Abstract
Ecological communities may be resistant to invasive species through a combination of top-down and bottom-up mechanisms, including predation, competition, parasitism, and disease. In particular, natural enemies that cross over from native species to use newly introduced non-native species as hosts can influence invasive species population dynamics and may slow down invasions. However, research on parasitism in biological invasions is lagging behind research on biological invasions in general. We used the model species winter moth (Operophtera brumata) to study the effect of recruitment of native parasitoids on an invasive population of winter moth in the northeastern United States. We deployed sentinel pupae over 4 years across this population’s range, identified recovered parasitoids, and measured the rate of parasitism by native sources across years, seasons, invasion history, and host densities. Native Pimpla wasps inflicted 98% of the parasitism detected, resulting in an annual average of 15–40% mortality on pupae not depredated. Pimpla were present across all years, seasons, and sites. Where winter moth has invaded, parasitism was greatest when winter moth pupal density was high (i.e., positive density-dependent mortality) suggesting that Pimpla is helping to regulate the population. The wasps were morphologically identified as Pimpla aequalis Provancher; however, using a multilocus genetic comparison approach, they were determined to comprise two cryptic species. Overall, this study shows that recruitment of these native wasps to the invasive winter moth population is likely playing a significant role in regulating population outbreaks and is aiding in biological control of winter moth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The introduction of non-native species to new ecological communities is creating novel and altered predator–prey and parasite–host interactions (Faillace et al. 2017; Garnas et al. 2016; Hobbs et al. 2009; Pearson et al. 2018; Shea and Chesson 2002; Strauss et al. 2012). The species richness of a community can predict the chance that an invasive species will successfully establish. This hypothesis, known as the biotic resistance hypothesis, holds that communities may resist invasions through a combination of factors including predation, competition, parasitism, and disease (Elton 1958; Jeschke et al. 2012; Levine et al. 2004; Maron and Vilà 2001; Sakai et al. 2001; Shea and Chesson 2002). Natural enemies of native or resident species that cross over to use non-native species can influence invasions in complex ways (Dearborn et al. 2016; Faillace et al. 2017; Grabenweger et al. 2010; Strauss et al. 2012) and have the potential to slow down invasions and aid in biological control (Dearborn et al. 2016; Kenis et al. 2008; Maron et al. 2001; Vindstad et al. 2013). These interactions are particularly strong for non-native species with sympatric native congeners and confamilials (Dearborn et al. 2016; Grabenweger et al. 2010; Strauss et al. 2012; Vindstad et al. 2013). Further, biotic resistance may especially affect invasive insects because they are typically r-selected (Sakai et al. 2001) and their population dynamics are closely related to natural enemies, predominantly parasitoids (Hassell 2000; Myers 2018; Waage and Greathead 1985). While it is clear that naturally-occurring biological controls play an important role in pest suppression (Heimpel and Mills 2017), research on the role of parasitism in biological invasions is lagging behind research on biological invasions in general (Poulin 2017) and particularly so for studies of invasive herbivores and their natural enemies (Bürgi et al. 2015).
The European winter moth, Operophtera brumata L. (Lepidoptera: Geometridae), is a well-known defoliator of forest, shade, and fruit trees with multiple invasive populations established in North America (Embree 1965; Myers 2018; Myers and Cory 2013; Roland and Embree 1995), including a recent (circa late 1990s) introduction in the northeastern United States (Elkinton et al. 2010, 2015). Following successful biological control of winter moth in Nova Scotia and British Columbia using the tachinid fly Cyzenis albicans (Fallén) (Diptera: Tachinidae) (Embree 1966; Murdoch et al. 1985; Roland and Embree 1995), similar efforts have been initiated in this most recent invasion (Elkinton et al. 2015). While importation biological control of winter moth in the northeastern United States has shown promising results (Elkinton et al. 2015, 2018), as previously found in Canada (Roland 1988, 1990), the overall success will likely depend on additional mortality from native natural enemies. Parasitoid recruitment from related native species can have significant effects on invasive populations in other insect study systems (e.g. Duan et al. 2013, 2014; Grabenweger et al. 2010; Matosevic and Melika 2013; Schonrogge et al. 1995; Zappala et al. 2012). This is especially true if the parasitoids limit the establishment or facilitate the eradication of a new exotic species (Tobin et al. 2011), if they respond to increases in the invasive population’s densities with density-dependent mortality (Holling 1973), or if the parasitoids parasitize at high rates at the invasion front such that they can slow or stop the spread of the invasive population (Lewis and Kareiva 1993).
Non-native species with native congeners in the introduced range may be less likely to establish an invasive population than species introduced to a range without a native congener; these invasive species face top-down pressure from the natural enemies of their congener (Callaway et al. 2013; Carrillo-Gavilan et al. 2012; Diez et al. 2008; Keane and Crawley 2002; Richardson and Pysek 2006). The native congener of winter moth, Bruce spanworm (Operophtera bruceata Hulst), is a potential source of native parasitoid recruitment to invasive populations of winter moth in North America. Bruce spanworm is present in all regions winter moth has invaded. In addition to having similar life-cycle dynamics, these two congeners use similar hosts, are present at similar times of the year, and can hybridize in the field (Gwiazdowski et al. 2013; Havill et al. 2017). Thus, it is likely that native natural enemies that parasitize Bruce spanworm could use winter moth as a host. Additionally, the life histories of winter moth and Bruce spanworm make their populations particularly vulnerable to pupal mortality by predation and parasitism from native natural enemies. Both winter moth and Bruce spanworm have a long pupation period (6–7 months during the summer, representing the vast majority of its life span) and pupates in the top layer of soil or leaf litter. Together this renders both species particularly vulnerable to pupal mortality by predation, parasitism, and disease.
Parasitoid wasps in the genus Pimpla Fabricius (Hymenoptera: Ichneumonidae) might be an important source of mortality for invasive populations of winter moth in North America. Species of Pimpla are found in all zoogeographic regions, including 27 species in the Nearctic Region (Yu et al. 2012), and typically parasitize Lepidoptera prepupae and pupae (Bennett 2008; Carlson 1979; Gauld 1991; Goulet and Huber 1993). Pimpla species are known to use geometrid pupae concealed in moss or soil (Fitton et al. 1988), suggesting that they may be important natural enemies of Bruce spanworm and winter moth, which fit both of these criteria. Pimpla species are known from the region of this study and have been associated with winter moth populations. Pimpla turionellae L., Pimpla contemplator Müller, and Pimpla flavicoxis Thompson have been recorded as attacking winter moth in its native range in Europe (Fitton et al. 1988; Silvestri 1941; Wylie 1960). P. flavicoxis is currently considered a valid species (Yu et al. 2012) but has also been treated as a junior synonym of Pimpla aquilonia Cresson (Carlson 1979). P. aquilonia is a broadly distributed Holarctic species (covering the region of this study) reported from multiple families of Lepidoptera, including geometrids, but not yet from winter moth (Yu et al. 2012). A small release of P. turionellae to Nova Scotia, Canada in 1955 was conducted to control winter moth; however, recovery collections revealed no evidence of establishment (Graham 1958; Humble 1985). Similarly, P. contemplator was released in Nova Scotia, Canada in 1964 to control winter moth but with no evidence of establishment (Carlson 1979). Pimpla hesperus Townes is native to North America and has been reported as a parasitoid of winter moth and Bruce spanworm in British Columbia, Canada (Humble 1985). Recently, an undetermined species of Pimpla in the United States in Maine was found to be more abundant at sites with high winter moth infestation than moderately infested sites (Morin 2015), and another, or potentially the same, undetermined species of Pimpla has been reported in the northeastern United States as a hyperparasitoid of C. albicans, an introduced biological control agent of winter moth (Broadley et al. 2018). These latter two studies revealed an association of Pimpla species with the invasive population of winter moth in the northeastern U.S., but neither study directly assessed Pimpla wasps as primary parasitoids of winter moth. The assemblage and origin of Pimpla species associated with invasive winter moth populations in the northeastern U.S. is unknown, as is their prevalence, role in causing winter moth mortality, and potential to regulate winter moth densities.
In this study we aimed to (1) quantify parasitism by Pimpla wasps on winter moth pupae and C. albicans puparia across a spatial and temporal gradient, (2) test for a density dependent effect of pupal parasitism, and (3) identify the Pimpla species using morphological and molecular characteristics. We discuss our results in relation to their implications for understanding the role and origin of this parasitoid in the control of an introduced lepidopteran pest. These findings demonstrate the importance of evaluating native parasitoids in the establishment, spread, and population dynamics of other invasive species.
Methods
Pupal deployment
To acquire winter moth for deployment as sentinel pupae, we collected winter moth larvae from long-term study sites across eastern Massachusetts each spring from 2014 to 2017 (Elkinton et al. 2015). Larvae were reared in batches of 500 or fewer individuals in ventilated 20 L (5 gallon) buckets with the foliage from the tree species on which they were found. Mortality from viruses, other diseases, and larval parasitism in these collections was minimal (Broadley et al. 2017; Donahue et al. 2018). When the larvae showed signs of pupating (thickening body shape), sifted peat moss was added to the bottom of the buckets for pupation. All winter moth pupae were non-destructively checked under a dissecting microscope (Wild Heerbrugg M5 stereo) for parasitism by C. albicans or other larval parasitoids. The unparasitized winter moth pupae and pupae parasitized by C. albicans were stored at 12 °C in a growth chamber (Percival Scientific) until deployment.
To study pupal parasitism by native parasitoids, winter moth pupae were deployed at sites across eastern Massachusetts, Rhode Island, and Connecticut from 2014 to 2017 (Table 1). The study sites were chosen to coincide with winter moth long-term study sites and to reflect a range of winter moth and C. albicans establishment histories (Elkinton et al. 2014, 2015). The study sites were all in mixed hardwood forests dominated by red oak (Quercus rubra). The pupae were deployed in three to five consecutive rounds from mid-June until the end of October with five deployments (one every 3 weeks) in 2014 and three deployments (one every 6 weeks) in 2015–2017. Each deployment consisted of 100 winter moth pupae attached to small burlap squares with beeswax; these were buried 2 cm below the soil surface haphazardly under the drip line of a red oak tree as had been done for previous studies (Broadley et al. 2018; Whited 2007). The placement depth was chosen to mimic natural pupa depths (East 1974; Embree 1965; Holliday 1977). All pupae in each deployment were the same age; all had pupated at the end of May from larvae collected mid-May. In total 12,420 winter moth pupae were deployed.
To evaluate the effect of native parasitoids on C. albicans puparia (winter moth pupae that were parasitized by C. albicans), in 2014–2016 C. albicans puparia were also deployed at a subset of sites (Table 1). C. albicans eggs are laid on the surface of defoliated leaves, and winter moth can become parasitized by C. albicans if they inadvertently consume a C. albicans egg while feeding. The C. albicans hatches when inside its host, and when the host winter moth pupates in the soil, the fly larva develops and forms a puparium inside the host pupa. Only one puparium can form inside each host (Wylie 1960). The fly puparium is inside the winter moth cocoon and is readily visible by mid- or late June. For this study, a total of 3400 C. albicans puparia were deployed.
Pupal dissections, incubation, and parasitoid collection
After each 3- or 6-week pupal deployment, we retrieved the sentinel pupae and characterized their fate as alive (intact) or dead (consumed by predators, parasitized, or diseased). Without destructive dissection, it is not possible to determine if the retrieved pupae may have a developing endoparasitoid. Thus, the intact pupae were stored in an incubator (Percival Scientific) until the following spring to allow any further parasitoid development. Pupae were stored at 12 °C until the beginning of December, at 9.5 °C until the end of December, and at 4 °C until late March. The pupae were kept in dark with no day/night cycle, and once a month they were sprayed with a sodium propionate solution (5 g sodium propionate/l of water) to prevent mold. Starting in late March, the temperatures were increased 4 °C every 3 days to 22 °C when the pupae were taken out of storage and kept at room temperature. The parasitoids were identified as Pimpla using keys in Townes et al. (1960), Townes (1969) and stored in 95% ethanol at − 20 °C for further molecular or morphological identification.
Monthly and annual parasitism rate estimates
To calculate monthly and annual mortality from parasitism, our estimate of parasitism included pupae that had developing wasps (larvae and pharate adults) and pupae with wasp emergence holes (as shown in Fig. S1). The proportion of pupae parasitized by Pimpla wasps for each deployment was calculated by dividing the total number of parasitized pupae by the total number of intact pupae retrieved from the field (i.e., number of pupae retrieved excluding the number of pupae that were lost due to predation). This method of calculating percent parasitism incorporates the fact that parasitism rates can be obscured by predation rates because predation typically occurs on the pupae whether or not they were parasitized. This method aims to estimate the true underlying mortality rate of each source in the system (Buonaccorsi and Elkinton 1990; Elkinton et al. 1996; Royama 1981; Van Driesche 1983). To test for any relationship between the rates of Pimpla wasp parasitism and predation, we regressed the proportion parasitized by the proportion lost to predation and no trend was detected (Fig. S2); this further suggests that predators do not discriminate between parasitized and unparasitized pupae.
To calculate the standardized mortality from parasitism for each deployment over 31 days (S31), we used the following equation:
where Sp is the pupal survivorship from parasitism as a proportion and n is the true number of days the pupae were deployed (which ranged from 19 to 45 days with a mean of 31 days). To calculate the cumulative pupal stage parasitism (Pc), we used the following pair of equations:
and
where Sc is the cumulative pupal stage survivorship from parasitism and Sp1 to Sp5 are the proportion survival from parasitism for successive deployments for a particular site and year. No value was included for deployments four and five when only three deployments were used to span the pupal life stage.
Host selection, development, and sex ratio
In 2016, we deployed an additional set of 2000 winter moth pupae to study parasitism depth and host searching behavior of Pimpla wasps. The pupae were spread one layer thick across the base of wire mesh cages (mesh size: 6.4 mm sides with a 17 mm mesh lid) to keep predators out. Two cages each were deployed in two of the study sites, Wellesley and Hanson, Massachusetts (Table 1) for three consecutive rounds for 35 days each (deployment 1: 7 July–8 August; deployment 2: 8 August–9 September; deployment 3: 9 September–11 October). Half of the pupae were deployed with a thin layer of leaves over top, and the other half had 2 cm of soil then leaves over top. After deployment, the retrieved pupae were sifted from the soil and stored at room temperature with natural light cycling to allow wasps to complete their development and emerge. Every 3 days, the pupae were sprayed with the water-sodium propionate solution, and wasp emergence was recorded. We tested for an effect of soil and leaf coverage on the emergence counts using a generalized linear model (GLM) with a negative binomial fit.
To study wasp development time, sex ratio, and host choice, Pimpla wasps that emerged from field-deployed pupae in 2016 were reared in the laboratory in cages (BugDorm 4F4545 Insect Rearing Cage). Dead wasps were replaced, and newly emerged wasps were added, but we always kept 30 wasps per cage with an equal number of males and females. At night, the cages were stored in full dark at 12 °C; during the day, they were kept at 23 °C and exposed to ambient light. The wasps were sprayed with water twice a day and given honey water. To study development time, the wasps were given access to 100–200 unparasitized winter moth pupae, which were replaced every 5–6 days for a total of 12 rounds between 15 August and 17 October. Oviposition was monitored during the first hour of exposure to new pupae. When a female oviposited into a pupa, we moved the pupa singly to a tube (15 ml Falcon centrifuge tube with a ventilation hole). The exposed pupae were stored at room temperature in ambient light and monitored for subsequent wasp emergence. Of these 120 pupae, 42 wasps emerged. Wasp development time was calculated. The sex of wasps that emerged from the field-deployed pupae and lab colony was noted and sex-ratio was evaluated by exposure treatment (field or lab pupae), by date of exposure to parasitism, and by season of emergence (fall or spring) using binomial GLM. To assess host choice, a subset of 30 wasps was given both winter moth pupae and C. albicans puparia in a choice test. We ran three trials in September, each with an equal number of winter moth pupae and C. albicans puparia ranging from 100 to 200 pupae for each pupa type. We also compared the monthly parasitism rates from pupae and puparia deployed in the main study plots that received C. albicans puparia (Table 1, sites A–F, 2014–2016). We analyzed both monthly and cumulative pupal stage parasitism by pupal type (winter moth pupae or parasitized by C. albicans), year, site, and deployment using a GLM with a quasibinomial fit.
Parasitism seasonality and year-to-year variation
To assess seasonality of parasitism rates, we used a logistic regression to analyze the monthly rate of parasitism on winter moth pupae weighted by the total pupae analyzed against the main effect of deployment date and included year and site. We used data from the main study sites (Table 1, sites A–H). Similarly, to assess seasonality of parasitism, we used a negative binomial GLM to evaluate counts of adult wasps that emerged from the additional pupae deployed in 2016 (“Pimpla host selection, development time, and sex ratio” section) by date of exposure to parasitism. To assess pupal parasitism across years, parasitism rates from the winter moth deployed across all years and sites (Table 1, sites A–H) were compared using a logistic model using both our estimation of monthly and annual parasitism rates weighted by the total pupae analyzed. For our analysis of monthly parasitism, we included year, site, and deployment as predictors. For our analysis of cumulative pupal stage parasitism, we weighted the logistic regression by the average number of pupae analyzed across the deployments and used year and site as predictors.
Parasitism with winter moth spread and density
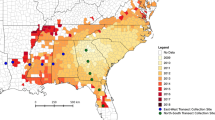
To compare Pimpla wasp parasitism on winter moth pupae between sites infested by winter moth (the first eight sites listed in Table 1, to the east of the light dotted line in Fig. 1) compared to sites on the edge of the infestation or outside the infestation area (the last six sites listed in Table 1, to the west of the light dotted line in Fig. 1), we used a logistic regression to analyze the monthly rate of parasitism weighted by the total pupae analyzed with infestation status, year, and site as predictors. We also analyzed the cumulative rate of parasitism weighted by the average number of pupae. Only years 2015 and 2016 were used for these comparisons as they included both heavily infested sites and sites outside the heavily infested area. The designation of a site as winter moth-infested was determined from previous studies of winter moth spread (Elkinton et al. 2014, 2015, 2018).
Average (2014–2017) percent parasitism by Pimpla on winter moth pupae across the pupal deployment sites. The letters for each study site correspond to Table 1 with the six main study sites (A–F) indicated by the gray boxes. The area to the right of the dashed lines approximates the winter moth infestation area for 2007 and 2014 (Elkinton et al. 2014, 2015)
To estimate winter moth pupal density at each long-term study plot (Table 1 sites A–H), 16 plastic buckets (16 cm width × 28 cm length × 10 cm height) filled 3 cm deep with sifted peat moss and rainwater drainage holes were placed under each study tree in late May before pre-pupal winter moth caterpillars began to spin down from the tree canopies. Each bucket was placed at a randomly selected distance between the tree stem and the edge of the tree canopy along one of eight evenly spaced directions. To test for density-dependence, we analyzed Pimpla wasp parasitism by winter moth pupae density using a logistic regression of monthly parasitism rates weighted by the total pupae analyzed regressed against the corresponding density of winter moth pupae (log-transformed) with site, year, and deployment as predictors. We also analyzed the cumulative pupal stage parasitism against the log-transformed pupal density with year and site effects.
Statistical analyses
All analyses were performed in R 3.4.4 (RCoreTeam 2013) using RStudio, version 1.1.442 (RstudioTeam 2015). For each analysis, the full model was run initially (including site, year, and deployment effects, etc.), the model was evaluated for evidence skew in the residuals or outliers, and any insignificant predictors were dropped sequentially until the best fit model was selected using AIC comparisons. We checked for overdispersion, and when evidence of overdispersion was noted, we applied a quasibinomial or quasipoisson distribution. Quasibinomial and quasipoisson analyses do not generate AIC values; thus, to select the best fit model, we compared the residual deviance of the fit model to that of the null model. A pseudo-R2 was calculated by comparing the residual deviance of the fit model against the null model (deviance null model − deviance fit model/deviance null model). All graphical data were displayed using ggplot2 (Wickham 2009).
Morphological comparative analyses
Following initial identification of our specimens as belonging to the genus Pimpla, further morphological and molecular identification was conducted using a subset of 302 samples (289 from winter moth and 13 from C. albicans puparia). This subset included wasps reared from all collection sites, seasons, and years, and included males and females. The specimens were initially sorted into putative species based on morphology; species identification was attempted using keys and diagnostic information in Townes (1940) and Townes et al. (1960). Specimens were also compared with authoritatively determined specimens of Pimpla turionellae L., Pimpla contemplator Müller, Pimpla hesperus Townes, Pimpla aquilonia Cresson, and Pimpla disparis Viereck in the Smithsonian Institution National Museum of Natural History, Washington, DC (USNM). All but the last two species have been recorded as attacking winter moth (Silvestri 1941; Wylie 1960; Humble 1985). Pimpla flavicoxis has been reported from winter moth in the British Isles and was previously treated as a junior synonym of P. aquilonia. However, there are no specimens at the USNM identified as P. flavicoxis. P. disparis was introduced into Canada and the U.S. to control other lepidopteran pests. It has been reported as a parasitoid of lepidopterans in multiple families, including geometrids (Yu et al. 2012). We also compared our specimens to the lectotype for Pimpla aequalis Provancher from the Université Laval, Quebec City, Quebec, Canada (ULQC). Vouchers for parasitoid species in this research are deposited in the University of Massachusetts Insect Collection, Amherst, MA (UMEC).
Molecular comparative analyses
DNA extraction, amplification, and sequencing
A subset of the Pimpla wasps that emerged or were dissected from winter moth or C. albicans pupae were selected for molecular analyses. When possible, three adult samples and one larval sample were selected for each location and study year; otherwise, up to four wasps of any life stage were selected for a total of 77 field-collected individuals and 20 laboratory-reared wasps (Table S1). DNA was extracted using the QIAGEN DNeasy Blood and Tissue Kits following the company protocol with the following modifications: for larvae, individuals were destructively sampled by grinding with a mortar and pestle; for adults, DNA was extracted from a single leg removed from the specimen; for both life stages, DNA was eluted twice in 100 μl Buffer AE instead of once with 200 μl. All DNA extractions were stored at − 20 °C for subsequent analysis.
A portion of the mitochondrial locus cytochrome c oxidase subunit I (COI) was amplified using standard PCR techniques. For a subset of individuals collected across years, sites, life stages, and life history (Table S1) fragments from three additional nuclear gene regions were amplified: the carbomoylphosphate synthase domain (Cadherin, rudimentary, CAD), elongation factor 1-α (EF1-α), and the D2 and D3 expansion segments of the large subunit ribosomal RNA gene (28S). The primers and temperature profiles used are outlined in Table S2. For each locus, a master mix was prepared using the following ratios of reagents per sample: 17.3 μl nuclease free water, 0.5 μl dNTPs, 5 μl 5 × GoTaq Buffer (Promega), 0.2 μl GoTaq (Promega), 0.5 μl of both the forward and reverse primer (10 μM each), and 1 μl of eluted DNA. PCR reactions were run on a BioRad T100 thermocycler, and the resulting PCR products were visualized on a 1.5% agarose gel stained with SYBERsafe (Invitrogen, Carlsbad, CA) to verify amplification. Samples that produced bands of the expected fragment size for each locus were then cleaned prior to sequencing using Exonuclease 1 (ThermoScientific) and Thermolabile Recombinant Shrimp Alkaline Phosphatase (New England BioLabs). The resulting products were submitted to The Yale University DNA Analysis Facility on Science Hill for Sanger sequencing in both sense and anti-sense orientations.
The resulting sequences were visualized, and the forward and reverse sequences aligned and edited using Geneious R8.1.8 and R9 (Biomatters Ltd.). The ends of the aligned sequences were trimmed by hand to remove primer sequences and so that all sequences had a high-quality score (> 90% HQ nucleotide reads). The presence of heterozygous sites was determined by Geneious and encoded using IUPAC-IUB ambiguity codes. All ambiguous regions were subsequently inspected by eye. For our COI fragment sequences, we looked for evidence of nuclear mitochondrial DNAs (NUMTs) or pseudogenes by examining for the presence of stop codons based on translation with Invertebrate Mitochondrial DNA genetic code.
Phylogenetic analysis
If any of our sequences were identical, we only included a single representative haplotype. The number of samples and sample identification for each haplotype is outlined in Table S3. To compare our Pimpla COI sequences to previously published Pimpla COI sequences, we searched the National Center for Biotechnology Information (NCBI) GenBank database and the University of Guelph Centre for Biodiversity Genomics’s Barcode of Life Data Systems (BOLD). We initially downloaded triplicate sequences from each Pimpla species available across the two repositories, but when the triplicates were identical to each other or nearly identical (> 99% identical), we then retained one representative sequence for each Pimpla species available. From the BOLD sequences, we prioritized sequences acquired from Pimpla samples in the hymenopteran collection of the Canadian Natural Collection of Insects, Arachnids and Nematodes (Agriculture and Agri-Food Canada) accessed by A. Bennett (accession numbers start with ‘BOLD HYCNG’). These sequences included 57 Pimpla specimens representing 13 of the 19 extant described species in the Nearctic Region (Yu et al. 2012). When no representative sequence was available for a particular Pimpla species by the Canadian National Collection, we searched GenBank for a representative sample, followed by any other sequences available in BOLD. Sequences identified as P. aequalis and as Pimpla sp. that were the closest matches in GenBank to sequences of our each of our two Pimpla clades were included; these were associated with publications (Carpenter and Wheeler 1999; Hebert et al. 2016) and from the International Barcode of Life and NCBI GenBank.
JModelTest was used to select the best substitution model for nucleotide evolution, as implemented in the CIPRES Science Gateway (Miller et al. 2010). We performed neighbor-joining, maximum likelihood, and Bayesian reconstructions using the GTR substitution model. Neighbor-joining analyses were run in Geneious using 1000 bootstrap replications and a majority rule (50%) consensus threshold. Maximum likelihood analyses were run using PhyML (Guindon et al. 2010) with 100 bootstrap replications. Bayesian analyses were run using MrBayes 3.2.6 (Huelsenbeck and Ronquist 2001) with a MCMC chain length of 1,000,000 and a burn in length of 10%. The resulting gene trees were then visualized using FigTree Version 1.4.2 (Rambaut 2014).
To determine whether specimens identified as P. aequalis might be members of a cryptic species complex, we used a multilocus genealogical concordance approach (Andersen et al. 2010; Dettman et al. 2006; Groeneveld et al. 2009; Starrett and Hedin 2007) to estimate the number of species present in our dataset. This method considers lineage sorting in multiple, independent loci and has become a common approach for species delineation. For these analyses, we created separate alignments for each gene fragment including each specimen from which all target loci were successfully amplified. In addition, we included publicly available sequences from Labena grallator (Say) (Hymenoptera: Ichneumonidae) as the outgroup for each alignment. Individual gene trees were estimated for each locus, and the congruence of the topologies of the reconstructed gene trees were then visualized by inferring a majority-rule consensus tree using PAUP (Swofford 2003).
Results
Parasitoid collection
Of the 6580 retrieved pupae and puparia that escaped predation (5009 winter moth pupae and 1571 C. albicans puparia, Table 1) over the study period (2014–2017), 342 were parasitized by Pimpla wasps (305 winter moth pupae and 37 C. albicans puparia). Besides the Pimpla wasps, we recovered only two other species: two winter moth pupae (Wellesley, MA and Pawckatuck, CT; 24 June–5 August 2015) were parasitized by a species of Cratichneumon, and four C. albicans puparia (Kingston, RI; 5 August–18 September 2015) were parasitized by a brood of diapriid wasps. Of the Pimpla wasps recovered from field-deployed sentinel pupae and puparia, 46% were adults and the rest larvae.
Parasitism seasonality, overwintering, and year-to-year variation
Monthly parasitism on winter moth pupae varied from 0 to 52% (Fig. S3). Deployment was marginally significant with parasitism rates from early August to mid-September slighter higher than those of either late June to early August or mid-September to late October (df = 100, pseudoR2 = 0.34, p = 0.06). Pupae exposed to Pimpla wasps after the first week of August had wasps that did not emerge until the following spring (the overwintering generation), and most of the overwintering wasps (91%) were from winter moth pupae that were exposed to wasps after the first week of September. Cumulative pupal stage parasitism (one minus the product of the survivorship of each pupal deployment for the duration of the pupal life stage, see “Monthly and annual parasitism rate estimates” section) ranged from 0 to 92% (Table 1). Pimpla wasps were recovered from all years and sites, though some sites had a year without recoveries. There was a significant effect of year but not site or deployment date when analyzing monthly parasitism rates (df = 57, pseudoR2 = 0.40, p = 0.034), and site was significant when analyzing the cumulative pupal stage parasitism rates (Fig. S4; df = 13, pseudoR2 = 0.72, p = 0.009). Parasitism rates were highest in 2015.
Parasitism with winter moth spread and density
Pupal density of the study sites had a significant effect on the monthly (df = 62, pseudoR2 = 0.25, p = 0.039) and cumulative pupal stage parasitism (df = 19, pseudoR2 = 0.39, p = 0.036), with a significant effect of year in both models (Fig. 2). No significant difference was found in percent Pimpla wasp parasitism on winter moth pupae that were deployed in sites on the edge of the current winter moth infestation area compared to the heavy infestation area (Fig. 1). However, there was a trend toward higher parasitism rates at the edge of the winter moth infestation (Fig. S5). Pupae deployed in sites at the edge of the winter moth infestation had a mean cumulative pupal stage parasitism rate of 0.53 ± 0.08 (mean ± SE), while the cumulative parasitism was 0.32 ± 0.03 in the infested sites.
Logistic relationship between monthly (left) and cumulative pupal stage (right) Pimpla parasitism on pupae by winter moth pupal density across sites for the six main study sites. Each point indicates each site for each year, the solid lines show the fit model, and the dashed lines show confidence intervals
Wasp development time and sex ratio
It took 21.2 ± 0.6 SE days from the date of parasitism to adult wasp emergence (n = 39) for pupae parasitized in the laboratory. No wasp emergence was noted after 9 October until spring. Thus, if Pimpla wasps parasitize between 1 June and 1 October (122 days) and if wasps take 21 days from oviposition to emergence, then we can expect up to 5 generations per season and a final 6th overwintering generation. This estimate is from individuals held at a constant 23 °C temperature; however, in the field average temperatures are slightly cooler (~ 19 °C, Table S4), so development may be slower under field conditions. Across studies (field deployed pupae and laboratory rearing), there were 305 Pimpla females and 238 Pimpla males, which suggests a 1:1 female to male ratio.
Pimpla host selection
From the three deployments of pupae placed in Wellesley, MA and Hanson, MA in 2016, there was a significant effect of soil treatment (df = 8, pseudoR2 = 0.57, p = 0.0043) and deployment (p = 0.0018). More wasps emerged from the pupae that were covered only by a layer of leaves than from those buried under soil and leaves. In the host choice study, we did not observe any wasps attempting to oviposit in the C. albicans puparia, and no wasps emerged from these trials. However, from the field studies, we found that C. albicans puparia can be parasitized by Pimpla spp. but at a significantly lower rate than winter moth; this was true for the model that included monthly parasitism rates (df = 83, pseudoR2 = 0.39, p = 0.00012) and the model with cumulative pupal stage parasitism rates (df = 22, pseudoR2 = 0.64, p = 0.0018). Mean cumulative parasitism by Pimpla on C. albicans puparia was 0.15 as compared to 0.27 for winter moth pupae.
Molecular and morphological comparative analyses
Based on morphological features, a subset of Pimpla wasps were identified by Dr. David Wahl (Utah State University) as P. aequalis. Subsequently, additional samples were identified by the second author (RRK) as P. aequalis using the key presented in Townes (1940) and Townes et al. (1960) and comparison with the lectotype for P. aequalis (a female, Fig. S7). The identification was confirmed by another ichneumonid systematist (B. Santos, Smithsonian Institution NMNH) through examination of a subset of the identified specimens. However, results from analysis of molecular data (see below) suggest that the Pimpla wasps in this study actually comprise two species hereafter referred to as Pimpla sp. 1 and Pimpla sp. 2. Images of a female of Pimpla sp. 1 and a male of Pimpla sp. 2 are presented in Fig. 5a–f, respectively (note: we did not rear females of Pimpla sp. 2). While there appear to be subtle morphological differences between Pimpla sp. 1 and Pimpla sp. 2, particularly shape of the mesosoma, whether they are reliable for differentiating these species is equivocal because we have only one adult male of Pimpla sp. 2. All other specimens of Pimpla sp. 2 were larvae from parasitized hosts.
We acquired high quality COI sequences for 74 individuals representing all sites and years (50 adults and 24 larvae; Table S1). Based on reconstruction of the phylogeny using the COI gene fragment, our samples separated into two distinct clades that exhibited 9.7–10.1% sequence divergence (Fig. 3). All nucleotide differences between the two clades represented third-codon substitutions and thus likely represent genetic differences accumulated due to genetic drift and not selection. Both Pimpla clades included wasps acting as primary parasitoids and hyperparasitoids (49 primary and 26 hyperparasitoids; Table S1). Additionally, we obtained high quality sequences for fragments of CAD, EF1-α, and 28S from 26 Pimpla wasps (19 Pimpla sp. 1 individuals and 7 Pimpla sp. 2 individuals as from the COI analyses). For CAD, sequences from the two Pimpla clades were 1.5–3.4% different from each other, with six base-pair differences fixed between the two Pimpla clades (Fig. 4, Table S7). Similarly, for EF1-α the two clades were between 0.7 and 1.6% different with 3 base-pairs consistently different between clades. See Tables S6–S8 for distance matrices for each alignment. For 28S, there were no differences between individuals from the two COI clades, with all but two individuals having identical sequences; the two individuals differed from the other by a single base-pair substitution. Because 28S was invariant, it was left out of the multilocus analyses. The trees constructed from each of these three loci (Fig. 4) and the majority rule consensus tree (Fig. S6) all supported the presence in our samples of two cryptic Pimpla species within what we considered P. aequalis based on morphology (Fig. 5).
Phylogentic inference of our Pimpla samples (bolded) and representative sequences. Representative sequences were downloaded from NCBI GenBank and BOLD. The tree was constructed using a Bayesian analysis with a 604 bp region of the COI locus. Where our sequences were identical, the groups were collapsed as outlined in Table S2. Pimpla aequalis sp. 1 are noted with red and sp. 2 with blue. Branch lengths are drawn proportional to the rate of change observed. The number to the left each node represents the bootstrap support value for the branch (Bayesian over the Maximum Likelihood)
Phylogentic inferences using a COI, b CAD, and c EF1-α gene regions for only our P. aequalis sp. 1 (red) and sp. 2 (blue) samples. Branch lengths are drawn proportional to the rate of change observed. The number to the left each node represents the bootstrap support value for the branch (Bayesian over the Maximum Likelihood)
Based on these comparisons, we appear to have two distinct species of Pimpla that fit P. aequalis sensu Townes (1940) and Townes et al. (1960), and these species are not any Palearctic species available to us known to attack winter moth in Europe. It is likely that either Pimpla sp. 1 or Pimpla sp. 2 is P. aequalis. The lectotype of P. aequalis is more similar morphologically to specimens of Pimpla sp. 1 than Pimpla sp. 2; however, Pimpla sp. 2 is known from a male only, and the P. aequalis lectotype is a female. Determining the identities of these clades, and discerning the identities of Holarctic species of Pimpla in general, would require extensive analysis of morphological and molecular data for Pimpla species in the Nearctic and Palearctic regions, including primary type specimens. The sequences generated from our specimens did not match any published sequences available for other Pimpla species, which includes 13 of the 19 extant described species in the Nearctic Region (Yu et al. 2012) and an additional six species of Pimpla from outside the Nearctic Region. The only molecular matches were for other unknown Pimpla species collected from the northeastern United States and southeastern Canada. Thus, Pimpla sp. 1 and Pimpla sp. 2 are presumably native to North America.
Discussion
Biotic resistance is the process by which native natural enemies spill over from native species to attack an invasive species and reduce the success of that invader (Diez et al. 2008; Elton 1958; Levine et al. 2004; Sakai et al. 2001; Shea and Chesson 2002). We found evidence of biotic resistance to invasive winter moth populations in the northeastern United States; in this most recent invasion, winter moth populations are sustaining heavy, density dependent parasitism by two cryptic species of Pimpla. One of the Pimpla wasps is likely P. aequalis, while the other appears to be a related unknown species. While the association of these Pimpla wasps with winter moth is recent, their impact is notable; estimates of parasitism across our study sites and years were on average 6% and were found to be as high as 52% on winter moth pupae in infested areas. Pimpla wasps were found across all of our study plots. Where winter moth has invaded, Pimpla wasp parasitism responded to winter moth pupal density in a positive density dependent manner and thus has the potential to be regulatory.
The ability of a native predator or parasitoid to respond functionally or numerically to a primary host population, while also using an alternative host when the primary host is at low densities, may result in particularly effective suppression of populations of non-native invader by a native species (Nechols et al. 1992; Shea and Chesson 2002). In this way, the native natural enemy has the ability to build up using the novel host but can also use other host species when this new host is less abundant. As a result, the natural enemy is maintained in the community and can aggregate or respond numerically when the invasive alien population outbreaks or spreads. This pattern of host use is common for a number of generalist predators, parasitoids, and pathogens (deRivera et al. 2005; Hassell and Rogers 1972; Holling 1973; Murdoch 1969; Oaten and Murdoch 1975; Schenk and Bacher 2002; Strauss et al. 2012). Parasitism by Pimpla wasps in this study exhibited these characteristics. While Pimpla wasps may build up using winter moth as a host species and respond to high densities with higher rates of parasitism, it also appears to be able to use other host species when winter moth populations are not at high densities.
We detected a positive density dependent relationship of parasitism to winter moth density, suggesting that Pimpla wasps may have a regulatory effect on the winter moth population densities. Further, since the Pimpla wasps are multivoltine, the wasp population may be able to build up in outbreak populations in a numerical response, which could control an outbreaking population (Holling 1973). However, we did not detect increased parasitism rates over either season or years and were not able to test for a numerical response to densities. In the absence of a documented numerical response, it may be that the density dependent response we found may arise from a Type III functional response driven by Pimpla wasp host switching behavior (Holling 1959; Murdoch 1969). While pimplines typically have a preferred host species (niche specialization), they are facultative generalists and can use a broad host range spanning multiple lepidopteran families (Bennett 2008; Fitton et al. 1988; Krombein et al. 1979). Parasitism of winter moth by Pimpla wasps is likely the result of natural enemy spillover from the native congener Bruce spanworm or other related lepidopteran species in the area. We found that Pimpla wasps exhibited particularly high parasitism 95 km beyond the nearest high density winter moth infestation (i.e., Framingham, MA) and 22 km from the nearest capture of winter moths in pheromone traps (i.e., Orange, MA). The finding that Pimpla wasps were present across the study area, including areas beyond the range that winter moth has spread, supports the hypothesis that the Pimpla species are native. Pimpla wasp population densities respond to, but do not depend on, the presence of winter moth. In fact, we found particular high parasitism rates at the leading edge of the winter moth infestation. This is likely because in these interior sites Pimpla wasps, as native generalist parasitoids, are maintained at high densities by a robust Lepidoptera community. In this way, Pimpla wasps may provide a biotic resistance barrier to the spread of winter moth, which was found by Elkinton et al. (2014) to be slowing. Pimpla wasps may help control winter moth spread, but this warrants further study.
If Pimpla wasps act as hyperparasitoids (a parasitoid of a parasitoid) of any native or introduced parasitoids (biological control agents), then they have the potential to reduce population control of the invasive species by inflicting more mortality on the biological control agent than on the invasive species itself. However, if a parasitoid inflicts more mortality on the invasive species than on any introduced biological control agent, then it aids in controlling the pest population (Brodeur 2000; Nechols et al. 1992; Sullivan 1987). While we found that Pimpla wasps can hyperparasitize, as is typical of many pimpline wasps (Bennett 2008; Fitton et al. 1988) and was found previously (Broadley et al. 2018), they appear to do so only facultatively. From our laboratory host range study, when given a choice of winter moth pupae or winter moth pupae parasitized by C. albicans, the wasps only parasitized winter moth pupae. From our field study, both species of Pimpla wasps can act as hyperparasitoids, but they do so at rates significantly lower than their rate of primary parasitism. Hyperparasitism on C. albicans at these sites was primarily caused by wasps from the genus Phygadeuon rather than Pimpla (Broadley et al. 2018). Together, this further demonstrates that Pimpla wasps contribute to the population control of winter moth.
We detected two species of Pimpla parasitizing winter moth pupae; one is likely P. aequalis, the other is unknown and is possibly an undescribed species. For our estimates of percent parasitism, we did not distinguish between the two species of Pimpla, but from the molecular work, one clade is better represented than the other; the majority (88%) of the randomized samples we tested belonged to Pimpla sp. 1. Both species were found across study site, season, and year, and both species acted as both primary and hyperparasitoids. While both species were initially considered P. aequalis based on morphology, we were not able to acquire DNA from the lectotype to discern if one of our two species is P. aequalis. Pimpla aequalis is known only from North America, and we consider both Pimpla species we recovered from winter moth as native to North America. However, some species of Pimpla are known to have a Holarctic distribution (Yu et al. 2012). The overall geographic distribution of the Pimpla species reported here cannot be determined until their identities have been discerned; this would require more extensive taxonomic research on Pimpla species in the Nearctic and Palearctic regions, such as a revision that includes both morphological and molecular data.
Winter moth has been extensively studied in its native range in Europe (e.g. Klemola et al. 2008; Myers and Cory 2013; Tenow et al. 2013; Varley et al. 1973; Vindstad et al. 2011, 2013), as well as in the prior accidental introductions to North America (e.g. Embree 1965; Roland 1990; Roland and Embree 1995); however, this is the first report of Pimpla species as important parasitoids of winter moth. To our knowledge none of the studies of winter moth pupal mortality in Nova Scotia recorded parasitism by Pimpla wasps, although native parasitoids were noted (Embree 1965; Graham 1958; Macphee et al. 1988; Pearsall and Walde 1994; Roland 1990). In British Columbia, Coccygomimus (= Pimpla) hesperus was recorded from Operophtera spp. pupae (Humble 1985) but was not recorded in later studies (Roland 1990; Roland and Embree 1995). This suggests that the wasps were accidently overlooked in the prior studies, that Pimpla wasps now show more host switching to winter moth than when winter moth was first introduced to North America in the 1930s, or that the region of this study has more abundant Pimpla wasp populations with a wider host range than was found in Nova Scotia or British Columbia. Surveys were conducted in winter moth infested sites in coastal Maine, and species of Pimpla, likely P. aequalis and other morphologically similar congeners, were found there (Morin 2015); however, the study did not include sentinel winter moth and thus showed co-occurrence but not parasitism.
We were surprised that Pimpla sp. 1 detected in this study seemed to be the only major species that parasitized winter moth pupae. The lack of additional parasitoids and slow recruitment of Pimpla wasps may help explain why winter moth has been such a high density pest in its introduced region. We looked for parasitism by P. contemplator and P. turionellae, which are known parasitoids of winter moth in Europe (Wylie 1960) and were introduced to southeastern Canada in an attempt to control winter moth, but there is no evidence that they established (Carlson 1979; Graham 1958). Further, P. disparis, a parasitoid introduced in this region to control gypsy moth, was also a potential candidate since P. disparis has a broad host range, attacking lepidopterans of at least 14 families (Schaefer et al. 1989). However, these Pimpla species were not detected. Besides the two species of Pimpla reported here, we only had a few cases of parasitism by diapriid and Cratichneumon wasps. Cratichneumon culex (Muell.) has been recorded as an important parasitoid of winter moth pupae in Europe (East 1974; Hassell 1969; Varley et al. 1973; Wylie 1960), and an undescribed Cratichneumon species was reared from winter moth in British Columbia (Humble 1985). As far as we know, C. culex has not been introduced to North America (Embree 1966; Graham 1958).
Conclusions
Our findings suggest an important role of Pimpla wasps in the population dynamics of winter moth, an invasive forest pest. Overall, this study shows that biotic resistance from a native parasitoid is affecting the dynamics of the winter moth invasion. We urge future research on parasitism by native species in the study of biological invasions, as knowledge of biotic resistance from native natural enemies on invasive populations is essential to our overall understanding of invasions. We also encourage insect biological control practitioners to consider not only the effect of an introduced biocontrol agent but also the effect of native parasitoids and the potential interactions between introduced biocontrol agents and native natural enemies.
References
Andersen JC, Gruwell ME, Morse GE, Normark BB (2010) Cryptic diversity in the Aspidiotus nerii complex in Australia. Ann Entomol Soc Am 103:844–854
Bennett AMR (2008) Review and identification keys to the ichneumonid parasitoids (Hymenoptera: Ichneumonidae) of Nearctic Choristoneura species (Lepidoptera: Tortricidae). Can Entomol 140:1–47
Broadley HJ, Boucher M, Burand JP, Elkinton JS (2017) The phylogenetic relationship and cross-infection of nucleopolyhedroviruses between the invasive winter moth (Operophtera brumata) and its native congener, Bruce spanworm (O. bruceata). J Invertebr Pathol 143:61–68
Broadley HJ, Kelly EA, Elkinton JS, Kula RR, Boettner GH (2018) Identification and impact of hyperparasitoids and predators affecting Cyzenis albicans (Tachinidae), a recently introduced biological control agent of winter moth (Operophtera brumata L.) in the northeastern U.S.A. Biol Control 121:99–108
Brodeur J (2000) Host specificity and trophic relationships of hyperparasitoids. In: Hochberg ME, Ives AR (eds) Parasitoid population ecology. Princeton University Press, Princeton
Buonaccorsi JP, Elkinton JS (1990) Estimation of contemporaneous mortality factors. Res Popul Ecol 32:151–171
Bürgi LP, Roltsch WJ, Mills NJ (2015) Allee effects and population regulation: a test for biotic resistance against an invasive leafroller by resident parasitoids. Popul Ecol 57(1):215–225. https://doi.org/10.1007/s10144-014-0451-4
Callaway RM, Montesinos D, Williams K, Maron JL (2013) Native congeners provide biotic resistance to invasive Potentilla through soil biota. Ecology 94:1223–1229
Carlson RW (1979) Family Ichneumonidae. In: Krombein KV, Hurd PD, Smith DR, Burks BD (eds) Catalog of Hymenoptera in America north of Mexico. Smithsonian Institution Press, Washington, pp 315–741
Carpenter JM, Wheeler WC (1999) Towards simultaneous analysis of morphological and molecular data in Hymenoptera. Zool Scr 28:251–260
Carrillo-Gavilan A, Moreira X, Zas R, Vila M, Sampedro L (2012) Early resistance of alien and native pines against two native generalist insect herbivores: no support for the natural enemy hypothesis. Funct Ecol 26:283–293
Dearborn KW, Heard SB, Sweeney J, Pureswaran DS (2016) Displacement of Tetropium cinnamopterum (Coleoptera: Cerambycidae) by its Invasive Congener Tetropium fuscum. Environ Entomol 45:848–854
deRivera CE, Ruiz GM, Hines AH, Jivoff P (2005) Biotic resistance to invasion: native predator limits abundance and distribution of an introduced crab. Ecology 86:3364–3376
Dettman JR, Jacobson DJ, Taylor JW (2006) Multilocus sequence data reveal extensive phylogenetic species diversity within the Neurospora discreta complex. Mycologia 98:436–446
Diez JM, Sullivan JJ, Hulme PE, Edwards G, Duncan RP (2008) Darwin’s naturalization conundrum: dissecting taxonomic patterns of species invasions. Ecol Lett 11:674–681
Donahue KL, Broadley HJ, Elkinton JS, Burand JP, Huang WF, Andersen JC (2018) Using the SSU, ITS, and ribosomal DNA operon arrangement to characterize two microsporidia infecting Bruce spanworm, Operophtera bruceata (Lepidoptera: Geometridae). J Eukaryot Microbiol. https://doi.org/10.1111/jeu.12685
Duan JJ, Taylor PB, Fuester RW, Kula RR, Marsh PM (2013) Hymenopteran parasitoids attacking the invasive emerald ash boror (Coleoptera: Buprestidae) in western and central Pennsylvania. Fla Entomol 96:166–172
Duan JJ, Abell KJ, Bauer LS, Gould J, Van Driesche R (2014) Natural enemies implicated in the regulation of an invasive pest: a life table analysis of the population dynamics of the emerald ash borer. Agric For Entomol 16:406–416
East R (1974) Predation on soil-dwelling stages of winter moth at Wytham Woods Berkshire. J Anim Ecol 43:611–626
Elkinton JS, Healy WM, Buonaccorsi JP, Boettner GH, Hazzard AM, Smith HR, Liebhold AM (1996) Interactions among gypsy moths, white-footed mice, and acorns. Ecology 77:2332–2342
Elkinton JS, Boettner GH, Sermac M, Gwiazdowski R, Hunkins RR, Callahan J, Scheufele SB, Donahue CP, Porter AH, Khrimian A, Whited BM, Campbell NK (2010) Survey for winter moth (Lepidoptera: Geometridae) in northeastern North America with pheromone-baited traps and hybridization with the native Bruce spanworm (Lepidoptera: Geometridae). Ann Entomol Soc Am 103:135–145
Elkinton JS, Liebhold A, Boettner GH, Sremac M (2014) Invasion spread of Operophtera brumata in northeastern United States and hybridization with O. bruceata. Biol Invasions 16:2263–2272
Elkinton JS, Boettner GH, Liebhold A, Gwiazdowski R (2015) Biology, spread, and biological control of winter moth in the eastern United States. In: Team TFHTE (ed). USDA Forest Service Publication, Morgantown
Elkinton JS, Boettner GH, Broadley HJ, Reardon R, Weeks Jr RD (2018) Biological control of the winter moth in the northeastern North America. USDA Forest Service Forest Health Assessment and Applied Science Team 2018-03:0-9
Elton CS (1958) The ecology of invasions by animals and plants. Methuen, London
Embree DG (1965) The population dynamics of the winter moth in Nova Scotia, 1954–1962. Mem Entomol Soc Can 97:5–57
Embree DG (1966) The role of introduced parasites in the control of the winter moth in Nova Scotia. Can Entomol 98:1159–1168
Faillace CA, Lorusso NS, Duffy S (2017) Overlooking the smallest matter: viruses impact biological invasions. Ecol Lett 20:524–538
Fitton MG, Shaw MR, Gauld ID (1988) Pimpline ichneumon-flies: Hymenoptera, Ichneumonidae (Pimplinae). Royal Entomological Society of London, London
Garnas JR, Auger-Rozenberg MA, Roques A, Bertelsmeier C, Wingfield MJ, Saccaggi DL, Roy HE, Slippers B (2016) Complex patterns of global spread in invasive insects: eco-evolutionary and management consequences. Biol Invasions 18:935–952
Gauld ID (1991) The Ichneumonidae of Costa Rica, 1. Introduction, keys to subfamilies, and keys to the species of the lower pimpliform subfamilies Rhyssinae, Poemeniinae, Acaenitinae and Cylloceriinae. Mem Am Entomol Inst 47:1–589
Goulet H, Huber JT (1993) Hymenoptera of the world: an identification guide to families. Centre for Land and Biological Resources Research, Ottawa
Grabenweger G, Kehrli P, Zweimuller I, Augustin S, Avtzis N, Bacher S, Freise J, Girardoz S, Guichard S, Heitland W, Lethmayer C, Stolz M, Tomov R, Volter L, Kenis M (2010) Temporal and spatial variations in the parasitoid complex of the horse chestnut leafminer during its invasion of Europe. Biol Invasions 12:2797–2813
Graham AR (1958) Recoveries of introduced species of parasites of the winter moth, Operophtera brumata (L.) (Lepidoptera: Geometridae), in Nova Scotia. Can Entomol 90:595–596
Groeneveld LF, Weisrock DW, Rasoloarison RM, Yoder AD, Kappeler PM (2009) Species delimitation in lemurs: multiple genetic loci reveal low levels of species diversity in the genus Cheirogaleus. BMC Evol Biol 9:30
Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321
Gwiazdowski RA, Elkinton JS, Dewaard JR, Sremac M (2013) Phylogeographic Diversity of the Winter Moths Operophtera brumata and O. bruceata (Lepidoptera: Geometridae) in Europe and North America. Ann Entomol Soc Am 106:143–151
Hassell MP (1969) A study of mortality factors acting upon Cyzenis albicans (Fall), a tachinid parasite of winter moth (Operophtera brumata (L)). J Anim Ecol 38:329–339
Hassell MP (2000) Host-parasitoid population dynamics. J Anim Ecol 69:543–566
Hassell MP, Rogers DJ (1972) Insect parasite responses in the development of population models. J Anim Ecol 41:661–676
Havill NP, Elkinton J, Andersen JC, Hagen SB, Broadley HJ, Boettner GJ, Caccone A (2017) Asymmetric hybridization between non-native winter moth, Operophtera brumata (Lepidoptera: Geometridae), and native Bruce spanworm, Operophtera bruceata, in the Northeastern United States, assessed with novel microsatellites and SNPs. Bull Entomol Res 107:241–250
Hebert PDN, Ratnasingham S, Zakharov EV, Telfer AC, Levesque-Beaudin V, Milton MA, Pedersen S, Jannetta P, deWaard JR (2016) Counting animal species with DNA barcodes: Canadian insects. Philos Trans R Soc B 371:20150333
Heimpel G, Mills N (2017) Biological control: ecology and applications. Cambridge University Press, Cambridge. https://doi.org/10.1017/9781139029117
Hobbs RJ, Higgs E, Harris JA (2009) Novel ecosystems: implications for conservation and restoration. Trends Ecol Evol 24:599–605
Holliday NJ (1977) Population ecology of winter moth (Operophtera brumata) on apple in relation to larval dispersal and time of bud burst. J Appl Ecol 14:803–813
Holling CS (1959) The components of predation as revealed by a study of small mammal predation of the European pine sawfly. Can Entomol 91:293–320
Holling CS (1973) Resilience and stability of ecological systems. Annu Rev Ecol Syst 4:1–23
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogeny. Bioinformatics 17:754–755
Humble LM (1985) Final-instar larvae of native pupal parasites and hyperparasites of Operophtera spp. (Lepidoptera, Geometridae) on southern Vancouver Island. Can Entomol 117:525–534
Jeschke J, Gómez Aparicio L, Haider S, Heger T, Lortie C, Pyšek P, Strayer D (2012) Support for major hypotheses in invasion biology is uneven and declining. NeoBiota 14:1
Keane RM, Crawley MJ (2002) Exotic plant invasions and the enemy release hypothesis. Trends Ecol Evol 17:164–170
Kenis M, Roy HE, Zindel R, Majerus MEN (2008) Current and potential management strategies against Harmonia axyridis. Biocontrol 53:235–252
Klemola T, Andersson T, Ruohomaki K (2008) Fecundity of the autumnal moth depends on pooled geometrid abundance without a time lag: implications for cyclic population dynamics. J Anim Ecol 77:597–604
Krombein KV, Hurd PD, Smith DR, Burks BD (1979) Symphyta and Apocrita (Parasitica). Catalog of Hymenoptera in America North of Mexico. Smithsonian Institute Press, Washington
Levine JM, Adler PB, Yelenik SG (2004) A meta-analysis of biotic resistance to exotic plant invasions. Ecol Lett 7:975–989
Lewis MA, Kareiva P (1993) Allee dynamics and the spread of invading organisms. Theor Popul Biol 43:141–158
Macphee A, Newton A, McRae KB (1988) Population studies on the winter moth Operophtera brumata (L.) (Lepidoptera, Geometridae) in apple orchards in Nova Scotia. Can Entomol 120:73–83
Maron JL, Vilà M (2001) When do herbivores affect plant invasion? Evidence for the natural enemies and biotic resistance hypotheses. Oikos 95:361–373
Maron JL, Harrison S, Greaves M (2001) Origin of an insect outbreak: escape in space or time from natural enemies? Oecologia 126:595–602
Matosevic D, Melika G (2013) Recruitment of native parasitoids to a new invasive host: first results of Diyocosmus kuriphilus parasitoid assemblage in Croatia. Bull Insectol 66:231–238
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Proceedings of the Gateway computing environments workshop (GCE). New Orleans
Morin H (2015) Winter moth (Operophtera brumata L.) natural enemy diversity and abundance in infested areas in Midcoast Maine. Thesis in Biology, The University of Maine
Murdoch WW (1969) Switching in general predators: experiments on predator specificity and stability of prey populations. Ecol Monogr 39:335
Murdoch WW, Chesson J, Chesson PL (1985) Biological-control in theory and practice. Am Nat 125:344–366
Myers JH (2018) Population cycles: generalities, exceptions and remaining mysteries. P R Soc B 285:20172841
Myers JH, Cory JS (2013) Population cycles in forest lepidoptera revisited. Ann Rev Ecol Evol Syst 44:565–592. https://doi.org/10.1146/annurev-ecolsys-110512-135858
Nechols JR, Kauffman WC, Schaefer PW (1992) Significance of host-specificity in classical biological control. In: Kauffman WC, Nechols JR (eds) Selection criteria and ecological consequences of importing natural enemies. Entomological Society of America, Lanham, pp 41–52
Oaten A, Murdoch WW (1975) Switching, functional response, and stability in predator–prey systems. Am Nat 109:299–318
Pearsall IA, Walde SJ (1994) Parasitism and predation as agents of mortality of winter moth populations in neglected apple orchards in Nova Scotia. Ecol Entomol 19:190–198
Pearson DE, Ortega YK, Eren O, Hierro JL (2018) Community assembly theory as a framework for biological invasions. Trends Ecol Evol 33:313–325
Poulin R (2017) Invasion ecology meets parasitology: advances and challenges. Int J Parasitol Parasites Wildl 6:361–363
Rambaut A (2014) FigTree Tree Figure Drawing Tool http://tree.bio.ed.ac.uk/software/figtree/. Accessed 2 May 2018
RCoreTeam (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Richardson DM, Pysek P (2006) Plant invasions: merging the concepts of species invasiveness and community invasibility. Prog Phys Geogr 30:409–431
Roland J (1988) Decline in winter moth populations in North America—direct versus indirect effect of introduced parasites. J Anim Ecol 57:523–531
Roland J (1990) Interaction of parasitism and predation in the decline of winter moth in Canada. In: Watt A, Leather SR, Hunter AF (eds) Population dynamics of forest insects. Intercept Ltd, Andover, pp 289–301
Roland J, Embree DG (1995) Biological control of the winter moth. Annu Rev Entomol 40:475–492
Royama T (1981) Evaluation of mortality factors in insect life table analysis. Ecol Monogr 51:495–505
RstudioTeam (2015) RStudio: integrated development environment for R. RStudio, Inc., Boston
Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J, With KA, Baughman S, Cabin RJ, Cohen JE, Ellstrand NC, McCauley DE, O’Neil P, Parker IM, Thompson JN, Weller SG (2001) The population biology of invasive species. Annu Rev Ecol Syst 32:305–332
Schaefer PW, Fuester RW, Chianese RJ, Rhoads LD, Tichenor RB (1989) Introduction and North-Amherican establishment of Coccygomimus disparis (Hymenoptera, Ichneumonidae), a polyphagus pupal parasite of Lepidoptera, including gypsy moth. Environ Entomol 18:1117–1125
Schenk D, Bacher S (2002) Functional response of a generalist insect predator to one of its prey species in the field. J Anim Ecol 71:524–531
Schonrogge K, Stone GN, Crawley MJ (1995) Spatial and temporal variation in guild structure—parasitoids and inquilines of Andricus quercuscalicis (Hymenoptera, Cynipidae) in its native and alien ranges. Oikos 72:51–60
Shea K, Chesson P (2002) Community ecology theory as a framework for biological invasions. Trends Ecol Evol 17:170–176
Silvestri F (1941) Contribuzioni alla conoscenza degli insetti dannosi e dei loro simbionti. VI. La falena brumale o la brumale (Operophtera brumata L.). Bolle Lab Entomol Portici 5:61–120
Starrett J, Hedin M (2007) Multilocus genealogies reveal multiple cryptic species and biogeographical complexity in the California turret spider Antrodiaetus riversi (Mygalomorphae, Antrodiaetidae). Mol Ecol 16:583–604
Strauss A, White A, Boots M (2012) Invading with biological weapons: the importance of disease-mediated invasions. Funct Ecol 26:1249–1261
Sullivan DJ (1987) Insect hyperparasitism. Annu Rev Entomol 32:49–70
Swofford DL (2003) PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4. Sinauer Associates, Sunderland
Tenow O, Nilssen AC, Bylund H, Pettersson R, Battisti A, Bohn U, Caroulle F, Ciornei C, Csoka G, Delb H, De Prins W, Glavendekic M, Gninenko YI, Hrasovec B, Matosevic D, Meshkova V, Moraal L, Netoiu C, Pajares J, Rubtsov V, Tomescu R, Utkina I (2013) Geometrid outbreak waves travel across Europe. J Anim Ecol 82:84–95
Tobin PC, Berec L, Liebhold AM (2011) Exploiting Allee effects for managing biological invasions. Ecol Lett 14:615–624
Townes HK (1940) A revision of the Pimplini of eastern North America (Hymenoptera: Ichneumonidae). Ann Entomol Soc Am 33:283–323
Townes H (1969) The genera of Ichneumonidae, part 1. Mem.Am Entomol Inst 11:i–ii +1–300
Townes HK, Townes M, Walley GS, Townes G (1960) Ichneumon-flies of America north of Mexico: 2 Subfamily Ephialtinae, Xoridinaem Acaenitinae. US Natl Mus Bull 216:1–676
Van Driesche RG (1983) Meaning of “percent parasitism” in studies of insect parasitoids. Environ Entomol 12:1611–1622
Varley GC, Gradwell GR, Hassell MP (1973) Insect population ecology: an analytical approach. Blackwell Scientific, Oxford, England, 212 pp
Vindstad OPL, Hagen SB, Jepsen JU, Kapari L, Schott T, Ims RA (2011) Phenological diversity in the interactions between winter moth (Operophtera brumata) larvae and parasitoid wasps in sub-arctic mountain birch forest. Bull Entomol Res 101:705–714
Vindstad OPL, Schott T, Hagen SB, Jepsen JU, Kapari L, Ims RA (2013) How rapidly do invasive birch forest geometrids recruit larval parasitoids? Insights from comparison with a sympatric native geometrid. Biol Invasions 15:1573–1589
Waage J, Greathead D (1985) Insect parasitoids. Academic Press Limited, San Diego
Whited BM (2007) The population ecology of winter moth (Operophtera brumata) in eastern Massachusetts. Masters thesis in Organismic and Evolutionary Biology, University of Massachusetts, Amherst
Wickham H (2009) ggplot2: elegant graphics for data analysis. Springer, New York
Wylie HG (1960) Insect parasites of the winter moth, Operophtera brumata (L.) (Lepidoptera: Geometridae) in western Europe. Entomophaga 5:111–129
Yu DSK, van Achterberg C, Horstmann K (2012) World Ichneumonoidea 2011. Taxonomy, biology, morphology and distribution. Taxapad. Home of Ichneumonoidea, Nepean, Ontario
Zappala L, Bernardo U, Biondi A, Cocco A, Deliperi S, Delrio G, Giorgini M, Pedata P, Rapisarda C, Garzia GT, Siscaro G (2012) Recruitment of native parasitoids by the exotic pest Tuta absoluta in Southern Italy. Bull Insectol 65:51–61
Acknowledgements
The authors thank R. Crandall, N. Ayres, A. Roehrig, T. Dowling, N. Milano, Q. Dupupet, R. Hennessy, C. Camp, E. Mooshian, K. Donahue, E. Lee, J. Cox, E. Kelly, and G. Greenberg, D. Swanson, D. Adams, R. Casagrande, E. Amore, and T. Peckham for their assistance with this research. We are grateful to B. Santos (USNM) and A. Bennett (CNC) for advice on ichneumonid identification and taxonomy. We thank T. Litwak (Systematic Entomology Laboratory, USDA-ARS) for imaging the Pimpla specimens. We thank S. Klopfstein and J. Mottern for their suggestions on primers and DNA amplification. Lastly, we thank Drs. Van Driesche, Adler, Normark, and Burand for comments on earlier versions of the manuscript. This material is based on work supported by Cooperative Agreements from the USDA APHIS [12 13 14-8225-0464-CA] and from the USDA Forest Service [13-CA-1140004-236-CA] as well as a Summer Research Scholarship from the University of Massachusetts Amherst Natural History Collections and an Irwin Martin Award from the Graduate Program in Organismic and Evolutionary Biology, University of Massachusetts Amherst. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA. USDA is an equal opportunity provider and employer.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Broadley, H.J., Kula, R.R., Boettner, G.H. et al. Recruitment of native parasitic wasps to populations of the invasive winter moth in the northeastern United States. Biol Invasions 21, 2871–2890 (2019). https://doi.org/10.1007/s10530-019-02019-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-019-02019-4