). The action plan proposed to protect biological genetic diversity of natural populations and species representing a precious resource for future generations, takes into account the uneven distribution over land of species, many of them being concentrated in relatively small habitats scattered on different continents. Such special places, identified by conservation biologists, have been defined “biodiversity hotspots” (Myers 1988), i.e., terrestrial habitats rich in endemic species with a high-priority for conservation. Myers and coauthors (2000) determined 25 hotspots which occupy 1.4% of the terrestrial surface, yet representing the exclusive homes of 44% of Earth’s plant species and 35% of birds, mammals, reptiles and amphibians (Myers et al. 2000). Moreover, two habitats–the Tropical Andes and the Mediterranean Basin–have been classified as “hyper-hot” for conservation priorities, because of their extremely high numbers of endemic plants: 20,000 and 13,000, respectively. Interestingly, the authors consider the possible application of the hotspot thesis also to other organisms, for example invertebrates: they claim that if 50% of endemic plant species are lost, a similar proportion of insect species will be lost. The paradigm of such claim is represented by the fig genus, the most widespread plant genus in the tropics, comprising 900 species each living associated with single wasp species, which act as pollinators but depend on fig’s ovaries for larval development (Myers et al. 2000).
The same approach has been proposed for microorganisms living in obligate symbioses with endangered plant species, which would rapidly become endangered together with their host plants (Staley 1997; Fuerst and Hugenholtz 2000). Among symbiotic microorganisms, mycorrhizal fungi are keystone mutualists of high ecological value, which largely contribute to plant nutrition by means of large mycelial networks which uptake and transfer phosphate, nitrogen and other mineral nutrients from the soil to host plants, in exchange of carbohydrates (Smith and Read 1997; Giovannetti and Avio 2002). Moreover they represent important contributors to plant species composition, variability, productivity and biodiversity, affecting ecosystem functioning and biodiversity maintenance (Myers 1993; Smith and Read 1997; van der Heijden et al. 1998). Consistently with the Convention on Biological Diversity, where genetic resources are defined as “any material of plant, animal and microbial or other origin containing functional units of heredity” (United Nations Environmental Programme 1992), mycorrhizal fungi should be included in global biodiversity assessments and conservation projects (Hawksworth 2004).
Among mycorrhizal symbionts, arbuscular mycorrhizal fungi (AMF) (Glomeromycota)–which form associations with about 80% of plant species–are evolutionarily ancient organisms and are considered “living fossils” (Giovannetti 2001), having survived mass extinctions ever since their origin, more than 460 million years ago (Simon et al. 1993; Redecker et al. 2000). About 200 glomeromycotan species, belonging to 12 mycorrhizal genera (Acaulospora, Appendicispora, Archaeospora, Diversispora, Entrophospora, Gigaspora, Glomus, Intraspora, Kuklospora, Pacispora, Paraglomus, Scutellospora) in nine families have been described. An updated species list is available on the web: (http://www.lrz-muenchen.de/~schuessler/amphylo/amphylo_species.html). AMF are obligate symbionts and are routinely propagated on living host plants in ex situ collections in many laboratories all over the world. Several isolates have been registered in international banks such as INVAM (http://invam.caf.wvu.edu) and IBG (http://www.kent.ac.uk/bio/beg): more than 1,200 living accessions belonging to 100 species and 221 accessions belonging to 50 species are present in INVAM and IBG, respectively. The distribution and abundance of AMF have been altered and many species have declined due to habitat loss. In particular, many species belonging to the genus Scutellospora are highly sensitive to anthropogenic disturbance and to agricultural practices such as tillage, chemical fertilizer and pesticide treatments (Giovannetti and Gianinazzi-Pearson 1994; Helgason et al. 1998; Daniell et al. 2001; Jansa et al. 2002; Johnson 1993; Blaszkowski 1993). Scutellospora species, originating from temperate, tropical, sub-tropical and Mediterranean regions are currently propagated in ex situ collections, where the strong selective pressure applied could lead to the conservation of the only strains able to survive and reproduce in standardized culture conditions (Giovannetti and Gianninazzi-Pearson 1994). Accordingly, concerns about the loss in genetic diversity of these beneficial microorganisms suggest to adopt a long-term strategy for safeguarding their biodiversity, represented by in situ conservation (Hawksworth 1991; Giovannetti and Gianinazzi-Pearson 1994).
Several species of the genus Scutellospora occur in coastal sand dunes of the Migliarino-San Rossore-Massaciuccoli Natural Park near Pisa, Tuscany, within the UNESCO Biosphere Reserve denominated “Selva Pisana” (http://www.unesco.org/mab/wnbrs.shtml) (Giovannetti and Nicolson 1983; Giovannetti and Avio 1983; Giovannetti 1985; Blaszkowski et al. 2004). In this work we report the establishment of the first in situ collection of AMF in the world and the characterization of two autochthonous Scutellospora species by their morphological identification and molecular analyses of SSU and ITS sequences.
Materials and methods
Selection of the site
The site for the establishment of the in situ collection was selected on the basis of the following criteria: level of habitat protection, anthropogenic disturbance, stability of the dune system, and occurrence of Scutellospora species.
Field sampling and spore counting
Nine samples of rhizospheric sand (about 1,000 g) from representative plant species, Ammophila arenaria, Helichrysum stoechas and Otanthus maritimus, were collected in June 2004 inside a 40 × 40 m collection area. They were stored in polyethylene bags at 4°C until processed. AM fungal spores were extracted from soil samples by wet-sieving and decanting, down to a mesh size of 50 μm (Gerdemann and Nicolson 1963). Spores and sporocarps retained on sieves of pore size 400, 250, 100 and 50 μm were flushed into Petri dishes, examined under a dissecting microscope (Wild, Leica, Milano, Italy) at magnifications up to 50× with illumination by incident light from a fibre-optic quartz-halogen light source and the numbers and types of AM fungal spores were recorded. Only intact, healthy spores of Gigasporaceae species were counted. Total spore numbers and distribution data were assessed on 100 g soil for each sample.
Morphological identification of AM fungi
Spores were manually isolated by using capillary pipettes according to their morphology and colour, mounted on microscope slides and examined under a Polyvar light microscope equipped with Nomarski differential interference contact optics (Reichert-Young, Vienna, Austria). For taxonomical identification spores were mounted both in polyvinyl alcohol lacto-glycerol (PVLG) (Omar et al. 1979) and in PVLG + Melzer’s reagent (1:1, v:v) (Gerdemann and Trappe 1974; Schenck and Perez 1990). Qualitative sporal traits were observed on at least 50 spores for each morphotype, and quantitative parameters were measured by using Quantimet 500 image analysis software (Leica).
With the aim of detecting auxiliary cells, Scutellospora spores were germinated in the dark at 24°C between two 47-mm-diameter cellulose nitrate Millipore™ (Millipore, Milan, Italy) membranes (pore diameter 0.45 μm), placed on moist sand originated from the in situ collection, in 14-cm-diameter Petri dishes. After 2 weeks, membranes were opened, AM mycelium stained with Trypan blue in lactic acid (0.05%) and observed under the dissecting microscope to check for auxiliary cells production. Membrane areas containing auxiliary cells were mounted on microscope slides and examined under the Polyvar light microscope. Images were acquired with a Reichert 100× oil immersion lens with a 1.25 numerical aperture.
Molecular identification of Scutellospora species
DNA extraction from spores
Intact, healthy spores of different Scutellospora morphotypes were manually collected with a capillary pipette under the dissecting microscope. They were sonicated two times (1 min each) in a B-1210 cleaner (Branson Ultrasonics, Soest, The Netherlands) and washed three times in sterile distilled water (SDW). Single spores were selected once more under the dissecting microscope and transferred in Eppendorf tubes before DNA extraction, according to the protocol described by Redecker et al. (1997). Briefly, a single spore was crushed using a glass pestle in 5 μl of 0.25 M NaOH. Other 5 μl of 0.25 M NaOH were added before incubation in boiling water (1 min). Five μl of 0.5 M Tris–HCl (pH 8) and 10 μl of 0.25 M HCl were added and the microtubes were dipped again in boiling water (2 min). Each extract (3 μl) was directly used for PCR amplification. Three single spores were analysed for each morphotype.
PCR conditions of partial SSU sequences
DNA extracts from single spores of Scutellospora morphotypes were used to analyse partial SSU sequences. A seminested PCR protocol was adopted. In the first PCR reaction DNA extracts (3 μl) were amplified in 25 μl of PCR reaction mix using 0.625 U of AmpliTaq Gold DNA Polymerase (Applied Biosystem, Milan, Italy), 10 pmol of the primers NS31 (Simon et al. 1992) and AM1 (Helgason et al. 1998), 0.2 mM (each) dNTPs, 1.5 mM MgCl2 and the manufacturer’s reaction buffer and the thermal cycler (Eppendorf Mastercycler® personal, Eppendorf, Milan, Italy) was programmed as follows: 95°C for 9 min, 10 cycles at 95°C for 1 min, 58°C for 1 min, 72°C for 1 min, 24 cycles at 95°C for 30 s, 58°C for 1 min, 72°C for 2 min, 1 cycle at 95°C for 30 s, 58°C for 1 min, 72°C for 10 min.
The seminested PCR reactions were performed diluting (1:100) the first PCR amplicons and using 2 μl of dilutions as template for the second reaction in a final volume of 50 μl. Twenty pmol of each primer pair, the newly designed SSU-1f (5′-AGC AGC CGC GGT AAT TCC A-3′) and AM1, were added in the PCR mix. Taq DNA polymerase, dNTPs and MgCl2 concentrations were as described above. Amplification conditions were as follows: 95°C for 5 min, 32 cycles at 95°C for 30 s, 58°C for 1 min, 72°C for 1 min, 1 cycle at 72°C for 10 min.
PCR conditions of internal trascribed spacer regions (ITS1-5.8S-ITS2)
The same DNA extracts utilised for partial SSU sequences analyses were used for analysing the internal trascribed spacer regions included between the primers ITS1F (5′-CTT GGT CAT TTA GAG GAA GTA A) and ITS4 (5′-TCC TCC GCT TAT TGA TAT GC-3′) (Gardes and Bruns 1993). ITS1F/ITS4 regions were amplified in 25 μl of PCR reaction mix. Final concentration of PCR mix components were 0.2 mM (each) dNTPs, 1.5 mM MgCl2, 10 pmol of each primer, 0.625 U of AmpliTaq Gold DNA Polymerase (Applied Biosystem) and the manufacturer’s reaction buffer. Reactions were carried out in a thermal cycler, which was programmed as follows: 95°C for 5 min, 4 cycles at 95°C for 30 s, 54°C for 30 s, 72°C for 1 min, 34 cycles at 95°C for 30 s, 53°C for 30 s, 72°C for 1 min, and a final extension step at 72°C for 10 min. Amplification products were diluted 1:100 and 2 μl were re-amplified in 50 μl of seminested-PCR reaction mix by using the primers ITS1 (5’-TCC GCA GGT TCA CCT ACG GA-3’) and ITS4. PCR mix components were 0.2 mM dNTPs, 2.5 mM MgCl2, 10 pmol of each primer, 0.625 U of AmpliTaq Gold DNA Polymerase and the manufacturer’s reaction buffer. Reactions were carried out following a touch down protocol to reduce the formation of spurious by-products during the amplification process. PCR reaction mix was preheated at 95°C for 5 min, then the annealing temperature was set 14°C above the expected annealing temperature (55°C) and lowered with 2°C every third cycle until 55°C, at which temperature 14 additional cycles were carried out. Cycles were performed as follows: 95°C for 30 s, annealing temperature 30 s, 72°C for 1 min. The end of the last cycle was followed by an extension step at 72°C for 10 min.
Cloning and sequencing
Amplified DNA fragments of both SSU and ITS1-5.8S-ITS2 regions were purified by using Montage™ PCR Centrifugal Filter Devices (Millipore) with a final elution volume of 20 μl. Purified products were cloned using Qiagen PCR Cloning Kit according to the manufacture’s instructions (Qiagen, Milan, Italy). Putative positive clones containing recombinant plasmid were purified by the GenElute Plasmid Miniprep Kit (Sigma, Milan Italy) and screened using standard SP6/T7 amplifications, followed by a nested PCR using SSU-1f/AM1 or ITS1/ITS4, as described above. SSU-1f/AM1 and ITS1/ITS4 amplification products were sequenced forward and reverse at BMR Genomics s.r.l. (University of Padua, Italy).
Phylogenetic analyses
Sequences obtained from the two morphotypes were aligned, using the ClustalW program (Chenna et al. 2003), with sequences of Scutellospora available in GenBank. After alignement, a dendrogram was generated by TREECON for Windows software with the Neighbour-Joining method by using the Kimura distance model, where the value ratio of transition/transversion was set to 2 (van de Peer and de Wachter 1994). The confidence of branching was assessed using 1000 bootstrap resamplings.
Results
The in situ collection
The first step of the work was represented by the identification of the site for the establishment of the in situ collection, whose boundaries were delimited by a fence surrounding an area of 1,600 m2 , located at 43°46′29′′N, 10°16′11′′E (Fig. 1). The soil pH (H2O) was 8.6 and available P (Olsen) was 5.0 ppm.
Maps showing the location of the UNESCO Biosphere Reserve “Selva Pisana” in Tuscany (Italy) and of the in situ collection area (square) within the Migliarino-San Rossore-Massaciuccoli Natural Park, close to the river Serchio. Source of the air photo on the left: Progetto TERRAFlyer http://www.geografia.toscana.it/terraflyer.htm
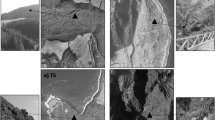
The selected site is characterised by a very high level of habitat protection, since it is located in an integrally protected area within the Migliarino-San Rossore-Massaciuccoli Natural Park, inside the UNESCO Biosphere Reserve “Selva Pisana”, in the north-western coast of Tuscany, near Pisa (Italy). The site is an old fixed dune system, with many small dunes not exceeding 2 m in height, extending for about 200 m inland, where a pine forest rises. It has a stable and well characterized herbaceous plant association–Echinophoro-Ammophiletum arenariae (Br. Bl. Gehu)–(Arrigoni 1990), mainly represented by A. arenaria, H. stoechas and O. maritimus (Fig. 2). In this area the occurrence of Scutellospora species was previously reported (Giovannetti and Nicolson 1983; Giovannetti and Avio 1983; Giovannetti 1985).
(a–c) Pictures of the area which hosts the in situ collection of AM fungi, within the UNESCO Biosphere Reserve “Selva Pisana” in the Migliarino-San Rossore-Massaciuccoli Natural Park. Plant community of the dune system is mainly composed of Ammophila arenaria (d), Helichrysum stoechas (e) and Otanthus maritimus (f) plants
Occurrence of Scutellospora species in rhizosphere samples
Scutellospora species were a regular component of A. arenaria and H. stoechas rhizospheres, occurring in all the samples distributed in the whole area of the in situ collection (Table 1). Though, spore numbers varied greatly from one sample to another. Most spores were recovered from A. arenaria (45.0 ± 11.5 spores/100 g soil) (mean ± standard error of the mean) and H. stoechas (40.9 ± 3.8 /100 g) rhizosphere, while O. maritimus showed a very low number of spores (7.3 ± 1.1/100 g).
Two Scutellospora species were retrieved, which were morphologically identified as Scutellospora persica, and Scutellospora fulgida, according to their original descriptions (Koske and Walker 1985; 1986) (Figs. 3–4). S. fulgida was recovered from 89% and 67% of A. arenaria and H. stoechas rhizosphere samples, respectively. S. persica was recovered from 100% of A. arenaria and H. stoechas, and from 67% of O. maritimus rhizosphere samples. Spore numbers showed a high variability among samples, S. fulgida ranging from 0 to 68/100 g and from 0 to 62/100 g in A. arenaria and H. stoechas samples, respectively, and S. persica ranging from 9 to 61/100 g and from 3 to 52/100 g, in A. arenaria and H. stoechas samples, respectively (Table 1). A few spores were retrieved from O. maritimus rhizosphere, mostly belonging to S. persica.
Light micrographs of Scutellospora fulgida. (a) Whole spore showing the bulbous sporogenous cell. Scale bar: 44 μm. (b) Morphological features of the spore wall. Scale bar: 5.5 μm. (c–d) Details of the boulbous sporogenous cell. Scale bars: 40 μm (c) and 9 μm (d). (e) Whole spore showing the germination shield. Scale bar: 44 μm. (f) Knobby auxiliary cells produced on coiled hyphae by germinating spores. Scale bar: 5 μm
Light micrographs of Scutellospora persica. (a) Spore showing the bulbous sporogenous cell. Scale bar: 34 μm. (b) Morphological features of the spore wall. Scale bar: 22 μm. (c) Germination shield. Scale bar: 44 μm. (d) Knobby auxiliary cells produced on straight hyphae by germinating spores. Scale bar: 5 μm
Molecular characterization of Scutellospora species by using partial SSU and ITS sequences
SSU sequence analysis
Seminested PCR produced fragments of ∼530 bp, from spores of both Scutellospora morphotypes. The following sequences were deposited in EMBL database (accession number AM503931, and AM503932 from S. fulgida spores morphotype; AM503933 and AM503934 from S. persica spores morphotype). BLASTn (02–2007) procedure confirmed the homology with Scutellospora sequences.
The only sequence of S. fulgida deposited in GenBank (AJ306435), obtained from spores collected directly from a field soil in Argentina (Schüβler et al. 2001), had an identity of more than 99% with our sequences. S. persica sequences from GenBank (AY882583, AY882584), obtained from spores directly collected from sand dunes, Portugal (Rodriguez-Echeverrìa and Freitas 2006) showed an identity of more than 99% with our sequences.
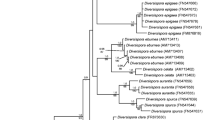
S. persica sequence AM503933 was obtained from six different clones from three spores and differed only by one nucleotide compared with the other S. persica morphotype sequence. The two S. fulgida sequences differed by one nucleotide and were similar to those of S. persica, differing by only one nucleotide. After alignement, the neighbour-joining phylogenetic analysis of the partial SSU region produced a tree, where our sequences fell in a branch comprising S. persica, S. fulgida, S. castanea, S. gregaria and S. weresubiae sequences (Fig. 5).
Neighbour-joining phylogenetic tree of Gigasporaceae, focusing on Scutellospora genus, obtained from the alignment of partial SSU rDNA sequences. Bootstrap values (out of 1000) are shown when they exceed 50%. Scutellospora sequences were clustered in four clades denominated A, B, C1 and C2 (after de Souza et al. 2005). Diversispora spurca and Acaulospora laevis were used as outgroups. Sequences obtained in this work are in bold and accession numbers are indicated
ITS sequence analysis
Seminested PCR produced fragments of ∼510 bp from spores of S. fulgida morphotype and of ∼490 bp from S. persica morphotype. The following sequences were deposited in EMBL database (accession numbers AM503935, AM503936, AM503937 and AM503938 from S. fulgida spores morphotype; AM503939, AM503940 and AM503941 from S. persica spores morphotype). The Scutellospora origin of the sequences was confirmed by the BLASTn procedure. Sequences from S. persica morphotype showed more than 98% identities with S. persica sequences present in GenBank (AJ410739, AJ410740). No S. fulgida sequences were available in public databases and our sequences are the first ones to be analysed, showing a high level of sequence identity with S. castanea.
The ITS neighbour-joining analysis of Scutellospora ITS1-5.8S-ITS2 sequences clearly distinguished between the two Scutellospora morphotypes of the in situ collection. S. persica sequences formed a separate cluster, supported by a high bootstrap value, including database sequences of S. persica spores (E28) originated from eastern Italian sand dunes (Fig. 6).
Neighbour-joining phylogenetic tree of Gigasporaceae, focusing on Scutellospora genus, obtained from the alignment of ITS sequences. Bootstrap values (out of 1000) are shown when they exceed 50%. Scutellospora sequences were clustered in three clades denominated B, C1 and C2 (after de Souza et al. 2005). Acaulospora denticulata was used as outgroup. Sequences obtained in this work are in bold and accession number are indicated
Discussion
This work represents the first report, to our knowledge, of the establishment of an in situ collection of AMF in the world. It is located in the coastal sand dunes of the Migliarino-San Rossore-Massaciuccoli Natural Park near Pisa, Tuscany, within the UNESCO Biosphere Reserve denominated “Selva Pisana”. In this collection the genus Scutellospora, reported to be threatened by anthropogenic disturbance (Giovannetti and Gianinazzi-Pearson 1994; Helgason et al. 1998; Daniell et al., 2001; Jansa et al., 2002; Johnson 1993; Blaszkowski 1993), is represented by two species, S. fulgida and S. persica, which were morphologically identified and molecularly characterised by SSU and ITS sequence analyses. The low diversity of Scutellospora species retrieved by spore collection is in agreement with previous works (Giovannetti and Avio 1983; Giovannetti and Nicolson 1983; Blaszkowski et al. 2004). Since this study is a first step aimed at retrieving Scutellospora spores of the in situ collection, a greater number of AMF species could be found by surveying plant roots using PCR techniques.
Scutellospora species are currently propagated in ex situ collections in many laboratories all over the world. Such collections are essential tools for the proper description of taxa, representing a fundamental step to build a picture of the abundance and distribution of Scutellospora species worldwide, and could be utilised to obtain the baseline data for ecological management and conservation strategies. Ex situ germplasm collections maintain the different isolates, originated from the most diverse environments, in growth conditions and soil-free substrates or soil mixtures, which may differ from those they were isolated from. These procedures represent a strong selective pressure, which may preserve only species and isolates able to tolerate environmental stresses caused by culture conditions (Giovannetti and Gianninazzi-Pearson 1994). Moreover many isolates are presently obtained from single spores, for preserving the genetic integrity of individuals. Such technique may further contribute to the decrease of the overall genomic diversity of isolates, since spores show large intrasporal molecular diversity (Hijri et al. 1999; Hosny et al. 1999; Khun et al. 2001; Jansa et al. 2002; Koch et al. 2004; Rosendahl and Stukenbrock 2004; Pawloska and Taylor 2004).
In situ conservation of AMF in natural reserves, characterized by the absence of anthropogenic disturbance, may represent the most suitable and long-term strategy for safeguarding their biodiversity (Hawksworth 1991; Giovannetti and Gianinazzi-Pearson 1994). Actually, many authors reported that AMF biodiversity is reduced in ecosystems with high anthropogenic disturbance such as arable lands, where ploughing, fertilizers and fungicides are applied. In these soils non-Glomus fungi, such as Scutellospora spp., Gigaspora spp., Acaulospora spp. and Entrophospora spp. were lost, compared to uncultivated soils (Johnson 1993; Blaszkowski 1993; Helgason et al. 1998; Jansa et al. 2002). Moreover, Oehl and co-authors (2005) observed that in intensively managed maize fields Scutellospora species (S. calospora and S. castanea) were more abundant in deeper soils, when compared with less intensively managed vineyards and extensive grasslands, confirming the responsiveness of these AMF species to anthropogenic disturbance.
The two Scutellospora species occurring in the in situ collection, S. fulgida and S. persica, have been rarely recovered, especially when compared to other globally distributed AMF species, such as Glomus mosseae, which has been isolated from at least 474 sites (Avio, personal communication). Accordingly, the number of isolates maintained in ex situ collections is very low: for instance in the INVAM collection S. fulgida and S. persica isolates have less than five accessions deposited, compared to more than 20 isolates for S. calospora and S. heterogama (http://invam.caf.wvu.edu/collection/generalinfo/isol_diversity.htm).
S. fulgida was described for the first time by Koske and Walker (1986) from maritime sand dunes of the east coast of the USA. There are only a few records of S. fulgida in the world: from Argentina (Schüβler et al. 2001), India and Thailand (Selvam and Mahadevan 2002; Bhadalung et al. 2005). Interestingly, S. fulgida was found in sand dunes of Calambrone (43°35′N, 10°18′E) (Blaszkowski et al. 2004), an area close to the UNESCO Biosphere Reserve “Selva Pisana”, where the in situ collection is located.
S. persica was first described by Koske and Walker (1985) from sand dunes of New Jersey, USA, and by now it has been recorded only in a few reports, mainly from sand dune systems. It occurs in Maryland, Massachusetts, Rhode Island and Virginia, USA (Bergen and Koske 1984; Koske 1987; Gemma and Koske 1989; Gemma et al. 1989; Friese and Koske 1991), in Poland (Blaszkowski and Tadych 1997), Portugal (Rodriguez-Echeverrìa and Freitas 2006), Italy (Puppi and Riess 1987; Lanfranco et al. 2001; Blaszkowski et al. 2004) and India (Selvam and Mahadevan 2002).
Our identification of Scutellospora species living in the in situ collection was based on a multidisciplinary approach, by combining morphological and molecular analyses of spores isolated from sand dunes. Actually, the discrimination of different species of Scutellospora may be difficult when based on field collected spores, where fine details of wall structures could be damaged. Moreover, such spores could show a poor germination, which is an important step for the detection of auxiliary cells. Accordingly, we utilised also a molecular approach to support morphological identification.
We analysed the portion of SSU amplified by NS31/AM1 primers (Helgason et al. 1998), usually utilised for differentiating AM fungal taxa colonizing plant roots and belonging to Glomeraceae, Acaulosporaceae and Gigasporaceae. These primers have successfully been used in many works to study the structure of AM fungal communities growing in natural and agricultural ecosystems or in polluted soils (Opik et al. 2006).
In a recent work, de Souza and co-authors (2005) showed that the nearly full-length SSU rDNA phylogenetic analysis of Gigasporaceae sequences grouped these species in four distinct clades, which were confirmed by the partial SSU phylogenetic analysis carried out in this work, even if supported by weaker boostrap values. The use of this primer set did not clearly separate discrete sequence groups inside the group C. S. fulgida and S. persica sequences were very similar and clustered together with some sequences of the same species but also with S. castanea, S. gregaria, S. weresubiae. This result may be explained by the fact that Gigasporaceae are not well discriminated by the V3–V4 region of the 18S rRNA, which is not sufficiently variable in this taxon (de Souza et al. 2004). For this reason we also analysed the ITS1-5.8S-ITS2 portion, which is highly variable even if a poor database of Scutellospora species is available. Sequences of S. persica spores retrieved in the in situ collection clustered with other S. persica sequences (Lanfranco et al. 2001) and a clearcut separation (76% bootstrap value) with S. fulgida sequences was obtained.
In conclusion, our findings highlight the importance of the establishment of in situ collections for the conservation of rare and possibly endangered AM fungi in protected areas, where anthropogenic impact is under control of national and international authorities. The in situ collection may represent a genetic reserve where the evolutionary history of these obligate symbionts can be preserved, along with the ecosystem where they live.
References
Arrigoni PV (1990) Flora e vegetazione della macchia Lucchese di Viareggio (Toscana). Webbia 44:1–62
Bergen M, Koske RE (1984) Vesicular arbuscular mycorrhizal fungi from sand dunes of Cape Cod, Massachusetts. Trans Br Mycol Soc 83:157–158
Bhadalung NN, Suwanarit A, Dell B, Nopamornbodi O, Thamchaipenet A, Rungchuang J (2005) Effects of long-term NP fertilization on abundance and diversity of arbuscular mycorrhizal fungi under a maize cropping system. Plant Soil 270:371–382
Blaszkowski J (1993) Comparative studies of the occurence of arbuscular fungi and mycorrhizae (Glomales) in cultivated and uncultivated soils of Poland. Acta Mycol 28:93–140
Blaszkowski J, Tadych M (1997) Scutellospora persica (Glomales, Zygomycetes), an arbuscular mycorrhizal fungus new to the mycota of Poland. Mycotaxon 65:379–390
Blaszkowski J, Blanke V, Renker C, Buscot F (2004) Glomus aurantium and G. xanthium, new species in Glomeromycota. Mycotaxon 90:447–467
Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, Higgins DG, Thompson JD (2003) Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res 31:3497–3500
Daniell TJ, Husband R, Fitter AH, Young JPW (2001) Molecular diversity of arbuscular mycorrhizal fungi colonising arable crops. FEMS Microbiol Ecol 36:203–209
de Souza FA, Kowalchuk GA, Leeflang P, van Veen JA, Smit E (2004) PCR-denaturing gradient gel electrophoresis profiling of inter- and intraspecies 18S rRNA gene sequence heterogeneity is an accurate and sensitive method to assess species diversity of arbuscular mycorrhizal fungi of the genus Gigaspora. Appl Environ Microbiol 70:1413–1424
de Souza FA, Declerck S, Smit E, Kowalchuk GA (2005) Morphological, ontogenetic and molecular characterization of Scutellospora reticulata (Glomeromycota). Mycol Res 109:697–706
Friese CF, Koske RE (1991) The spatial dispersion of spores of vesicular-arbuscular mycorrhizal fungi in a sand dune: microscale patterns associated with the root architecture of American beachgrass. Mycol Res 95:952–957
Fuerst JA, Hugenholtz P (2000) Microorganisms should be high on DNA preservation list. Science 290:1503
Gardes M, Bruns TD (1993) ITS primers with henanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–122
Gemma JN, Koske RE (1989) Field inoculation of American beachgrass (Ammophila breviligulata). J Environ Manage 29:173–182
Gemma JN, Koske RE, Carreiro M (1989) Seasonal dynamics of selected species of VA mycorrhizal fungi in a sand dune. Mycol Res 92:317–321
Gerdemann JW, Nicolson TH (1963) Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans Br Mycol Soc 46:235–244
Gerdemann JW, Trappe JM (1974) The Endogonaceae in the Pacific Northwest. Mycol Mem 5:1–76
Giovannetti M (1985) Seasonal variations of vesicular-arbuscular mycorrhizas and Endogonaceous spores in a maritime sand dune. Trans Br Mycol Soc 84:679–684
Giovannetti M (2001) Survival strategies in arbuscular mycorrhizal symbionts. In: Seckbach J (ed) Cellular origin and life in extreme habitats. Kluwer Academic Publishers, Dordrecht, NL, pp 293–307
Giovannetti M, Avio L (1983) Endogonaceae spores in marine sand dunes in Italy. Ann Microbiol 33:129–135
Giovannetti M, Avio L (2002) Biotechnology of arbuscular mycorrhizas. In: Khachatourians GG, Arora DK (eds) Applied mycology and biotechnology. Agriculture and Food Production, vol. 2. Elsevier, Amsterdam, NL, pp. 275–310
Giovannetti M, Nicolson TH (1983) Vesicular-arbuscular mycorrhizas in Italian sand dunes. Trans Br Mycol Soc 80:552–557
Giovannetti M, Gianinazzi-Pearson V (1994) Biodiversity in arbuscular mycorrhizal fungi. Mycol Res 98:705–715
Hawksworth DL (1991) The fungal dimension of biodiversity: mignitude, significance and conservation. Mycol Res 95:641–655
Hawksworth DL (2004) Fungal diversity and its implications for genetic resource collections. Stud Mycol 50:9–18
Helgason T, Daniell TJ, Husband R, Fitter AH, Young JPW (1998) Ploughing up the wood-wide web? Nature 394:431
Hijri M, Hosny M, Van Tuinen D, Dulieu H (1999) Intraspecific ITS polymorphism in Scutellospora castanea (Glomales, Zygomycota) is structured within multinucleate spores. Fungal Genet Biol 26:141–151
Hosny M, Hijri M, Passerieux E, Dulieu H (1999) rDNA units are highly polymorphic in Scutellospora castanea (Glomales, Zygomycetes). Gene 226:61–71
Jansa J, Mozafar A, Anken T, Ruh R, Sanders IR, Frossard E (2002) Diversity and structure of AMF communities as affected by tillage in a temperate soil. Mycorrhiza 12:225–234
Johnson NC (1993) Can fertilization of soil select less mutualistic mycorrhizae? Ecol Appl 3:749–757
Koch AM, Kuhn G, Fontanillas P, Fumagalli L, Goudet J, Sanders IR (2004) High genetic variability and low local diversity in a population of arbuscular mycorrhizal fungi. Proc Acad Sci USA 101:2369–2374
Koske RE (1987) Distribution of VA mycorrhizal fungi along a latitudinal temperature gradient. Mycologia 79:55–68
Koske RE, Walker C (1985) Species of Gigaspora (Endogonaceae) with roughened outer walls. Mycologia 77:702–720
Koske RE, Walker C (1986) Species of Scutellospora (Endogonaceae) with smooth- walled spores from maritime sand dunes: two new species and redescription of the spores of Scutellospora pellucida and Scutellospora calospora. Mycotaxon 27:219–235
Kuhn G, Hijri M, Sanders IR (2001) Evidence for the evolution of multiple genomes in arbuscular mycorrhizal fungi. Nature 414:745–748
Lanfranco L, Bianciotto V, Lumini E, Souza M, Morton JB, Bonfante P (2001) A combined morphological and molecular approach to characterize isolates of arbuscular mycorrhizal fungi in Gigaspora (Glomales). New Phytol 152:169–179
Myers N (1988) Threatened biotas: ‘hotspots’ in tropical forests. Environmentalists 8:187–208
Myers N (1993) Questions on mass extinction. Biodivers Conserv 2:2–17
Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858
Oehl F, Sieverding E, Ineichen K, Ris EA, Boller T, Wiemken A (2005) Community structure of arbuscular mycorrhizal fungi at different soil depths in extensively and intensively managed agroecosystems. New Phytol 165:273–283
Omar MB, Bolland L, Heather WA (1979) A permanent mounting medium for fungi. Bull Br Mycol Soc 13:31–32
Opik M, Moora M, Liira J, Zobel M (2006) Composition of root-colonizing arbuscular mycorrhizal fungal communities ecosystems around the globe. J Ecol 94:778–790
Pimm SL, Raven P (2000) Extinction by numbers. Nature 403:843–845
Pawlowska TE, Taylor JW (2004) Organization of genetic variation in individuals of arbuscular mycorrhizal fungi. Nature 427:733–737
Puppi G, Riess S (1987) Role and ecology of VA mycorrhizae in sand dunes. Angew Botanik 61:115–126
Redecker D, Thierfelder H, Walker C, Werner D (1997) Restriction analysis of PCR-amplified internal transcribed spacers of ribosomal DNA as a tool for species identification in different genera of the order Glomales. Appl Environ Microbiol 63:1756–1761
Redecker D, Kodner R, Graham LE (2000) Glomalean fungi from the Ordovician. Science 289:1920–1921
Rodriguez-Echeverrìa S, Freitas H (2006) Diversity of AMF associated with Ammophila arenaria ssp. arundinacea in Portuguese sand dunes. Mycorrhiza 16:543–552
Rosendahl S, Stukenbrock EH (2004) Community structure of arbuscular mycorrhizal fungi in undisturbed vegetation revealed by analyses of LSU rDNA sequences. Mol Ecol 13:3179–86
Schenck NC, Perez Y (1990) Manual for the identification of VA mycorrhizal fungi, 3rd edn. Synergistic publications, INVAM, Gainesville
Schüßler A, Schwarzott D, Walker C (2001) A new fungal phylum, The Glomeromycota: phylogeny and evolution. Mycol Res 105:1414–1421
Selvam A, Mahadevan A (2002) Distribution of mycorrhizas in an abandoned fly ash pond and mined sites of Neyveli Lignite Corporation, Tamil Nadu, India. Basic Appl Ecol 3:277–284
Simon L, Lalonde M, Bruns TD (1992) Specific amplification of 18S fungal ribosomal genes from vesicular-arbuscular endomycorrhizal fungi colonizing roots. Appl Environ Microbiol 58:291–295
Simon L, Bousquet J, Levesque RC, Lalonde M (1993) Origin and diversification of endomycorrhizal fungi and coincidence with vascular land plants. Nature 363:67–69
Smith SE, Read DJ (1997) Mycorrhizal symbiosis, 2nd edn. Academic Press, London
Staley JT (1997) Biodiversity: are microbial species threatened? Curr Op Biotech 8:340–345
United Nations Environmental Programme (1992) Convention on biological diversity, Nairobi, Kenya
van der Heijden MGA, Klironomos JN, Ursic M, Moutoglis P, Streitwolf-Engel R, Boller T, Wiemken A, Sanders IR (1998) Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 396:69–72
van de Peer Y, de Wachter R (1994) TREECON for Windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Comput Appl Biosci 10:569–570
Acknowledgements
This work was supported by the Migliarino-San Rossore-Massaciuccoli Natural Park and by a University of Pisa grant (Fondi di Ateneo).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Turrini, A., Avio, L., Bedini, S. et al. In situ collection of endangered arbuscular mychorrhizal fungi in a Mediterranean UNESCO Biosphere Reserve. Biodivers Conserv 17, 643–657 (2008). https://doi.org/10.1007/s10531-007-9288-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-007-9288-x