Abstract
Species invasions can alter food web structure and change ecosystem-level functioning, but it is often unclear how these invasions may affect the life history of native species. The Lake Sturgeon (Acipenser fulvescens), a large long-lived native fish species in the Great Lakes, has increased in abundance in the lower Niagara River and nearby Lake Ontario during a period of invasive species-induced ecosystem change precipitated most recently by Dreissenid mussels (Driessena polymorpha and Driessena bugensis) and Round Goby (Neogobius melanostomus). Material taken from cross-sections of archived pectoral spines from Niagara River Lake Sturgeon captured in 1998–2000 and 2010–2012 were analyzed for stable isotopes across discrete growth zones to provide an ontogenetic assessment of diet, and diet analysis of Lake Sturgeon captured in 2014 was conducted to assess the contribution of invasive prey. Round Goby was the most important Lake Sturgeon prey item (86% by weight) in 2014, which corroborated results of δ15N and δ13C. Lake Sturgeon captured after the invasion of Round Goby exhibited ontogenetic changes in δ15N that differed from pre-Round Goby patterns, though this effect was weaker for δ13C. Values of δ15N from spine growth zones indicated non-linear increases in trophic position with age and increased rate of δ15N enrichment after the Round Goby invasion. We conclude that Round Goby establishment in western Lake Ontario changed the feeding ecology of Lake Sturgeon, which may have a positive effect on population growth for this native species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-native species can have profound effects on the ecosystems and food webs they invade. Across systems, invasive species have been shown to cause declines in the abundance of native species (Barbiero and Tuchman 2004; Dorcas et al. 2012; Vilà et al. 2011), as well as alter food web structure (Ozersky et al. 2012; Vander Zanden et al. 1999) and ecosystem-level processes such as primary production and nutrient cycling (Capps and Flecker 2013; Hecky et al. 2004; Vilà et al. 2011). In the Laurentian Great Lakes, two recent species invasions, the Dreissenid mussels (Driessena polymorpha and Driessena bugensis) and Round Goby (Neogobius melanostomus), have exerted broad, among-lake effects. The invasion of Dreissenid mussels beginning in the late 1980s has resulted in the exclusion of native benthic amphipods (e.g. Diporeia spp.), reductions in pelagic phytoplankton and zooplankton biomass, and the sequestration of biomass in the nearshore zone (Higgins and Vander Zanden 2010; Nalepa et al. 2009). The subsequent invasion of the Round Goby, a sympatric predator of Dreissenid mussels, has also influenced the ecology of the Great Lakes through the competitive exclusion of native sculpins and other small-bodied fishes (Balshine et al. 2005; Janssen and Jude 2001), by emerging as an important prey source for piscivorous fishes such as Smallmouth Bass (Micropterus dolomieu), Walleye (Sander vitreus), Burbot (Lota lota), and Lake Trout (Salvelinus namaycush) (Crane and Einhouse 2016; Madenjian et al. 2011; Steinhart et al. 2004a), and by mobilizing Dreissenid biomass and activating a nearshore benthic energy subsidy pathway (Turschak et al. 2014). Evidence suggests that these invasions have strongly influenced food web dynamics across the Great Lakes, a phenomenon perhaps best illustrated by the shifts in trophic dynamics and relative abundance of fishes in Lake Huron during the 2000s (Madenjian et al. 2013). However, there are a number of native species for which the effects of these recent invasions on trophic ecology are understudied (Turschak and Bootsma 2015).
Ontogenetic shifts in diet and habitat use are common in fishes and have received considerable attention (Post 2003). Many secondary and tertiary consumers in the Great Lakes have exhibited invasion-induced changes in diet, which may influence ontogenetic variation in diet. For instance, both native Lake Whitefish (Coregonus clupeaformis) and naturalized non-native Alewife (Alosa pseudoharengus) in Lakes Michigan and Huron exhibited changes in diet after the invasion of Dreissenids in 1995 (Pothoven and Madenjian 2008). In fact, pervasive shifts in carbon source and trophic position of many species in Lakes Michigan and Ontario have been observed following Round Goby invasions (Paterson et al. 2014; Turschak et al. 2014). The relationship between body size and trophic position for many species has shifted in response to species invasions (Turschak and Bootsma 2015). For long-lived large bodied species that develop slowly and occupy intermediate life history stages for relatively long periods of time, this phenomenon may have important consequences for juvenile survival and reproductive effort. Differences in somatic growth may be expected to affect the onset of maturity according to life history theory, and indeed higher fitness at earlier age in response to higher somatic growth rates has been documented in fish (Hutchings 1993). In long-lived species with late age at maturation, even small increases in growth may significantly shorten the juvenile period and hasten the onset of maturity.
One native Great Lakes species on which the effects of Round Goby and Dreissenid invasions are unclear is the Lake Sturgeon, a long-lived, large-bodied, nearshore benthic consumer. Habitat degradation and overexploitation in the 19th and 20th centuries led to near-complete extirpation of Lake Sturgeon from the Great Lakes (Carlson 1995). Currently there is widespread interest in the recovery of this species across the Great Lakes, and efforts to aid recovery have included fishing closures, state or province-level “threatened” or “endangered” species designations, and stocking programs in the United States and Canada (Chalupnicki et al. 2011; Peterson et al. 2007). Though there are few detailed studies of Lake Sturgeon trophic ecology in the primary literature, available evidence suggests that this species is an opportunistic benthic forager that preys on invertebrates, bivalves, and small fishes (Nilo et al. 2006), and that shifts in diet toward larger prey items occur with increased size (Nilo et al. 2006; Jackson et al. 2002).
Trophic interactions between recent invasive species and Lake Sturgeon populations may affect sturgeon growth and reproduction, as well as interactions among native and previously naturalized predators and prey. This may be especially true in areas where Lake Sturgeon populations comprise a significant proportion of the consumer community biomass, like during spring spawning aggregations in the lower Niagara River (Biesinger et al. 2014). Interactions between Lake Sturgeon and Round Goby invaders may be driven by competition, where Round Goby and Lake Sturgeon compete for Dreissenids and other benthic resources; or by egg predation, where Round Goby consume Lake Sturgeon eggs and reduce recruitment. However, Lake Sturgeon certainly reach a large enough size to opportunistically take both Round Goby and Dreissenid mussels as prey, so it is possible that either or both of these invasive species represent an important prey resource for Lake Sturgeon populations.
Herein, we focus on a relict population of Lake Sturgeon in the lower Niagara River and Lake Ontario, where historical overharvest and habitat destruction nearly extirpated the species by the mid-1900s (Carlson 1995). Since 1998 however, Hughes et al. (2005) and Biesinger et al. (2014) have documented a recovery in this Lake Sturgeon population characterized by strong year classes produced in the late 1990s and high somatic growth rates. The Round Goby invasion reported by Walsh et al. (2007) and Paterson et al. (2014) then represents a transition between two ecosystem states that occurred during an observed Lake Sturgeon recovery period. First observations of Dreissenid mussels in Lake Ontario occurred in 1989 for Dreissena polymorpha and in 1991 for Dreissena bugensis (Mills et al. 1993), whereas Round Goby were first observed in open water monitoring surveys in 2002 (Walsh et al. 2007). Thus, increased recruitment of Lake Sturgeon in the 1990s reported by Hughes et al. (2005) occurred under Dreissenid mussel influence but Round Goby absence from Lake Ontario. Reduced recruitment but high somatic growth rate and adult survival reported during the 2000s (Biesinger et al. 2014) may be related to the recent establishment of the Round Goby. It is important to test whether interspecific interactions might have contributed to these changes in sturgeon demography in order to inform population and ecosystem management and contribute to our understanding of invasive species effects on established food webs.
We investigated the effects of size, age, and time period on the trophic ecology (i.e., trophic position and carbon source) of Lake Sturgeon inferred from the nitrogen (δ15N) and carbon (δ13C) stable isotope content of archived pectoral fin spine samples collected in 1998–2000 (pre-Round Goby) and 2010–2012 (post-Round Goby). Although Round Goby were first observed in western Lake Ontario near the Welland Canal, Ontario in 1998, we consider 2002 to be the operative transition year because this is when round gobies first appeared in annual lake-wide fish community monitoring surveys (Walsh et al. 2007). The survey consists of an annual bottom trawl assessment conducted at fixed stations in late April at depths from 8 to 170 m (O’Gorman et al. 2000). We consider occurrence and persistence of round goby captures in this survey to indicate expansion into Lake Ontario habitats because the lake-wide scale and repeated monitoring through time show that the first occurrence was not an isolated event. Lake Sturgeon pectoral fin spine cross-sections display annual growth rings that can be used to estimate age (Bruch et al. 2009), and though otoliths may produce a more accurate age estimate in older fish, fin spines are preferred because they can be collected non-lethally. Stable isotope values of the most recent growth at the edge of these spines, as well as in discrete growth zones within the spine from the origin (first year of growth) radiating outward, can elucidate age-specific changes in trophic position and carbon sources over time on an individual basis. This approach allowed us to evaluate shifts in diet across a wider range of ages than were encountered at the time of capture during our Lake Sturgeon sampling. Though we know of no similar studies in bony fishes, these same tools have been used to elucidate ontogenetic variation in feeding ecology from continuously-growing skeletal structures of a wide variety of species including sperm whale teeth (Physeter Macrocephalus) (Mendes et al. 2007) and white shark vertebrae (Carcharodon carcharias) (Estrada et al. 2006; Kim et al. 2012). Further, we know of no similar studies that have compared intra-individual variation in trophic position between time periods with different ecosystem states.
We hypothesized that similar to other nearshore Great Lakes fishes, the invasion of the Round Goby has provided a new piscine prey item to Lake Sturgeon, mobilizing previously-sequestered Dreissenid mussel biomass and allowing sturgeon to access higher trophic level prey at younger age and smaller size. This could lead to increased growth, earlier maturation, and potential increased lifetime reproductive output for Lake Sturgeon. We expected an increased rate of trophic level change in juvenile sturgeon and decreased variation across body size in larger sub-adult and adult sturgeon. Herein we evaluate Lake Sturgeon diet and linear models of stable isotope concentration across age and size to test three hypotheses: (1) Lake Sturgeon in the lower Niagara River currently exploit invasive Round Gobies as a prey resource; (2) variation in Lake Sturgeon trophic position across body size among sub-adult and adult individuals decreased after Round Goby invasion; and (3) the rate of increase in Lake Sturgeon trophic position with age, from the first year of growth to the year of capture, increased after the invasion of Round Goby.
Methods
Sample collection
Lake Sturgeon were captured from the lower Niagara River and Lake Ontario near the Niagara River mouth during spring and summer of 1998–2000 (Hughes et al. 2005) and 2010–2012. Fish were captured using gill nets and baited set lines following methods described by Hughes et al. (2005) and Thomas and Haas (2002). Captured fish were tagged with passive integrated transponder (PIT) tags and external floy tags and measured to the nearest mm total length (TL) and 0.5 kg weight. A 1 cm section of the leading pectoral fin spine was taken from the left pectoral fin approximately 1 cm distal to the basal propterygium of each fish for age estimation. All fin spines were air dried at 23 °C in a fume hood for 24–48 h and archived in paper envelopes until analysis. Pectoral fin spines were cross-sectioned with a low speed saw, mounted on glass slides, and aged under transmitted light at 1.25–10× magnification. Ages were estimated by 2 experienced readers independently. We limited inclusion of spine samples in this study to only those with high agreement between readers: either no difference in estimated age, or a difference of 1 year. Since we found no systematic bias in reader estimates (unpublished data) and since there was little or no difference between age estimates in our analysis set, we used ages assigned by a single reader for analysis. A majority of fish from each time period that were included in this study were age 14 or younger, within the range of ages Bruch et al. (2009) showed could be reliably estimated using pectoral fin spines.
We were interested primarily in assessing the interaction between age or size and the time periods associated with pre-Round Goby and post-Round goby invasion system states. Though we did not have baseline isotopic information (i.e. δ15N and δ13C of the first trophic level) prior to Round Goby invasion in this system, we assumed that lake sturgeon were exposed to similar isotopic baselines within a time period because the time periods differed predominantly in the occurrence of Round Goby. We used stable isotopes to help quantify contemporary Lake Sturgeon diet in the lower Niagara River and nearby Lake Ontario food web. Round Goby, Dreissenid mussels, Amphipoda, Gastropoda, and Diptera were collected from nearshore and offshore sites for analysis of δ15N and δ13C during 2013. Samples were collected from the Niagara River using dip nets and a petit ponar dredge in 2–5 m water depths near the mouth, and nearshore Lake Ontario samples were collected using petit ponar at 3–15 m depths. Samples were bagged separately by taxon and frozen at −20 °C until analysis. In 2014, Lake Sturgeon diets were quantified using gastric lavage methods adapted from Haley (1998). Fish were placed in a sling and anesthetized with a solution of Niagara River water and tricaine methanesulfonate (MS-222) pumped over the gills. An induction dose of 200 mg/L buffered with sodium bicarbonate was used initially, followed by a maintenance dose of 87 mg/L buffered MS-222 during lavage. A modified 7 L garden sprayer was used as a water delivery device attached to 6 mm outer diameter aquarium tubing of 1.5 m length. The tube was inserted into the esophagus, through the alimentary canal, and into the stomach where water was carefully pumped causing the fish to regurgitate and expel water and food particles. Regurgitated water was sieved through a 500 μm mesh screen and collected material was preserved in a 10% buffered formalin solution for identification. Following Bowen (1996), stomach contents were identified to the lowest practical taxonomic group (e.g. Dreissena, and Neogobius melanostomus), and quantified by frequency of occurrence, percent composition by number, and percent composition by wet weight.
Fin ray preparation
We quantified δ15N and δ13C of archived fin spines from fish collected in 1998–2000 by Hughes et al. (2005) and 2010–2012, as well as baseline food web items collected in 2013. Spine material immediately distal to the thin sections used for age estimation was used for isotopic analysis. Pectoral fin spine material was sub-sampled using a Brasseler 1/4 RA round carbide drill bit on a mounted drill system (Jensen Microdrill 334E500) to separate epithelial tissue (hereafter, fin tissue) on the outer surface of pectoral spines, spine surface tissue (most recent bone growth), and discrete within-spine growth zones. After sample material was taken from discrete growth zones, minimum, maximum, and median age of growth increments were assigned to each discrete growth zone sample under reflected light at 1.25–10× magnification. Reflected light was used in lieu of transmitted light due to sample thickness. Samples that included more than 3 growth zones were excluded from further analysis.
Stable isotope analysis
Food web samples for stable isotope analysis were freeze dried and homogenized to a powder using a glass mortar and pestle. As lipids can confound interpretation of δ13C (Post 2002), lipids were removed from whole fish and invertebrate baseline food web samples using a 2:1 chloroform:methanol solvent similar to the Bligh and Dyer (1959) method by adding 2 mL of solvent to each sample, vortexed for 30 s and then placed in a hot water bath (approximately 30 °C) for 24 h and decanting. The process was repeated and samples were then dried under a fume hood for at least 48 h to remove solvent. Sturgeon spine and fin samples do not have lipids (C:N < 3.5) and were not lipid extracted. Powdered samples were weighed into 5 × 9 mm tin capsules on a microbalance with weights between 400 and 600 µg for prey samples and 600–800 µg for spines and δ13C and δ15N were determined using a Thermo Finnigan Delta V Advantage Mass spectrometer and Thermo Finnigan Conflo IV gas interface (Thermo Finnigan, San Jose, CA, USA) equipped with a Costech 4010 Elemental Combustion System (Costech, Valencia, CA, USA).
Standard delta notation (δ) was used to express δ15N and δ13C in parts per thousand (‰) differences from a standard material as follows:
where R = 13C/12C or 15N/14N (Fry 1991; Hobson and Clark 1992). Pee Dee Belemnite for carbon and atmospheric nitrogen were used as standard reference materials. National Institute of Standards, Technology (NIST) standards, and internal lab standards were used to calculate the precision and accuracy of analysis. NIST standards analyzed to check instrument accuracy were NIST 8573 (l-glutamic acid), 8548 and 8547 (both ammonium sulphate) for δ15N (n = 45) and for δ13C (n = 45) were NIST 8573 (l-glutamic acid) and 8542 (sucrose), and had a difference of <0.2‰ for δ15N and <0.1‰ for δ13C from the certified values. The analytical precision based on NIST 1577c (n = 61) and the internal lab standard Tilapia (n = 68) had a standard deviation of <0.2‰ for δ15N and <0.1‰ for δ13C. All stable isotope analysis were carried out at the Chemical Tracers Laboratory, University of Windsor.
Fin tissue has been shown to be a good alternative to muscle tissue for determination of dietary δ13C and δ15N in fish (Kelly et al. 2006), but others have found that sturgeon spine tissue may not share that utility (DeVries and Schramm 2015). We assumed fin tissue was an accurate representation of whole body stable isotope content in Lake Sturgeon and used values from these tissues for analysis of Lake Sturgeon stable isotope fractions at time of capture. To test for differences in isotopic signature among tissue types, variation of δ13C and δ15N in the most recent bone growth from spine samples were compared to fin sample δ13C and δ15N using Pearson’s correlation (r). Relationships between fin tissue and most recent tissue growth of spines were significant for both δ13C (r = 0.549) and δ15N (r = 0.760). Though these relationships are not as highly correlated as those reported in Kelly et al. (2006), correlations still suggest that isotopic signature of spine tissue varies with that of fin tissue in Lake Sturgeon.
Statistical analysis
We first investigated differences in ontogenetic relationships of Lake Sturgeon stable nitrogen and carbon fractions between pre- and post-Round Goby invasion periods (hereafter referred to as “time period”). Because no baseline food-web samples were available from the lower Niagara River in 1998–2000, trophic level could not be estimated and analyses were conducted on raw isotope fractions only. Variation in stable nitrogen and carbon isotope fractions across Lake Sturgeon TL at time-of-capture and between time periods was investigated using general linear model analysis to infer changes in trophic position and carbon source, respectively. Separate analyses were conducted for fin tissue variation in δ13C and δ15N. For each stable isotope response variable, we evaluated 5 linear models constructed from intercept, total length (L), time period (Z), and L × Z effect terms using R version 3.3.0 (R Core Team 2016), to test whether size-dependent variation in stable isotope fractions changed between time periods. Models for δ13C and δ15N response variables included (1) an intercept only model, (2) a body size model, (3) a time period model, (4) a body size and time period model, and (5) a body size, time period, and L × Z interaction model. The model with the lowest AICC score was considered the best fitting model given our data (Burnham and Anderson 2002), and our hypothesis was that the best fitting model would include the L × Z interaction term indicating differences in ontogeny by time period.
We then investigated intra-individual variation in stable nitrogen and carbon fractions across Lake Sturgeon age and time period. Our age variable was defined as the median age of each drilled spine sample. The time period variable corresponded to the estimated year of growth, calculated by subtracting the drilled sample’s median age from the fish’s age at capture. We then categorized each drilled sample’s year of growth into either pre-Round Goby (≤2002) or post-Round Goby (>2002) time periods based on first observations of round gobies in Lake Ontario trawl surveys (Walsh et al. 2007). Therefore, age, time period, δ13C, and δ15N variables were all drilled sample-level variables nested within individual fish such that fish captured in 2010–2012 that were alive in both time periods yielded samples from both time periods. For analysis of δ15N across age and time period, the age covariate was natural log-transformed to account for an apparent non-linear relationship between δ15N and age. We used linear mixed-effects models to evaluate the fixed effects of age and time on δ13C and δ15N while accommodating repeated measurements of each fish. We evaluated 5 models for each stable isotope (δ13C and δ15N) to test whether age-dependent variation in stable isotope fractions changed between time periods. These models were constructed from the fixed effect terms: intercept (M), age (A), time period of growth (Z), and an age by time interaction (A × Z); with a random intercept term for each fish. Mixed effects models were constructed and evaluated using the “nlme” package (Pinheiro et al. 2015) in R version 3.3.0 (R Core Team 2016). Models for δ13C and δ15N response variables included (1) an intercept only model, (2) an age model, (3) a time period model, (4) an age and time period model, and (5) an age, time period, and A × Z interaction model. Models were first estimated using maximum likelihood estimation (ML), and comparisons between models were conducted using AICC as in our fin tissue linear model analysis. Once we arrived at a best-fitting model, parameters were estimated using restricted ML to best illustrate the main effects on Lake Sturgeon trophic position and carbon source. Our hypothesis that shifts in Lake Sturgeon trophic position would occur at earlier age after Round Goby invasion would be supported if the best fitting models of δ13C and δ15N contained an A × Z interaction term indicating accelerated change in δ13C or δ15N-at-age (i.e. an A × Z term with the same sign as the A term) in post-Round Goby time periods.
Results
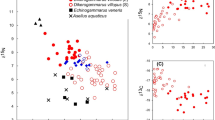
Lake Sturgeon included in the analysis averaged 1290 ± 60 SE mm TL (range: 854–1573) in 1998–2000, and 1216 ± 42 SE mm TL (range: 760–1639) in 2010–2012. Age estimates for these fish averaged 14.7 ± 1.7 SE years old (range: 6–27) in 1998–2000, and 13.1 ± 1.4 SE years (range: 5–31) in 2010–2012. In 2014, we examined the stomach contents of 33 Lake Sturgeon from the lower Niagara River, of which 24 contained prey. Lake Sturgeon in our diet analysis averaged 1435 ± 28 SE mm TL, and there was no significant difference in fish size between fish with empty and non-empty stomachs (t = 0.867, p = 0.4). Lake Sturgeon with empty stomachs were omitted from further analyses. Round Goby was the most important prey item by weight for Lake Sturgeon in 2014, comprising over 86% of the total diet. Though Amphipoda was the most numerically dominant diet item (91%) and occurred in the highest proportion of stomachs (88%), round goby was the second most dominant in percentage by number (4%) and by occurrence (48%) (Table 1). Lake Sturgeon were higher than Round Goby in δ15N and similar in δ13C, which supports our diet results under the assumption that δ15N (but not δ13C) is enriched from prey to predator: Round Goby were more important to Lake Sturgeon diet and growth than the available invertebrate prey in this system (Fig. 1). Lake Sturgeon and Round Goby δ13C were intermediate between Dreissenids and Gastropods. Values of δ15N were similar among Lake Sturgeon fin and spine tissues, but spine δ13C was higher than fin tissue δ13C (Table 2). The mismatch in spine δ13C was likely due to high carbonate concentration in spine tissue, which has been observed in other high-carbonate tissue such as fish scales (Perga and Gerdeaux 2003). However, the remainder of our analysis contained no cross-tissue type comparisons so no correction for the effect of carbonates on stable isotopes was necessary.
Best fit general linear models investigating the effects of Lake Sturgeon total length, sampling period, and their interaction on δ15N and δ13C in fin tissues included the interaction term for length × time period (Table 3). The best model for δ15N and for δ13C indicated that there was only a very small effect of total length on δ13C or δ15N in the post-Round Goby time period, whereas there was much stronger variation in stable isotopes across length in the pre-Round Goby time period (Table 4; Fig. 2).
Model results for size dependence (mm total length) of fin tissue δ15N (panel a) and δ13C (panel b) between fish captured in 1998–2000 and in 2010–2012 (n = 45). Filled circles and solid lines denote Lake Sturgeon captured in 1998–2000, open circles and blue dashed lines denote Lake Sturgeon captured in 2010–2012
Model selection by AICC resulted in the inclusion of intercept, age, time period, and age × time period effects in our best model for spine tissue δ15N but not for δ13C (Table 3). The age × time period interaction effect in the best mixed-effects model for spine δ15N suggested that fish captured in the post-Round Goby time period increased in trophic level at a faster rate with age than fish captured prior to Round Gobies (Table 5; Fig. 3a). Because the independent variable age was natural log-transformed but the response variable was not, this interaction term suggests that for any given age interval, the increase in δ15N was 86% greater in the post-Round Goby period than before the Round Goby invasion (95% confidence interval: 26.1–147.7%). For δ13C spine chronology analysis, there was no apparent non-linearity with age, so the age covariate was left untransformed. The δ13C model with the lowest AICC value included intercept and age main effects, though this result is equivocal in that 3 of the 5 model hypotheses had ΔAIC scores of <2. The full model with the age × time period interaction term was not among the top models. The best model suggests that Lake Sturgeon δ13C decreased at a 0.028‰ * year−1 faster rate in the post-Round Goby period versus the pre-Round Goby period, though the standard deviation is high, and the effect would be considered non-significant at α = 0.05 (Table 5; Fig. 3b). Again, the similarity in AICC values of competing models suggests that no clearly dominant δ13C pattern exists across the variables evaluated in the spine analysis (Table 3).
Results of linear mixed-effects model analysis of isotope fractions in lake sturgeon spine cross-sections versus age, time period, and their interaction. Panel a displays δ15N results (126 observations, 39 fish) and panel b displays δ13C results (126 observations, 39 fish). Model results using a natural log transformation of the age variable are plotted on an arithmetic scale in panel a. Open circles denote post-Round Goby time period fraction-at-age and filled circles denote pre-Round Goby time period fraction-at-age. In panel a, the black solid line and blue dashed line illustrate the final model main effect (group average) for differences in pre-Round Goby and post-Round Goby time periods, respectively. Panel b displays a single dotted black line for all samples, as our best model suggested no differences by time period
Discussion
Niagara River Lake Sturgeon δ15N increased with size and age in both time periods but the rate of increase changed between 1998–2000 and 2010–2012, coincident with Round Goby invasion and exploitation in the Lake Sturgeon diet. Round Goby have very likely become the most important prey item for Niagara River Lake Sturgeon based on diet analysis, δ15N, and δ13C (Fig. 1; Table 1). Since 2002, Lake Sturgeon accessed a higher trophic level at a younger age and smaller size than prior to the invasion (Figs. 2, 3). Our analysis of spine growth chronologies was able to detect this increased rate of ascension through the food web at younger ages, but did not contain enough data from older fish to fully evaluate the growth chronologies of older, larger fish. Interestingly, variance in δ15N at older age in the post-Round Goby time period appears reduced compared with similarly aged fish in the pre-Round Goby period (Fig. 3a). This suggests that Lake Sturgeon in this system may have exploited a higher diversity of prey items prior to Round Goby invasion, resulting in a wider inter-individual isotopic variance but a lower population-level δ15N relative to the post-invasion time period. Our analysis of ontogeny in spine tissue failed to detect variation in δ15N with size for post-invasion fish because Lake Sturgeon had evidently begun accessing higher trophic level prey at sizes below those vulnerable to our sampling gear (Table 1).
In lake ecosystems such as the Great Lakes, variation in δ13C is generally considered reflective of carbon sources from pelagic (lower δ13C from higher proportion of suspended algal productivity) versus near-shore habitats (higher δ13C from recycling of algal carbon and allochthonous inputs) (Turschak and Bootsma 2015). Since the Niagara River is a relatively small connecting channel between two large lake ecosystems, Lake Erie and Lake Ontario, we assume that organisms found there are primarily subject to lake processes.
We then conclude that Lake Sturgeon used an increasingly pelagic carbon source based on lower δ13C with increased age and size. Unlike the results for δ15N, variation in δ13C in the spine analysis showed a weak pattern of change in δ13C with age in 2010–2012 versus 1998–2002, despite significant differences in δ13C across size between groups in the fin tissue analysis. Differences in diet between periods produced shifts in Lake Sturgeon carbon sourcing at smaller sizes and younger ages, and reduced variability of δ13C among the larger-sized fish evaluated in the fin tissue analysis. This earlier pelagic carbon sourcing may signify a more rapid Lake Sturgeon growth rate and earlier emigration from more nearshore foraging environments than in previous time periods. In addition to increased Lake Sturgeon growth leading to greater lifetime reproductive effort, this pattern may also release nearshore benthic communities from predation pressure as Lake Sturgeon specialize on Round Goby prey and forage further offshore at a younger age. Notably, there was a subset of fish from both time periods that exhibited low δ13C (i.e. −21.2 ± 0.4 SE‰) during the formation of their first growth bands compared to other initial values. These values could be excluded as outliers in our analysis, perhaps because they may reflect variation in post-larva settlement location or the contribution of multiple spawning populations or sites with different background δ13C in the environment. If these points are excluded from the analysis, the best model contains an age × time period interaction term but there is again little difference among top models, corroborating our results that there is little to no difference in δ13C change across age between time periods.
Lake Sturgeon may be expected to occupy multiple niches throughout life as resource use optima change with size-dependent foraging ability and mortality risk (sensu Werner and Gilliam 1984). Consistent with this expectation, Lake Sturgeon spine tissue δ15N varied widely across the range of ages and sizes we observed in our study (Figs. 2, 3). This variation was explained by both size and age of Lake Sturgeon, suggesting that access to higher trophic level prey in our system is dependent on predator body size as it is in other species (Estrada et al. 2006; Vander Zanden et al. 2000). We suggest that the invasion of round goby in Lake Ontario and the lower Niagara River may have resulted not only in increased prey fish consumption, but also in higher somatic growth rates for juvenile and sub-adult Lake Sturgeon, as has been observed in Smallmouth Bass in Lake Erie (Crane and Einhouse 2016). An alternative explanation could be that our results reflect a new differential mortality regime, perhaps driven by increased competition for invertebrate prey between Round Goby and non-piscivorous Lake Sturgeon, a possibility that may warrant further investigation. Such a recruitment bottleneck could lead to increased growth rates due to reduced intraspecific competition among surviving juvenile Lake Sturgeon, but intraspecific competition is not expected to be strong in this system as Lake Sturgeon abundance appears quite low in both time periods compared to historical accounts. The dynamics of δ13C differed for Lake Sturgeon size between time periods, not for age, indicating that round goby invasion differentially affected δ13C and δ15N dynamics in Lake Sturgeon. Variation in δ13C was highest among young fish in our spine analysis (Fig. 3) which may be explained by variation in settlement location of young juveniles (Lugendo et al. 2006), though we cannot rule out other effects such as immigration from habitats across Lake Ontario and its tributaries which may differ in δ13C. The trend toward declining δ13C with size and age for older, larger individuals (Figs. 2, 3) supports an expectation that Lake Sturgeon select habitats further away from the littoral zone with increased age and size (Peterson et al. 2007).
In Lake Erie, Johnson et al. (2005) demonstrated that the invasion of Round Goby acted as an energetic pathway through which high biomass accumulated by Dreissenid mussels could now be exploited by the consumer community. By demonstrating that Lake Sturgeon in Lake Ontario showed increased trophic position from pre- to post-Round Goby invasion concomitant with high representation of Round Goby in their diets, our results support the findings of Johnson et al. (2005). Furthermore, our results suggest that Lake Sturgeon, which at large body size have few predators of their own, may act as a sink for the Dreissenid energetic pathway recently mobilized by Round Goby. In Lake Erie, the Round Goby invasion exerted top-down effects on Dreissenids leading to a density-dependent feedback on Round Goby population growth: apparently becoming food limited by 2002 (Barton et al. 2005). If under Round Goby predation pressure Dreissenid mussels maintain high production in the nearshore zone, then overall energy availability for Lake Sturgeon and other nearshore-associated fishes may remain high.
Though our study does not relate invasive species effects to a numerical response from Lake Sturgeon populations, it does show that the time period associated with Round Goby establishment in the Lake Ontario system corresponds to Lake Sturgeon reaching a higher trophic level at younger age and smaller size than Lake Sturgeon captured prior to the Round Goby invasion. Shifts in diet to larger, more energy-dense higher-trophic level prey is generally associated with higher fitness in fishes (Werner and Gilliam 1984), as exploitation of these resources confers a survival advantage over smaller conspecifics. In other fishes, this type of shift is often observed as a switch from invertivory to piscivory early in life (Post 2003). Largemouth Bass, for example, have been shown to exhibit increased growth and survival rates once switching to fish prey from invertebrate prey during their first year of life (Olson 1996). To generalize to other vertebrates, King et al. (2006) reported increased growth and body size in Lake Erie water snake (Nerodia sipedon insularum) after Round Goby invasion into that water body, suggesting that as water snakes increased their consumption of Round Goby they experienced higher growth rates. With the evidence we present here that Lake Sturgeon rely heavily on Round Goby as a prey source, we may expect that the availability of Round Goby prey has a positive effect on adult Lake Sturgeon growth and survival, if not on population size. Future efforts should focus on whether individual fitness benefits of Round Goby consumption translate to increased population growth, or whether these benefits are offset by other factors not investigated herein, including increased egg predation by Round Gobies or negative effects of interspecific competition at small Lake Sturgeon body size.
Predator–prey relationships between native and non-native species are an important means by which invasive species affect food webs and ecosystems. Species invasions have been shown to vastly alter trophic dynamics, such as the distinct trophic cascade caused by the invasion of the opossum shrimp (Mysis diluviana) in Flathead Lake, Montana in 1996 (Ellis et al. 2011), or in the shift in the Lake Huron fish community structure in the 2000s following a variety of species invasions (Madenjian et al. 2013). However, not all invasive species effects are negative, and in fact some studies have shown that invasive species can have equivocal, if not positive, influences on native species (e.g. Steinhart et al. 2004a, Crane and Einhouse 2016). Negative and positive interactions between many native and non-native species in the Great Lakes have been documented following decades of species introductions beginning as early as the 1800 s (Mills et al. 1993), but the overall effect of these invaders is often not readily apparent. For example, Alewife have a mix of positive and negative impacts on Lake Trout in the Great Lakes where adult Lake Trout have exploited Alewife as a high percentage of their diets (Jacobs et al. 2010; Miller and Holey 1992), but Lake Trout also suffer reduced larval survival due to Alewife predation on pelagic larvae and thiamine deficiency of Lake Trout eggs produced by adults that rely on Alewife for prey (Krueger et al. 1995; Madenjian et al. 2008). Evidence of increased Lake Trout recruitment and production following recent declines in Alewife abundance in Lake Huron suggests that the net effect of Alewife-Lake Trout interactions is negative (He et al. 2012), illustrating how the net influence of an invasive species-native species interaction can be comprised of a combination of positive and negative interactions. An important unknown that remains untested after our study is the extent to which negative effects of Round Goby may impair successful recruitment through egg predation or competitive exclusion of young Lake Sturgeon. It seems especially possible that Round Goby could represent a significant predation risk for Lake Sturgeon eggs, as in Smallmouth Bass (Steinhart et al. 2004b) but without the benefit of adult nest guarding behavior. Future work should focus on evaluating potential negative interactions between Round Gobies and Lake Sturgeon.
References
Balshine S, Verma A, Chant V, Theysmeyer T (2005) Competitive interactions between round gobies and logperch. J Great Lakes Res 31:68–77
Barbiero RP, Tuchman ML (2004) Changes in the crustacean communities of Lakes Michigan, Huron, and Erie following the invasion of the predatory cladoceran Bythotrephes longimanus. Can J Fish Aquat Sci 61:2111–2125
Barton DR, Johnson RA, Campbell L, Petruniak J, Patterson M (2005) Effects of round gobies (Neogobius melanostomus) on dreissenid mussels and other invertebrates in eastern Lake Erie, 2002–2004. J Great Lakes Res 31:252–261
Biesinger Z, Gorsky D, Jacobs GR, Sweka JA, Webb MAH, Talbott M (2014) Population assessment of Lake Sturgeon in the lower Niagara River. In: New York State Department of Environmental Conservation 2013 Annual Report to the Great lakes Fishery Commission’s Lake Ontario Committee, Windsor, Ontario, 26–27 March 2014
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Bowen SH (1996) Quantitative description of diet. In: Murphy BR, Willis DW (eds) Fisheries techniques, 2nd edn. American Fisheries Society, Bethesda, pp 513–532
Bruch RM, Campana SE, Davis-Foust SL, Hansen MJ, Janssen J (2009) Lake sturgeon age validation using bomb radiocarbon and known-age fish. Trans Am Fish Soc 138:361–372
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, New York
Capps KA, Flecker AS (2013) Invasive aquarium fish transform ecosystem nutrient dynamics. Proc R Soc B Biol Sci 280:20131520
Carlson D (1995) Lake Sturgeon waters and fisheries in New York State. J Great Lakes Res 21(1):35–41
Chalupnicki MA, Dittman DE, Carlson DM (2011) Distribution of Lake Sturgeon in New York: 11 years of restoration management. Am Midl Nat 165:364–371
Crane DP, Einhouse DW (2016) Changes in growth and diet of smallmouth bass following invasion of Lake Erie by the round goby. J Great Lakes Res 42(2):405–412
DeVries RJ, Schramm HL (2015) Similarities and differences in 13C and 15N stable isotope ratios in two non-lethal tissue types from shovelnose sturgeon Scaphirhynchus platorynchus (Rafinesque, 1820). J Appl Ichthyol 31:474–478
Dorcas ME, Willson JD, Reed RN, Snow RW, Rochford MR, Miller MA, Meshaka WE, Andreadis PT, Mazzotti FJ, Romagosa CM, Hart KM (2012) Severe mammal declines coincide with proliferation of invasive Burmese pythons in Everglades National Park. Proc Natl A Sci USA 109:2418–2422
Ellis BK, Stanford JA, Goodman D, Stafford CP, Gustafson DL, Beauchamp DA, Chess DW, Craft JA, Deleray MA, Hansen BS (2011) Long-term effects of a trophic cascade in a large lake ecosystem. Proc Natl A Sci USA 108:1070–1075
Estrada JA, Rice AN, Natanson LJ, Skomal GB (2006) Use of isotopic analysis of vertebrae in reconstructing ontogenetic feeding ecology in white sharks. Ecology 87:829–834
Fry B (1991) Stable isotope diagrams of freshwater food webs. Ecology 72:2293–2297
Haley N (1998) A gastric lavage technique for characterizing diets of sturgeons. N Am J Fish Manage 18:978–981
He JX, Ebener MP, Riley SC, Cottrill A, Kowalski A, Koproski S, Mohr L, Johnson JE (2012) Lake Trout status in the main basin of Lake Huron, 1973–2010. N Am J Fish Manag 32:402–412
Hecky RE, Smith RE, Barton DR, Guildford SJ, Taylor WD, Charlton MN, Howel T (2004) The nearshore phosphorus shunt: a consequence of ecosystem engineering by dreissenids in the Laurentian Great Lakes. Can J Fish Aquat Sci 61(7):1285–1293
Higgins SN, Vander Zanden MJ (2010) What a difference a species makes: a meta-analysis of dreissenid mussel impacts on freshwater ecosystems. Ecol Monogr 80:179–196
Hobson KA, Clark RG (1992) Assessing avian diets using stable isotopes I: turnover of 13C in tissues. Condor 1992:181–188
Hughes TC, Lowie CE, Haynes JM (2005) Age, growth, relative abundance, and scuba capture of a new or recovering spawning population of lake sturgeon in the lower Niagara River, New York. N Am J Fish Manag 25:1263–1272
Hutchings JA (1993) Adaptive life histories effected by age-specific survival and growth rate. Ecology 74(3):673–684
Jackson JR, VanDeValk AJ, Brooking TE, VanKeeken OA, Rudstam LG (2002) Growth and feeding dynamics of lake sturgeon, Acipenser fulvescens, in Oneida Lake, New York: results from the first five years of a restoration program. J Appl Ichthyol 18:439–443
Jacobs GR, Madenjian CP, Bunnell DB, Holuszko JD (2010) Diet of lake trout and burbot in northern Lake Michigan during spring: evidence of ecological interaction. J Great Lakes Res 36:312–317
Janssen J, Jude DJ (2001) Recruitment failure of mottled sculpin Cottus bairdi in Calumet Harbor, Southern Lake Michigan, Induced by the newly introduced Round Goby Neogobius melanostomus. J Great Lakes Res 27:319–328
Johnson TB, Bunnell DB, Knight CT (2005) A potential new energy pathway in central Lake Erie: the Round Goby connection. J Great Lakes Res 31:238–251
Kelly MH, Hagar WG, Jardine TD, Cunjak RA (2006) Nonlethal sampling of sunfish and slimy sculpin for stable isotope analysis: how scale and fin tissue compare with muscle tissue. N Am J Fish Manag 26:921–925
Kim SL, Tinker MT, Estes JA, Koch PL (2012) Ontogenetic and among-individual variation in foraging strategies of northeast pacific white sharks based on stable isotope analysis. PLoS ONE 7:e45068. doi:10.1371/journal.pone.0045068
King RB, Ray JM, Stanford KM (2006) Gorging on gobies: beneficial effects of alien prey on a threatened vertebrate. Can J Zool 84:108–115
Krueger CC, Perkins DL, Mills EL, Marsden JE (1995) Predation by alewives on lake trout fry in Lake Ontario: role of an exotic species in preventing restoration of a native species. International conference on restoration of Lake Trout in the Laurentian Great Lakes 21, Supplement 1:458–469
Lugendo BR, Nagelkerken I, Van Der Velde G, Mgaya YD (2006) The importance of mangroves, mud and sand flats, and seagrass beds as feeding areas for juvenile fishes in Chwaka Bay, Zanzibar: gut content and stable isotope analyses. J Fish Biol 69(6):1639–1661
Madenjian CP, O’Gorman R, Bunnell DB, Argyle RL, Roseman EF, Warner DM, Stockwell JD, Stapanian MA (2008) Adverse effects of alewives on Laurentian Great Lakes fish communities. N Am J Fish Manag 28:263–282
Madenjian CP, Stapanian M, Witzel L, Einhouse D, Pothoven S, Whitford H (2011) Evidence for predatory control of the invasive Round Goby. Biol Invasions 13:987–1002
Madenjian CP, Rutherford ES, Stow CA, Roseman EF, He JX (2013) Trophic Shift, Not Collapse. Environ Sci Technol 47:11915–11916
Mendes S, Newton J, Reid R, Zuur A, Pierce G (2007) Stable carbon and nitrogen isotope ratio profiling of sperm whale teeth reveals ontogenetic movements and trophic ecology. Oecologia 151:605–615
Miller MA, Holey ME (1992) Diets of Lake Trout inhabiting nearshore and offshore Lake Michigan environments. J Great Lakes Res 18:51–60
Mills EL, Leach JH, Carlton JT, Secor CL (1993) Exotic species in the Great Lakes: a history of biotic crises and anthropogenic introductions. J Great Lakes Res 19:1–54
Nalepa TF, Fanslow DL, Lang GA (2009) Transformation of the offshore benthic community in Lake Michigan: recent shift from the native amphipod Diporeia spp. To the invasive mussel Dreissena rostriformis bugensis. Freshw Biol 54:466–479
Nilo P, Tremblay S, Bolon A, Dodson J, Dumont P, Fortin R (2006) Feeding ecology of juvenile lake sturgeon in the St. Lawrence River System. Trans Am Fish Soc 135:1044–1055
Olson MH (1996) Ontogenetic niche shifts in largemouth bass: variability and consequences for first-year growth. Ecology 77:179–190
O'Gorman R, Elrod JH, Owens RW, Schneider CP, Eckert TH, Lantry BF (2000) Shifts in depth distributions of alewives, rainbow smelt, and age-2 lake trout in southern Lake Ontario following establishment of dreissenids. Trans Am Fish Soc 129(5):1096–1106
Ozersky T, Evans DO, Barton DR (2012) Invasive mussels alter the littoral food web of a large lake: stable isotopes reveal drastic shifts in sources and flow of energy. PLoS ONE 7:e51249
Paterson G, Rush SA, Arts MT, Drouillard KG, Haffner GD, Johnson TB, Lantry BF, Hebert CE, McGoldrick DJ, Backus SM, Fisk AT (2014) Ecological tracers reveal resource convergence among prey fish species in a large lake ecosystem. Freshw Biol 59:2150–2161
Perga ME, Gerdeaux D (2003) Using the δ13C and δ15N of whitefish scales for retrospective ecological studies: changes in isotope signatures during the restoration of Lake Geneva, 1980–2001. J Fish Biol 63:1197–1207
Peterson D, Vecsei P, Jennings C (2007) Ecology and biology of the Lake Sturgeon: a synthesis of current knowledge of a threatened North American Acipenseridae. Rev Fish Biol Fish 17:59–76. doi:10.1007/s11160-006-9018-6
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2015) nlme: linear and nonlinear mixed effects models. R package version 3.1-119. http://CRAN.R-project.org/package=nlme
Post DM (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83:703–718
Post DM (2003) Individual variation in the timing of ontogenetic niche shifts in largemouth bass. Ecology 84:1298–1310
Pothoven SA, Madenjian CP (2008) Changes in consumption by alewives and lake whitefish after dreissenid mussel invasions in Lakes Michigan and Huron. N Am J Fish Manag 28:308–320
R Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org/
Steinhart GB, Stein RA, Marschall EA (2004a) High growth rate of young-of-the-year smallmouth bass in Lake Erie: a result of the Round Goby invasion? J Great Lakes Res 30:381–389
Steinhart GB, Marschall EA, Stein RA (2004b) Round Goby predation on smallmouth bass offspring in nests during simulated catch-and-release angling. Trans Am Fish Soc 133:121–131
Thomas MV, Haas RC (2002) Abundance, age structure, and spatial distribution of lake sturgeon, Acipenser fulvescens, in the St Clair System. J Appl Ichthyol 18(4):495–501
Turschak BA, Bootsma HA (2015) Lake Michigan trophic structure as revealed by stable C and N isotopes. J Great Lakes Res. doi:10.1016/j.jglr.2015.04.004
Turschak BA, Bunnell D, Czesny S, Höök TO, Janssen J, Warner D, Bootsma HA (2014) Nearshore energy subsidies support Lake Michigan fishes and invertebrates following major changes in food web structure. Ecology 95:1243–1252
Vander Zanden MJ, Casselman JM, Rasmussen JB (1999) Stable isotope evidence for the food web consequences of species invasions in lakes. Nature 401:464–467
Vander Zanden MJ, Shuter BJ, Lester NP, Rasmussen JB (2000) Within-and among-population variation in the trophic position of a pelagic predator, lake trout (Salvelinus namaycush). Can J Fish Aquat Sci 57(4):725–731
Vilà M, Espinar JL, Hejda M, Hulme PE, Jarošík V, Maron JL, Pergl J, Schaffner U, Sun Y, Pyšek P (2011) Ecological impacts of invasive alien plants: a meta-analysis of their effects on species, communities and ecosystems. Ecol Lett 14:702–708
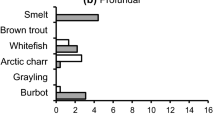
Walsh MG, Dittman DE, O’Gorman R (2007) Occurrence and food habits of the Round Goby in the Profundal Zone of Southwestern Lake Ontario. J Great Lakes Res 33:83–92
Werner EE, Gilliam JF (1984) The ontogenetic niche and species interactions in size-structured populations. Annu Rev Ecol Syst 15:393–425
Acknowledgements
This manuscript was much improved by the helpful comments provided by John Sweka, Mike Millard, and four anonymous reviewers. Brian Layton, Zy Biesinger, Zeb Woiak, Jonah Withers, and many others helped assist with fieldwork and sample processing. Mention of specific products does not constitute endorsement by the U.S. Government. This study was funded by the Great Lakes Restoration Initiative. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jacobs, G.R., Bruestle, E.L., Hussey, A. et al. Invasive species alter ontogenetic shifts in the trophic ecology of Lake Sturgeon (Acipenser fulvescens) in the Niagara River and Lake Ontario. Biol Invasions 19, 1533–1546 (2017). https://doi.org/10.1007/s10530-017-1376-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-017-1376-6