Abstract
Aquatic invasive species can affect food web structure, native fish growth, and production, depending on the traits of the invasive species and the pre-invasion conditions of the ecosystem. Thermal tolerances and behavioral traits can further influence differential exploitation of resources shared between native and invasive species. An unauthorized introduction of redside shiner (Richardsonius balteatus) into reservoirs in the Upper Skagit River, Washington, USA caused concern of potential competition, decreased production, and recruitment of rainbow trout (Oncorhynchus mykiss). We combined bioenergetics modeling and stable isotope analysis with field data to quantify consumption demand of native and invasive fishes and related consumption to the availability of key zooplankton prey. Per capita consumption on Daphnia by redside shiner was low; however, their high abundance imposed considerable demand on prey resources in Ross Lake. Although monthly consumption demand by the fish community was less than 50% of the monthly production and biomass of Daphnia in Ross Lake, the current Daphnia densities and growth of rainbow trout were considerably lower than before the invasion. These reductions correspond to lower annual consumption of Daphnia. Our study provides insight on mechanisms that influence food web impacts of an invasive omnivore in cold-water reservoirs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Freshwater ecosystems are threatened globally and suffering from disproportionate biodiversity loss as they are subjected to many degradative processes that reduce their functionality and ecosystem services (Reid et al., 2019). Reservoir ecosystems are particularly vulnerable to impacts of climate change (Miranda et al., 2020), invasive species (Johnson et al., 2008), and increasing water demand and scarcity (Boretti & Rosa, 2019). Reservoir ecosystems are artificial but can provide critical water storage and cold-water refuge for cool- and cold-water fish species under a warming climate. These systems often pose special management challenges associated with “hybrid” food webs, highlighting the role that food web research can play to support management decisions to identify and maintain productive and resilient ecosystems (Naiman et al., 2012).
Aquatic invasive species can disrupt native food webs and trophic structure through a variety of direct and indirect pathways (Jackson et al., 2017). These impacts are often context dependent, varying by characteristics, such as the invader’s taxon and the ecosystem’s food web structure and resources (Thomsen et al., 2011). Quantitative studies are needed to understand the mechanisms by which these invader-driven food web alterations may affect native species at the individual level to give context to resulting changes observed at the population and community level (Jackson et al., 2017). This knowledge supports choosing appropriate management actions for invasive species control, mitigation, or other restoration actions. For example, trophic overlap or separation can indicate different mechanisms at play: overlap could indicate exploitative competition in resource-poor environments, or it can also be a result of abundant resources. Similarly, trophic separation can result from competitive exclusion from a preferred prey or adaptive niche specialization (Rubenson et al., 2020). The impact of such trophic shifts on individuals thus depends on timing of availability and access to preferred and alternative prey. Knowledge of this trophic structure is needed within the context of resource availability, consumption demand, and observed growth to understand the resulting effects of invasive species on growth and survival of native species and the corresponding population-level effects.
Lakes and reservoirs experience different effects of invasive species compared to riverine habitats because thermal structure often drives fish habitat use in response to physiological constraints, such as metabolism, stress, and growth potential (Magnuson et al., 1979). Thermal stratification can constrain suitable habitat availability (and access to prey) for cold- and cool-water species during the growing season. By contrast, invasive species with higher thermal optima benefit from greater access to abundant prey resources in the epilimnion (Tunney et al., 2012) or refuge from cool- and cold-water predatory fish in thermally stratified waterbodies. Further, changing seasonal thermal structure due to a warming climate (Woolway et al., 2021) is altering spatial and temporal habitat availability and shifting phenological mismatches between consumers and prey which could lead to cascading effects throughout the food web by modifying habitat overlap and species interactions (Winder & Schindler, 2004; Ficke et al., 2007). For reservoirs in particular, evaluation of these thermally driven interactions within the context of how current and future climate conditions and water operations may interact to influence the timing, magnitude, and vertical structure of thermal stratification may inform management decisions (Moreno-Ostos et al., 2008; Feldbauer et al., 2020).
Redside shiner Richardsonius balteatus, a small-bodied minnow, have invaded numerous mid- and high-elevation, temperate lakes and reservoirs and have been associated with declines in salmonid growth rates, prompting some managers to seek eradication methods (Messner & Schoby, 2019; Smith et al., 2021). Negative interactions between redside shiner and rainbow trout Oncorhynchus mykiss have been well studied in a series of Canadian lakes, with declines in growth and production of rainbow trout following introductions of redside shiner (Lindsey, 1950; Larkin & Smith, 1954; Johannes & Larkin, 1961). Additionally, redside shiner have a higher thermal optimum than salmonid species—a potential advantage under warming conditions (Johnson et al., 2023). However, effects of redside shiner invasions on prey resources have been varied (Johannes & Larkin, 1961), and this species coexists naturally in salmonid-dominated systems throughout the western USA, leaving in question the mechanisms by which this species can affect native salmonids in invaded systems and the conditions which allow it. In Washington’s North Cascades mountains, an unauthorized (likely through baitfish release, Rahel & Smith, 2018) introduction of redside shiner in Ross Lake in the early 2000s quickly flourished and spread to downstream Diablo Lake, raising concerns among managers about potential impacts to native juvenile salmonids that may be competing for resources in these reservoirs. Although diet analysis suggested considerable diet overlap between redside shiner and native juvenile salmonids in Ross Lake (Welch, 2012), it is unknown whether redside shiner limit growth and survival of juvenile salmonids through resource competition or other means.
The goal of our study was to understand the role of an invasive omnivore in driving prey supply and altering the growth environment for a native species in a mid-elevation reservoir ecosystem. Specifically, we evaluated the hypothesis that redside shiner are limiting growth and survival of juvenile salmonids through resource competition. Using Ross Lake as a study system, our specific objectives were to (1) quantify seasonal zooplankton prey availability and seasonal consumption by native (rainbow trout) and nonnative (redside shiner) fishes in the reservoir and (2) compare seasonal prey availability and consumption demand to evaluate whether prey supply is a limiting factor for rainbow trout in the reservoirs. We leveraged historical growth and diet data for rainbow trout in the reservoir collected in the 1970s (pre-invasion) to compare annual energy budgets and evaluate changes to rainbow trout growth and energy acquisition between the two studies. These processes were examined within the context of seasonal thermal stratification and the associated constraints on habitat access and availability within the reservoirs.
Methods
Study system
Ross Lake is the largest and deepest of the three reservoirs impounded by hydroelectric projects in the upper Skagit River, WA (elevation at full pool = 489 m above sea level, storage at full pool = 1.78 km3, max depth = 116 m, mean depth = 37 m), extending northward from Ross Dam for approximately 37 km, just beyond the border with British Columbia at full pool (Fig. 1). Ross Lake is ultraoligotrophic (Total Dissolved Phosphorus < 2 µg/l), generally clear (Turbidity < 1.0 Nephelometric Turbidity Units) and shows strong thermal stratification from around June until October when the thermocline deepens and begins to destratify. Peak summer surface temperatures are 18–22 °C, depending on the year and region of the lake (Fig. 2). In addition to the seasonal thermal changes, Ross Lake is drawn down between 16 and 25 m every year during the fall and winter before refilling in the spring, and drawdowns in recent years have reached 40 m for maintenance purposes (Fig. S1). Native fish species include rainbow trout, bull trout Salvelinus confluentus, and Dolly Varden S. malma. Nonnative species include eastern brook trout S. fontinalis (introduced in early 1900s), cutthroat trout O. clarkii (introduced in early or mid-1900s), and redside shiner (introduced ca. 2000). Rainbow trout spawn in tributaries in the spring (May–June), emerge in the late summer, and most recruit to Ross Lake around age 2–3. Char spawn in streams during fall and also recruit to the reservoir at ages 2 and older, whereas redside shiner spawn during summer and complete their life cycle within the lake.
Map of the study system. Reprinted from Johnson et al. 2024b with permission
Isoclines for Ross Lake showing variation in thermal structure (°C) by year (a) and region (b). Data prior to 2019 were collected by the National Park Service (Johnson et al., 2024a). The Ross Lake South site is located mid-lake in the pelagic zone near the confluence with Big Beaver Creek, and the North site was located mid-lake in the pelagic zone near the confluence with Little Beaver Creek. Reprinted from Johnson et al. 2024b with permission
Fish collection
All data collected and described throughout these methods are available from Johnson et al. (2024a) unless otherwise cited. From 2019 to 2021, rainbow trout were collected in spring, summer, and fall using sinking multi-mesh gill nets set in three approximate depth strata corresponding to the epilimnion (0–10 m), metalimnion (10–20 m), and hypolimnion (20–30 m; Beauchamp et al., 2007a). Limitations to fish sampling in 2019 and 2020 due to limited take permitting for threatened species and COVID-19 restrictions resulted in low sample sizes or missing samples for some species/size class/season combinations, so data were pooled across all years. Angling was conducted opportunistically to increase sample sizes and minimize mortality risk to bull trout, which are listed as threatened under the Endangered Species Act. Redside shiner were also collected seasonally, primarily using minnow traps. Fish to be released were held in a live well with aerators, anesthetized with buffered MS-222 prior to processing, allowed to recover in the live well, and released; otherwise, fish were processed as described and then whole bodies were immediately frozen. All fish were measured for fork length (FL; mm), weight (0.1 g), and tissues were collected: fin tissue (for stable isotopes), and scale samples (plus otoliths from mortalities). Stomach contents from fish were collected using gastric lavage, with contents filtered into a 500-ug sieve and immediately placed on ice.
Size structure, abundance, and mortality
We used hydroacoustic surveys of the nearshore and slope zones in October 2021 to estimate a total abundance of approximately 12 million redside shiner > 40-mm FL in Ross Lake (Tables S1–S3; details provided in the Supplementary Methods). Population abundances of the salmonid species are not well studied in this system and not conducive to hydroacoustic techniques, thus we analyzed dietary consumption in Ross Lake based on size-structured unit populations of 1,000 fish > 200-mm FL (the modal size for recruitment of adfluvial salmonids to the reservoir was approximated at 200-mm FL) as well as an estimated population of 3,000 bull trout. Notably, we did not model bull trout in this study, but we used their estimated population abundance to set a realistic estimate for rainbow trout abundance. This estimated population size was generated from recent snorkel survey counts in the upper Skagit River in British Columbia assuming that the surveyed area represented 40% of the high-quality spawning habitat accessible from Ross Lake (Seattle City Light, 2012; Foster, 2020). This approach allows managers to easily scale the resulting consumption estimates up or down to evaluate different predation scenarios based on alternative population estimates and uncertainty (Beauchamp et al., 2007b). From the unit population of bull trout, we used relative catch frequencies from the gill-net sampling in 2018–2021 to estimate the relative abundance of the other salmonid species indexed to bull trout (Table 1; Johnson et al., 2024a).
We created age-structured populations for each species using annual survival rates (\(S\)) to allocate these total population abundances to each age class for each species. We used previously reported \(S\) for redside shiner in Ross Lake (Welch, 2012) and used weighted catch curve analysis on gill-net monitoring data from 2006 to 2018 (collected and maintained by the National Park Service, Johnson et al., 2024a) to estimate \(S\) for the salmonids. We used these data to estimate annual instantaneous mortality rates (Z) and annual survival rates for each species (Table 1; Miranda & Bettoli, 2007). We then created estimates of daily age class abundance for an annual cycle by applying the daily instantaneous mortality rate (Z/365) to the initial age class abundance estimate.
Prey availability
We conducted vertical zooplankton tows (net: 30 cm diameter, 90 cm length, 150 µm mesh) seasonally in 2019 (June, July, October) and monthly during 2021 (May–October) at two stations in Ross Lake (Fig. 1) to assess pelagic prey availability. Tows sampled two depth bins, 0–20 m and 0–10 m depth intervals, with two replicate tows for each depth interval. These depth bins were selected to correspond to the epilimnion and metalimnion so that we could quantify depth-specific prey availability and compare to depth distribution of fishes during thermal stratification (e.g., Sorel et al., 2016a). After rinsing from the net and cod end, zooplankton were stored in ethanol. Additional horizontal tows were conducted to collect zooplankton samples for stable isotope analysis.
In the laboratory, zooplankton samples were concentrated, and crustacean taxa and eggs were enumerated in 3–5 individual 5-ml aliquots for each sample. Cladocerans were identified to genus and all others identified to order. Preservation methods tend to expel eggs from cladoceran carapaces, making it challenging to identify loose eggs and obtain accurate egg counts when multiple taxa coexist in a sample. To address this issue, we proportionally applied loose cladoceran eggs to Daphnia based on relative frequency of adult cladoceran taxa in the sample. The first 50 Daphnia encountered in each sample were measured using Image Pro Premier digital image analysis software (version 10; Media Cybernetics, Inc.) for body length (BL; µm) from the top of the helmet to the base of the tail spine. BL was converted to wet weight (W; µg) with the following equations which estimates dry weight (DW; µg) from pooled Daphnia species (Dumont et al., 1975) and then converted from dry weight using a percent dry weight value of 10% (Luecke & Brandt, 1993):
Daphnia density (individuals/l) was calculated as an average between replicate samples for each sampling date, depth strata, and site. Areal density for each site and date (individuals/m2) was also calculated from volumetric density sampled in the 0–20 m depth strata, assuming that negligible densities of exploitable zooplankton were present below 20 m depth, to compare to historical estimates of zooplankton density (1971–1973, Seattle City Light, 1974). Standing stock biomass was estimated for each sampling date by multiplying the mean individual wet weight by densities. Production rates were estimated with the egg ratio method (Paloheimo, 1974; details available in the Supplemental Methods).
Pelagic volume was used to expand biomass and production to total lake-wide estimates. Pelagic volume was estimated in Ross Lake using surface area estimates at 1 ft (0.3 m) contours derived from the 2018 drawdown digital elevation model (DEM; provided by Seattle City Light). For each lake surface elevation specific to the date of sampling, we estimated volume of the pelagic epilimnion by multiplying the surface area of the elevation contour that was 10 m (33 ft) below surface elevation by 10 and volume of the metalimnion as the surface area of the elevation contour that was 20 m (66 ft) below surface elevation by 10 (Fig. S2). For each sampling date, whole-lake biomass and production were estimated for each depth range sampled (0–10 m and 0–20 m), averaged between the two sites, and estimates for the metalimnion (10–20 m) were calculated by subtracting lake-wide estimates for 0–10 m from those of 0–20 m.
Stable isotope analysis
Stable isotope analysis was conducted on fish fin tissue (Sanderson et al., 2009) and whole bodies of zooplankton and assorted benthic invertebrates (tissue removed from shells) to map the lake’s food web and to complement stomach content analysis. We aimed to analyze fin tissue from 5 to 10 fish of each species in each size class (100-mm FL increments for salmonids, 50-mm FL for redside shiners) during the summer. Most fish (primarily summer) and zooplankton (spring and fall) samples used for stable isotope analysis were collected in 2019, whereas all other invertebrate samples were collected in the fall of 2021. Other invertebrate herbivores and omnivores were collected for stable isotope analysis to provide representative samples for primary pelagic and benthic consumers in the food web.
All the fish consumer and invertebrate samples were measured for δ15N and δ13C by the University of Washington IsoLab using a ThermoFinnigan MAT253 isotope ratio mass spectrometer and Costech elemental analyzer or by the University of Washington Facility for Compound-Specific Isotope Analysis of Environmental Samples using a Thermo Scientific Delta V isotope ratio mass spectrometer and a CE elemental analyzer. Both labs referenced samples to 2 glutamic acid standards and Bristol Bay sockeye salmon. Stable isotope ratios are reported using delta (δ) notation in permil units (‰) compared to Vienna Pee Dee Belemite for C and air for N. Isotopic values of fish consumers were compared among size classes within each lake using nonparametric Kruskal–Wallis and Dunn’s multiple comparison test to evaluate ontogenetic differences in diet and guide how we pooled isotope and diet data across size classes within a species.
We implemented Bayesian stable isotope mixing models (SIMMs) using the MixSIAR package in R (R Core Team, 2023; Stock et al., 2018) to estimate diet proportions from stable isotope signatures (details available in the Supplemental Methods; mean and SD for consumers and prey categories in Table S4). These estimates of diet proportions integrate over longer periods and thus complement the finer-scale taxonomic resolution and short-term (e.g., within the previous ~ 24 h) diet proportions estimated from stomach content analysis. In each season, species were pooled within size classes according to multiple comparison test results described above. Most tissue samples analyzed were collected in the summer (most during gill-net surveys in late July), with some also collected in the fall (early October). Enough data existed to fit SIMMs with season as a fixed factor for rainbow trout 300–399-mm FL and redside shiner 100 + mm FL. Support for a seasonal model to describe diet proportions was evaluated using leave-one-out cross-validation (LOO). All SIMMs used in this study were thoroughly evaluated and successfully converged.
Bioenergetic model inputs
Bioenergetics models are energy balance equations that partition energy intake (i.e., food) into waste, metabolism, and growth, while accounting for their relationships with temperature and body size. Thus, using measurements of observed growth, these models can be used to predict total consumption on a daily time step in addition to an estimate of feeding rate (as a proportion of maximum theoretical consumption, Cmax). The required inputs for these models, described in detail below, include observed growth (initial and final weights) and energy density of the consumer, spawning losses, thermal experience, seasonal diet composition, and prey energy density (available in Johnson et al., 2024a).
Depth use and thermal experience
Vertical temperature profiles were conducted monthly at two stations in Ross Lake (Fig. 1) to measure the thermal structure from spring through fall. These data were supplemented by Seattle City Light temperature loggers in Ross Lake forebays for winter and early spring, and profiles collected by the National Park Service from 2010 to 2018 to generate estimates of average temperatures across this time span. Temperatures from discrete dates and depths were linearly interpolated in both dimensions to generate a symmetric grid of temperatures for computing mean temperatures across layers (epi-, meta-, and hypolimnion) and through time. For simplicity in bioenergetics simulations, we only used temperature data from the southern of the two limnological sites to estimate thermal experience.
Thermal experience was determined using patterns in depth distribution observed from gill-net catch data (rainbow trout) and hydroacoustic surveys (redside shiner). Due to permitting restrictions on gillnetting effort, depth distribution of rainbow trout was evaluated at 2 sites in Ross Lake only during 2021, when the southern region was sampled seasonally but the northern site was only sampled during summer. Thermal experience for rainbow trout was assigned by temperature-based rules developed from these depth distribution patterns (Fig. S3) and general knowledge about the species thermal requirements (Rand et al., 1993). If the mean temperature in the epilimnion was ≤ 18 °C, rainbow trout were assumed to be entirely within this layer, and the average epilimnion temperature was used. If the mean temperature in the epilimnion was > 18 °C, we applied a weighted average temperature assuming that 75% of rainbow trout were in the metalimnion and 25% in the epilimnion (Fig. S3a). We assumed that 80% of redside shiner occupied the epilimnion and 20% occupied the metalimnion during the growing season (Fig. S4). Daily temperatures were averaged across each layer and weighted average temperatures calculated based on proportion of fish assumed to occupy each depth layer (Table S5).
Diet composition and prey energy density
We analyzed diets from rainbow trout but relied on previously published diet information for redside shiner in Ross Lake (Welch, 2012) supplemented by stable isotope analysis. Stomach contents were identified using a dissecting microscope and sorted into functional taxonomic groups. Blotted wet weights (0.0001 g) were recorded for each taxonomic group identified, then pooled into broader key functional groups of prey. Wet weights were converted into proportions, which were then averaged within species, size class, and season sampling blocks. Diet data were pooled across size classes where stable isotope values were not significantly different. Prey energy densities were estimated using bomb calorimetry for fish prey and taken from the literature for all other prey groups (Table S6).
Diet proportions evaluated from Bayesian stable isotope mixing models (SIMMs) were compared to diet proportions from stomach content analysis assuming that stable isotope signatures from fin tissue incorporated diets from approximately the previous 1–3 months. This approximation was used for simplicity and loosely guided by isotopic half-life predictions ranging from 38 days for a 5-g fish to 73 days for a 100-g fish (Eq. 2 in Vander Zanden et al., 2015). Thus, SIMMs from summer-collected samples were compared to spring stomach contents, and SIMMs from fall-collected samples were compared to summer stomach contents. Details on how SIMMs (Figs. S5–S9) were incorporated with stomach content analysis (Tables S7–S8), ecological assumptions made to fill in seasonal diets where data did not exist, and the final diet inputs used in the bioenergetics simulations (Table S9) can be found in the Supplemental Methods.
Age, growth, and energy density
For each species, scales were imaged, and annuli were measured using Image Pro Premier software (Media Cybernetics Inc., version 10). When necessary and available, we corroborated scale-based ages and annuli markings with otoliths. Geometric mean regression (GMR) was used to evaluate the relationship between fish FL and scale radius (SR) to avoid biased estimates from either of the ordinary regressions of FL ~ SR or SR ~ FL (Ricker 1992). Scale data were pooled across sites within each species. The Fraser-Lee proportional method (Isley & Grabowski, 2007) was used to back calculate fish FL at each annulus using the following equation:
where \(F{L}_{i}\) is the back-calculated fork length (mm) at a given annulus, \(S{R}_{i}\) is the scale radius at that annulus, \(S{R}_{\text{cap}}\) is the scale radius (mm) at capture, \(F{L}_{\text{cap}}\) is the fork length (mm) at capture, and \(a\) is the intercept from the GMR lines (rainbow trout: \(FL=42.894+155.501\times SR\), \({R}^{2}\)=0.774; redside shiner: \(FL=10.599+68.114\times SR\), \({R}^{2}\)=0.884). Back-calculated FLs were converted to wet weight using wet weight-FL regressions (rainbow trout: \(W=1.27\times {10}^{-5}\times F{L}^{2.961}\), N = 1917, \({R}^{2}\)= 0.996; redside shiner: \(W=7.48\times {10}^{-6}\times F{L}^{3.119}\), N = 90, \({R}^{2}\)= 0.973).
Energy density (J/g wet weight) of consumers was estimated using bomb calorimetry (Parr model 6725 semi-micro-bomb calorimeter, Parr Instrument Company, Moline, IL) for a subset of whole bodies collected across seasons in Ross Lake (rainbow trout: N = 27, FL = 148–492 mm, W = 33–989 g; redside shiners: N = 46, FL = 37–125 mm, W = 0.4–29.1 g). We evaluated the relationship between energy density and body weight of the consumers within each species and set energy density inputs for the bioenergetics simulations depending on the initial and final weights for each age class (Table 2).
In the simulations, spawning was represented as a loss in weight occurring on a single day. We assumed that redside shiner lost approximately 11% body weight during spawning, which was based on gonad weights sampled in Ross Lake in June. We assumed that the mean spawning time occurred on July 1 (day 61 of the simulation), that this species matures at age 2, and spawns every year (Smith et al., 2021). For rainbow trout, we assumed that fish ≥ age 3 lost 8% body weight during spawning (Juncos et al., 2013) which occurred in mid-June (day 45 of the simulation).
Consumption demand and carrying capacity
Fish Bioenergetics 4.0 (Deslauriers et al., 2017) was used to run the simulations parameterized for rainbow trout (Rand et al., 1993) and redside shiner (Johnson et al., 2023) to estimate daily, seasonal, and annual consumption demand (g of each prey category). We fit the model to annual growth starting at the beginning of the growing season, May 1 (Woodin, 1974), using average back-calculated weight at annulus as the initial and final weights (g). Per capita consumption for each age class of each consumer was expanded to the population level by multiplying individual consumption by the daily age class abundance. Daphnia weigh about 50% less and contain about 50% less water in diet samples than they do fresh (Luecke & Brandt, 1993; Stockwell et al., 1999); therefore, simulated consumption of Daphnia (g) was multiplied by 2 to estimate the fresh weight consumed for direct comparison to biomass and production estimates.
Historical data from rainbow trout were used to run comparable simulations using the same methods described above to evaluate any changes in the growth environment and energy budgets, using back-calculated size at age in 1970–1972 (Woodin, 1974), vertical temperature profiles from a site near Devil’s Creek (approximately 6 km upstream of the southern limnological station) from Jun 1970 to Sep 1974 (Seattle City Light unpublished data, available from Johnson et al. 2024a), and monthly size-based diet composition from May to Oct 1970 (Seattle City Light, 1972). Sampling of tributary fish and scales during this study led us to conclude that the size at age 1 reported in Woodin (1974) was too large, and we believe that the first annulus was not counted in these data. Therefore, historical back-calculated size at age was adjusted by adding 1 year to each annulus to appropriately compare to our back-calculation data.
Monthly consumption demand was compared to Daphnia production and standing stock biomass to evaluate the carrying capacity of the reservoirs and food limitation. Daphnia were the dominant zooplankton in rainbow trout diets and were thus determined to be the primary driver of food limitation for rainbow trout in the reservoirs. Previous work in other reservoir systems have shown that rainbow trout consumption (% Cmax) was directly related to Daphnia biomass (Tabor et al., 1996), further supporting our use of this metric to evaluate feeding conditions. We evaluated consumption demand versus Daphnia availability for the whole water column (0–20 m depth) in addition to a depth use scenario to determine whether thermally driven behavior may be limiting access to food supplies. For this depth use scenario, we estimated consumption demand versus prey availability separately for the epilimnion (0–10 m) and the metalimnion (10–20 m) in Ross Lake, assuming that salmonids would be restricted to the metalimnion during peak stratification. We divided redside shiner consumption evenly between the two layers and restricted all rainbow trout consumption to the metalimnion during the growing season to estimate depth-specific consumption demand versus Daphnia production.
Results
Stable isotope analysis and food web structure
Ross Lake rainbow trout exhibited ontogenetic shifts in stable isotope signature and trophic position (Fig. 3; Supplement Tables S10 and S11). Smaller rainbow trout (100–199 mm FL) began at a moderate position along the benthic-pelagic axis and a low trophic position (N = 5; mean ± SE: δ15N = 7.2 ± 0.1, δ13C = − 26.7 ± 0.8) and then shifted to more pelagic prey as they increased to 200–299-mm FL (N = 10; mean ± SE: δ15N = 7.7 ± 0.2, δ13C = − 28.0 ± 0.3). They increased trophic position in the transition size of 300–399-mm FL (N = 26; mean ± SE: δ15N = 9.2 ± 0.1, δ13C = − 27.1 ± 0.2) before stabilizing at a top trophic position upon reaching 400 + mm FL (N = 9; mean ± SE: δ15N = 9.8 ± 0.2, δ13C = − 25.9 ± 0.3). Redside shiner in Ross Lake exhibited stable isotope signatures similar to smaller rainbow trout (N = 23; mean ± SE: δ15N = 7.2 ± 0.1, δ13C = − 25.7 ± 0.4), without significant differences in isotopic signature between size classes.
Stable isotope biplots (mean ± standard deviation) of the Ross Lake food web. Consumer species were separated by size classes if significant differences in stable isotope values were observed. BT-hybrid: bull trout and char hybrids, DV: Dolly Varden, EBT: brook trout, RBT: rainbow trout, RSS: redside shiner. Numbers following the species code represent the group’s size bin, in mm fork length. Reprinted from Johnson et al. 2024b with permission
Diet composition
Diet proportions estimated from SIMMs tended to estimate higher contributions of fish prey for smaller rainbow trout (< 300 mm FL) and all redside shiner in Ross Lake compared to stomach contents (Table S8). Due to gape limitations and prey access, we attributed this to predation on redside shiner eggs and larvae, which would have a much higher rate of digestion and thus a lower probability of detection in stomach contents (Legler et al., 2010), especially for fish collected in gill nets and minnow traps that were set for an extended amount of time. Alternatively, predation on eggs and larvae could be episodic, corresponding with spawn and emergence timing, making it difficult to detect in stomach contents. Therefore, we relied on SIMMs to inform the contribution of fish prey for these groups for the spring–summer interval and adjusted the other prey groups accordingly for bioenergetics simulations.
Diet proportions estimated from SIMMs generally aligned well for rainbow trout ≥ 300-mm FL in Ross Lake, except for the allocation between benthos/insects and zooplankton in spring for 300–399-mm FL fish (Table S8). Diet proportions in spring for this size class showed heavy reliance on immature insects (Table S7), and the benthic production fueling these insects could have been pelagically derived due to a combination of steep-walled bathymetry of the lake and interruptions to spring benthic production due to winter drawdowns via hydroelectric operations. We were unable to collect immature insects in the spring preventing us from evaluating potential seasonal differences in their stable isotope signatures. Therefore, we relied on stomach contents to inform the diet inputs used in the bioenergetics simulation for this season and size class.
Daphnia availability
Monthly zooplankton samples were only collected during 2021, thus our ability to statistically compare Daphnia densities across years was limited. However, densities and seasonal trends seemed similar among years, characterized by low availability of edible-sized Daphnia ≥ 1-mm BL (< 1 individual/l; Fig. 4). Daphnia densities peaked in the spring (May or June) at the south site, while densities in the north site were highest in October 2021. Densities were generally lower in the metalimnion (10–20 m) than in the epilimnion, although this was not always the case. Areal densities (individuals/m2) recorded during the current study are lower than observed in 1971–1973 in most months (Seattle City Light, 1974; Fig. S10). Mean Daphnia body lengths were ≥ 1 mm in all months (Fig. S11).
Rainbow trout growth
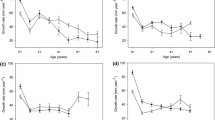
Rainbow trout in Ross Lake grew at the highest rate between annuli 2–3 and 3–4, corresponding to lake recruitment (Fig. 5). Rainbow trout in our study grew slower than fish sampled in 1971–73 at ages 2–5, but then reached a similar size at age 6 (mean initial FL: 374 mm), after fish became the dominant prey source (Fig. 5b). While 6 was the maximum age observed in 1971–73, rainbow trout in our study continued growing through age 7 (the maximum age observed) to a larger maximum size than observed historically.
Ross Lake rainbow trout back-calculated fork length (a) and weight (b) at annulus. Data derived from the current study (Johnson et al., 2024a) are shown by the black circles (mean) with error bars (2 standard error), and historical data (Woodin 1974) are shown by the gray and white symbols. Note that for the historical data, 1 year was added to each annulus to appropriately compare to our back-calculation data. Sampling of tributary fish and scales during this study led us to conclude that the size at age 1 reported in Woodin (1974) was too large, and we believe that the first annulus was not counted in these data
Consumption demand versus prey availability
Rainbow trout in Ross Lake fed at low rates (31–36%Cmax) and generally exhibited low growth efficiency (defined as total growth/total consumption; 4–10%; Table 2). Rainbow trout consumption was highest in the spring and summer (Apr-Jun, Jul-Sep) and lowest in the winter (Fig. S12). In Ross Lake, zooplankton was most important as prey during the growing season for rainbow trout 200–299-mm FL. Rainbow trout smaller than this (100–199 mm FL) consumed more insects and benthos during the spring and summer, potentially indicating differing habitat usage. Rainbow trout in the larger size classes progressively increased their reliance on fish prey, primarily identified in the diet samples as redside shiner. Trout 300–399-mm FL represented this transitional size, as evidenced in the stable isotope data (Fig. 3), and fish > 400-mm FL were consistently piscivorous.
Redside shiner fed at high rates but also experienced poor growth efficiency (112–122%Cmax; 8–10%; Table 2). Consumption peaked in the summer and was 4 × higher than spring and nearly 3 × higher than fall consumption (Fig. S12). Summer was the primary growing season for redside shiner in this system due to their higher and relatively narrow band of optimal growth temperatures (Johnson et al., 2023). Redside shiner consumed a mix of zooplankton, insects, and benthos across all seasons and fish (eggs/larvae) in the spring/summer. While zooplankton represented the highest proportion of their diets in the fall, total biomass of zooplankton consumed was highest in the summer.
Rainbow trout ate more Daphnia than did redside shiner on a per capita basis (Table 2); however, due to large differences in population size, the redside shiner population consumed the vast majority of Daphnia in Ross Lake. With an estimated population of around 12 million redside shiner > 40-mm FL, monthly population consumption of Daphnia by redside shiner ranged from 78 to 96% of the population consumption by salmonid and redside shiner combined.
Lake-wide Daphnia production and biomass in Ross Lake were lowest in May and peaked in August before a large decline in September (Fig. 6). Combined population consumption followed a similar trend, although consumption rates peaked in September before slightly declining in October. Consumption demand accounted for less than half of the lake-wide Daphnia production in all months except September, when Daphnia was most limited and consumption accounted for 48% of production and 28% of total production + biomass. When evaluating depth-specific Daphnia supply versus demand, prey resources in the metalimnion were most limited in June and September, when consumption demand accounted for 49% and 43% of Daphnia production, respectively.
Mean estimates of monthly Daphnia production and biomass compared to population consumption demand of rainbow trout and redside shiner in Ross Lake. Consumption demand versus prey supply is shown for the combined epi- and metalimnion (0–20 m depth), the epilimnion alone (0–10 m depth), and the metalimnion alone (10–20 m depth). Refer to the methods section for a description of the depth use scenarios used to divide consumption between the two depth layers. RBT: rainbow trout, RSS: redside shiner
Comparisons of historical energy budgets to those in the current study suggest that rainbow trout are not feeding as effectively as they did in 1971–73. Rainbow trout in the current study were smaller at annuli 2–5 and these lower growth rates were accompanied by lower total annual energy budgets compared to rainbow trout sampled in the 1970s (Fig. 7). The annual energy budget declined for nearly all prey categories, although this decline was largest for Daphnia. Lower contribution of Daphnia in the energy budgets of rainbow trout also coincided with lower Daphnia abundance (density per m2) measured in the current study compared to the 1970s (Fig. S10). Despite the addition of redside shiner to the energy budget for larger rainbow trout in the current study, the energetic contribution of this new prey source was not great enough to overcome the decline in the other prey categories.
Discussion
Our quantitative food web analysis highlighted the dominant role of the invasive redside shiner as a zooplankton consumer in Ross Lake. Although the fish community consumed less than 50% of the monthly production and biomass of Daphnia, the current densities of Daphnia were considerably lower than during 1971–1974. Rainbow trout in the current study experienced lower contributions of Daphnia to their annual energy budget and lower annual growth compared to the 1970s. These comparisons suggest that foraging success by rainbow trout has declined, especially for smaller rainbow trout feeding on Daphnia. Thus, for rainbow trout, growth and foraging performance may be more sensitive to reduced prey density than could be inferred simply by cruder measures of available prey supply. These findings support hypotheses regarding the role of redside shiner as a resource competitor limiting growth of rainbow trout (Larkin & Smith, 1954; Johannes & Larkin, 1961). While we did not explicitly evaluate the role of redside shiner as a novel prey for rainbow trout in this study (but see Johnson et al., 2024b), results from our energy budget analysis suggest that redside shiner do not provide substantial benefits to the rainbow trout as a novel prey source, except for the oldest age classes which represented a very small fraction of the population.
Areal densities of Daphnia (#/m2) were substantially higher in 1971–1973 than in 2019–2021 in multiple regions of the lake throughout the summer, supporting the hypothesis that prey density is currently too low for rainbow trout to feed as effectively. However, differences in sample collection depths between the studies confound this comparison of densities across time. We only collected zooplankton from 20 m to the surface, and we assumed zero individuals below 20 m; thus, if habitat occupancy extended below 20 m depth, our reported areal densities would be underestimated and the reported difference between the two studies would be inflated. However, even if we assume that our observed densities extended down to 50 m depth (rather than the 20 m we sampled), this still results in lower areal densities than observed in 1971–1973 summers; therefore, it is probable that Daphnia densities are lower now than they were historically, although the true magnitude of the difference is unknown.
This trend in Daphnia density could be the result of one or more environmental or ecological factors. One hypothesis is that increased consumption demand by redside shiner has suppressed population growth of Daphnia. Alternatively, decreased Daphnia abundance could be due to nutrient limitation and reduced productivity as the reservoir has aged; however, the largest productivity decline would likely have occurred during the first decade following inundation in the 1950s (Ney, 1996).
The thermal structure in current years compared to the 1971–1973 (Fig. S13) indicates that rainbow trout may be thermally excluded from the epilimnion for more of the growing season under current conditions, which could also be limiting their access to Daphnia. Daphnia densities in the metalimnion (10–20 m depth) were similar to or greater than in the epilimnion (0–10 m depth) in July and September at the northern site and in June, August, and September at the southern site. Thus, thermal exclusion is unlikely to limit rainbow trout consumption during these months. However, lower Daphnia densities in the metalimnion could exacerbate the existing limitation of low lake-wide Daphnia densities during July in the south and during August in the north.
Reduced Daphnia consumption by rainbow trout in Ross Lake could also result from predator-induced changes to habitat selection and behavior of the trout. Predation pressure on juvenile rainbow trout is higher now compared to the 1970s, as bull trout abundance in the upper Skagit River in British Columbia and presumably in Ross Lake as well, has increased since the introduction of redside shiner (Foster, 2020), and larger rainbow trout are now piscivorous (Johnson et al., 2024b). Small rainbow trout in lakes and reservoirs commonly seek predation refuge in nearshore habitats, which would limit their access to Daphnia, which are concentrated in the pelagic zone (Tabor & Wurtsbaugh, 1991; Biro et al., 2005). Supporting this hypothesis, we found that during the current study, benthos contributed a larger fraction of the energy budget for the younger, smaller trout and then declined with age, while benthos contributed the least to age 2 energy budgets and then increased with age in the 1970s.
Growth and consumption by rainbow trout in other reservoirs have been tightly linked with Daphnia biomass per unit volume (Tabor et al., 1996), aligning with our findings in Ross Lake. These pelagic resources play a particularly important role in reservoirs compared to natural lakes as reservoir water-level fluctuations can disrupt benthic productivity depending on the timing and extent of water drawdown (Hansen et al., 2018; Trottier et al., 2019). Previous studies in Ross Lake have shown low standing crop of benthic invertebrates in the drawdown zone (Seattle City Light, 1972). The contributions of immature insects and other benthos to the energy budget of rainbow trout in the current study were also lower than in the 1970s indicating that these prey resources are also currently limited. Benthos and immature insects were also major components of the redside shiner diet, suggesting the increased consumption demand could be putting further pressure on these already limited benthic resources.
Redside shiner were apparently not food limited in Ross Lake, despite low Daphnia densities that affected rainbow trout feeding, thus they may be able to limit rainbow trout in resource-poor systems. Part of this ability to withstand low Daphnia densities may result from their relatively lower metabolic activity, growth rates, and food requirements (Johnson et al., 2023). It is also possible that they can feed more effectively than trout at low prey densities. Observations from enclosure experiments supported this hypothesis; redside shiner fed more efficiently on Gammarus than rainbow trout (Johannes & Larkin, 1961), although these competitive interactions might also be influenced by environmental variables such as temperature (Reeves et al., 1987). Whether redside shiner are more efficient at feeding on zooplankton is uncertain. Functional response experiments could shed light on competitive advantages redside shiner may have over rainbow trout and elucidate any relationships between resource availability and impacts of invasion (Dick et al., 2014).
Interestingly, our analysis of annual energy budgets of rainbow trout highlighted that the addition of redside shiner as a novel prey source for rainbow trout did not compensate for the decline in energy acquisition from invertebrate prey categories for most age classes. This finding suggests that rainbow trout were not able to substantially benefit from redside shiner as a prey source until they were > 400-mm FL (age 6). Rainbow trout in the current study did attain an older maximum age and larger maximum weight compared to the 1970s, which could have implications for reproduction potential in the tributaries (Quinn, 2005). However, we presume that very few rainbow trout in this population survive to age 6 due to low estimated annual survival (only 3% of fish > 200-mm FL), suggesting that any benefits to rainbow trout growth are probably not realized at the population-level. Additionally, any increases in recruitment to the lake could be overwhelmed by increases to predation rate (Johnson et al., 2024b).
Limitations/assumptions
Population abundance
Population abundance exerted the strongest influence on predicted consumption demand; therefore, uncertainty around these estimates influenced our conclusions regarding consumption demand in these reservoirs. Despite considerable uncertainty regarding rainbow trout abundance in Ross Lake, the population is too small to strongly alter our conclusions. Our estimate of consumption by rainbow trout represented only 5–21% of the consumption demand imposed by redside shiners; therefore, the rainbow trout population would have to be many-fold larger than our current estimates to measurably change our conclusions about the relative consumption demand of these two species.
Our assessment of redside shiner abundance likely underestimated the actual abundance by some percentage due to the challenges of detecting the portion of fish close to the bottom. Consequently, we could have underestimated population-level consumption demand on Daphnia by redside shiner. Thus, consumption demand could have been closer to carrying capacity than was indicated by our estimates. If we use a conservative estimate of carrying capacity to be 50% of production + biomass, Ross Lake Daphnia supply in September would be at carrying capacity with a redside shiner population around 1.8 × our estimated size (i.e., 21.5 million), which is near the upper confidence interval of the redside shiner abundance estimate (21.1 million; Table S3).
Daphnia production
Although the egg ratio method for estimating production (Paloheimo, 1974) is standard in such fisheries studies, it is not without flaws. First, it assumes constant birth and death rates throughout each sampling interval. In reality, these dynamics are episodic – egg ratios change considerably over short periods. Considerable variability in these metrics may be lost when using longer sampling intervals, such as the monthly sampling used in this study (Brett et al., 1992). Challenges in identifying loose cladoceran eggs add another level of uncertainty to production estimates based on egg ratios. Nevertheless, we believe that our estimates of Daphnia biomass and production provide a useful guide for evaluating available prey supply in these reservoirs.
Management implications
Identifying the factors currently limiting this invasive population may improve understanding of future risks driven by a changing climate and environmental conditions as well as informing control strategies (Rahel & Olden, 2008). In contrast to rainbow trout, redside shiner in Ross Lake fed at or just exceeding their theoretical %Cmax, indicating they are probably limited by factors other than prey availability. Redside shiner experience a relatively short growing season in Ross Lake, with most consumption and growth occurring during June–September. Warming would increase consumption rates and likely heighten limitations they impose on shared prey supply; however, it could also increase growth and survival rates in the spring and fall, potentially facilitating further expansion into the southern region, where abundance is comparatively low. If warming was high enough, it could also shift redside shiner depth distributions into the thermocline during the growing season, increasing the spatial and diet overlap with native salmonids and intensifying competition for the limited zooplankton supply below the epilimnion. Bioenergetic simulations show that redside shiner growth potential is within 90% of its maximum from 16 to 20 °C with %Cmax and diet energy comparable to this study (Johnson et al., 2023), suggesting behavioral thermoregulation might induce vertical habitat shifts above these temperatures.
Stable isotope mixing models detected higher levels of cannibalism by redside shiner than expected based on diet analysis, which could represent an important and previously overlooked mechanism of population control. Extent of cannibalism reported in the literature is varied (Weisel & Newman, 1951; Johannes & Larkin, 1961; Welch, 2012), and Weisel & Newman (1951) suggested that redside shiners “are probably their own worst egg predators.” Cannibalism could indicate low resource availability or a natural response to predictable high-density pulses of high-energy food (Fox, 1975). Further insights into the role of cannibalism in regulating this population could be gained by quantifying predation mortality by other predators for comparison to annual biomass and production. This result could also be due to bias from the stable isotope data and mixing models. SIMMs could have modeled higher levels of fish consumption by redside shiner if their true trophic discrimination factor (TDF) for δ15N was higher than the assumed 3.4‰ ± 1.0‰ (Post, 2002; Hussey et al., 2014). In the absence of data on TDFs for redside shiner, we used average TDF values that are widely applied in similar fisheries studies, making these results comparable to others (e.g., Rubenson et al., 2020; Hansen et al., 2022).
High relative feeding rates (%Cmax) exhibited by redside shiner in this system suggest that management actions to reduce abundance could decrease exploitative competition with rainbow trout in Ross Lake. This is not always the case; for example, in Lake Tahoe high consumption demand and low %Cmax of invasive Mysis feeding on copepods indicated that decreases in their density could increase per capita consumption rates by Mysis rather than increase food available to kokanee Oncorhynchus nerka (Hansen et al., 2023). Uncertainties in the relative roles of predation versus environmental conditions in regulating zooplankton population dynamics makes it challenging to predict the magnitude of Daphnia response to a partial decrease in consumption demand.
Conclusion
Quantitative assessments of reservoir food webs, such as those presented here for Ross Lake, can provide data to inform management of novel or hybrid food webs (Naiman et al., 2012). As native species re-introductions and translocations become an increasingly popular conservation tool (Seddon et al., 2007)—including current proposals to explore introducing anadromous salmonids above Ross dam (Seattle City Light, 2023)—such studies can also be leveraged to evaluate whether food web capacity exists to support these often costly introductions (e.g., Sorel et al., 2016b; Hansen et al., 2023). By linking these food web processes to thermal conditions, water management strategies can be ecologically informed and optimize current and future operations to support robust ecosystems.
Data availability
Data generated as a part of this study described in the methods are published (Johnson et al., 2024a).
References
Beauchamp, D. A., A. D. Cross, J. L. Armstrong, K. W. Myers, J. H. H. Moss, J. L. Boldt & L. J. Haldorson, 2007a. Bioenergetic responses by Pacific salmon to climate and ecosystem variation. North Pacific Anadromous Fish Commission Bulletin 4: 257–269.
Beauchamp, D. A., D. H. Wahl & B. M. Johnson, 2007b. Predator-prey interactions. In Guy, C. S. & M. R. Brown (eds), Analysis and Interpretation of Freshwater Fisheries Data American Fisheries Society, Bethesda: 765–842.
Biro, P. A., J. R. Post & M. V. Abrahams, 2005. Ontogeny of energy allocation reveals selective pressure promoting risk-taking behaviour in young fish cohorts. Proceedings of the Royal Society B: Biological Sciences 272: 1443–1448. https://doi.org/10.1098/rspb.2005.3096.
Boretti, A. & L. Rosa, 2019. Reassessing the projections of the World Water Development Report. NPJ Clean Water 2: 15. https://doi.org/10.1038/s41545-019-0039-9.
Brett, M. T., L. Martin & T. J. Kawecki, 1992. An experimental test of the egg-ratio method: estimated versus observed death rates. Freshwater Biology 28: 237–248. https://doi.org/10.1111/j.1365-2427.1992.tb00580.x.
Deslauriers, D., S. R. Chipps, J. E. Breck, J. A. Rice & C. P. Madenjian, 2017. Fish Bioenergetics 4.0: an R-based modeling application. Fisheries 42: 586–596. https://doi.org/10.1080/03632415.2017.1377558.
Dick, J. T. A., M. E. Alexander, J. M. Jeschke, A. Ricciardi, H. J. MacIsaac, T. B. Robinson, S. Kumschick, O. L. F. Weyl, A. M. Dunn, M. J. Hatcher, R. A. Paterson, K. D. Farnsworth & D. M. Richardson, 2014. Advancing impact prediction and hypothesis testing in invasion ecology using a comparative functional response approach. Biological Invasions 16: 735–753. https://doi.org/10.1007/s10530-013-0550-8.
Dumont, H. J., I. Van de Velde & S. Dumont, 1975. The dry weight estimate of biomass in a selection of Cladocera, Copepoda and Rotifera from the plankton, periphyton and benthos of continental waters. Oecologia 19: 75–97. https://doi.org/10.1007/BF00377592.
Feldbauer, J., D. Kneis, T. Hegewald, T. U. Berendonk & T. Petzoldt, 2020. Managing climate change in drinking water reservoirs: Potentials and limitations of dynamic withdrawal strategies. Environmental Sciences Europe 32: 48. https://doi.org/10.1186/s12302-020-00324-7.
Ficke, A. D., C. A. Myrick & L. J. Hansen, 2007. Potential impacts of global climate change on freshwater fisheries. Reviews in Fish Biology and Fisheries 17: 581–613. https://doi.org/10.1007/s11160-007-9059-5.
Foster, J., 2020. 2020 Skagit River snorkel survey report, Triton Environmental Consultants Ltd., Vernon, British Columbia:
Fox, L. R., 1975. Cannibalism in natural populations. Annual Review of Ecology and Systematics 6: 87–106.
Hansen, A. G., J. R. Gardner, K. A. Connelly, M. Polacek & D. A. Beauchamp, 2018. Trophic compression of lake food webs under hydrologic disturbance. Ecosphere 9: 1–11. https://doi.org/10.1002/ecs2.2304.
Hansen, A. G., J. R. Gardner, K. A. Connelly, M. Polacek & D. A. Beauchamp, 2022. Resource use among top-level piscivores in a temperate reservoir: Implications for a threatened coldwater specialist. Ecology of Freshwater Fish 31: 469–491. https://doi.org/10.1111/eff.12644.
Hansen, A. G., A. McCoy, G. P. Thiede & D. A. Beauchamp, 2023. Pelagic food web interactions in a large invaded ecosystem: implications for reintroducing a native top predator. Ecology of Freshwater Fish Online. https://doi.org/10.1111/eff.12706.
Hussey, N. E., M. A. MacNeil, B. C. McMeans, J. A. Olin, S. F. J. Dudley, G. Cliff, S. P. Wintner, S. T. Fennessy & A. T. Fisk, 2014. Rescaling the trophic structure of marine food webs. Ecology Letters 17: 239–250. https://doi.org/10.1111/ele.12226.
Isley, J. J. & T. B. Grabowski, 2007. Age and growth. In Guy, C. S. & M. L. Brown (eds), Analysis and Interpretation of Freshwater Fisheries Data American Fisheries Society, Bethesda, Maryland: 187–228.
Jackson, M. C., R. J. Wasserman, J. Grey, A. Ricciardi, J. T. A. Dick & M. E. Alexander, 2017. Novel and disrupted trophic links following invasion in freshwater ecosystems. In Bohan, D., A. Dumbrell & F. Massol (eds), Networks of Invasion: Empirical Evidence and Case Studies Academic Press, Oxford: 55–97. https://doi.org/10.1016/bs.aecr.2016.10.006.
Johannes, R. E. & P. A. Larkin, 1961. Competition for food between redside shiners (Richardsonius balteatus) and rainbow trout (Salmo gairdneri) in two British Columbia lakes. Journal of the Fisheries Research Board of Canada 18: 203–220. https://doi.org/10.1139/f61-015.
Johnson, P. T. J., J. D. Olden & M. J. Vander Zanden, 2008. Dam invaders: Impoundments facilitate biological invasions into freshwaters. Frontiers in Ecology and the Environment 6: 357–363. https://doi.org/10.1890/070156.
Johnson, R. C., D. A. Beauchamp & J. D. Olden, 2023. Bioenergetics model for the nonnative redside shiner. Transactions of the American Fisheries Society 152: 94–113. https://doi.org/10.1002/tafs.10392.
Johnson, R. C., T. J. Code, M. S. Hoy, B. L. Jensen, K. D. Stenberg, C. O. Ostberg & J. J. Duda, 2024a. Upper Skagit reservoir food web data, 2005–2021: US. Geological Survey Data Release. https://doi.org/10.5066/P14FNXDV.
Johnson, R. C., M. S. Hoy, K. D. Stenberg, J. H. Mclean, B. L. Jensen, T. J. Code, C. O. Ostberg & D. A. Beauchamp, 2024b. Shift in piscivory by salmonids following invasion of a minnow in an oligotrophic reservoir. Ecology of Freshwater Fish. https://doi.org/10.1111/eff.12778.
Juncos, R., D. A. Beauchamp & P. H. Vigliano, 2013. Modeling prey consumption by native and nonnative piscivorous fishes: implications for competition and impacts on shared prey in an ultraoligotrophic lake in Patagonia. Transactions of the American Fisheries Society 142: 268–281. https://doi.org/10.1080/00028487.2012.730109.
Larkin, P. A. & S. B. Smith, 1954. Some effects of introduction of the redside shiner on the Kamloops trout in Paul Lake, British Columbia. Transactions of the American Fisheries Society 83: 161–175. https://doi.org/10.1577/1548-8659(1953)83[161:seoiot]2.0.co;2.
Legler, N. D., T. B. Johnson, D. D. Heath & S. A. Ludsin, 2010. Water temperature and prey size effects on the rate of digestion of larval and early juvenile fish. Transactions of the American Fisheries Society 139: 868–875. https://doi.org/10.1577/t09-212.1.
Lindsey, C. C., 1950. The relation of the redside shiner to production of trout in British Columbia. B.C. Game Commission, Scientific Report, https://a100.gov.bc.ca/pub/acat/public/viewReport.do?reportId=9082.
Luecke, C. & D. Brandt, 1993. Notes: Estimating the energy density of Daphnid prey for use with rainbow trout bioenergetics models. Transactions of the American Fisheries Society 122: 386–389. https://doi.org/10.1577/1548-8659(1993)122%3c0386:netedo%3e2.3.co;2.
Magnuson, J. J., L. B. Crowder & P. A. Medvick, 1979. Temperature as an ecological resource. Integrative and Comparative Biology 19: 331–343. https://doi.org/10.1093/icb/19.1.331.
Messner, J., & G. Schoby, 2019. Fisheries management Annual Report: Salmon Region 2017. Idaho Department of Fish and Game, 19–102.
Miranda, L. E. & P. W. Bettoli, 2007. Mortality. In Guy, C. S. & M. L. Brown (eds), Analysis and Interpretation of Freshwater Fisheries Data American Fisheries Society, Bethesda, Maryland: 229–277.
Miranda, L. E., G. Coppola & J. Boxrucker, 2020. Reservoir fish habitats: a perspective on coping with climate change. Reviews in Fisheries Science and Aquaculture 28: 478–498. https://doi.org/10.1080/23308249.2020.1767035.
Moreno-Ostos, E., R. Marcé, J. Ordóñez, J. Dolz & J. Armengol, 2008. Hydraulic management drives heat budgets and temperature trends in a Mediterranean reservoir. International Review of Hydrobiology 93: 131–147. https://doi.org/10.1002/iroh.200710965.
Naiman, R. J., J. R. Alldredge, D. A. Beauchamp, P. A. Bisson, J. Congleton, C. J. Henny, N. Huntly, R. Lamberson, C. Levings, E. N. Merrill, W. G. Pearcy, B. E. Rieman, G. T. Ruggerone, D. Scarnecchia, P. E. Smouse & C. C. Wood, 2012. Developing a broader scientific foundation for river restoration: Columbia River food webs. Proceedings of the National Academy of Sciences of the United States of America 109: 21201–21207. https://doi.org/10.1073/pnas.1213408109.
Ney, J. J., 1996. Oligotrophication and its discontents: effects of reduced nutrient loading on reservoir fisheries. In Miranda, L. E. & D. R. De Vries (eds), Multidimensional approaches to reservoir fisheries management American Fisheries Society, Bethesda, Maryland: 285–295.
Paloheimo, J. E., 1974. Calculation of instantaneous birth rate. Limnology and Oceanography 19: 692–694.
Post, D. M., 2002. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83: 703–718. https://doi.org/10.1890/0012-9658(2002)083[0703:USITET]2.0.CO;2.
Quinn, T. P., 2005. The Behavior and Ecology of Pacific Salmon and Trout, University of Washington Press, Seattle:
R Core Team, 2023. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
Rahel, F. J. & J. D. Olden, 2008. Assessing the effects of climate change on aquatic invasive species. Conservation Biology 22: 521–533. https://doi.org/10.1111/j.1523-1739.2008.00950.x.
Rahel, F. J. & M. A. Smith, 2018. Pathways of unauthorized fish introductions and types of management responses. Hydrobiologia 817: 41–56. https://doi.org/10.1007/s10750-018-3596-x.
Rand, P. S., D. J. Stewart, P. W. Seelbach, M. L. Jones & L. R. Wedge, 1993. Modeling steelhead population energetics in Lakes Michigan and Ontario. Transactions of the American Fisheries Society 122: 977–1001. https://doi.org/10.1577/1548-8659(1993)122%3c0977:mspeil%3e2.3.co;2.
Reeves, G. H., F. H. Everest & J. D. Hall, 1987. Interactions between the redside shiner (Richardsonius balteatus) and the steelhead trout (Salmo gairdneri) in western Oregon: The influence of water temperature. Canadian Journal of Fisheries and Aquatic Sciences 44: 1603–1613. https://doi.org/10.1139/f87-194.
Reid, A. J., A. K. Carlson, I. F. Creed, E. J. Eliason, P. A. Gell, P. T. J. Johnson, K. A. Kidd, T. J. MacCormack, J. D. Olden, S. J. Ormerod, J. P. Smol, W. W. Taylor, K. Tockner, J. C. Vermaire, D. Dudgeon & S. J. Cooke, 2019. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biological Reviews 94: 849–873. https://doi.org/10.1111/brv.12480.
Ricker, W. E., 1992. Back-calculation of fish lengths based on proportionality between scale and length increments. Canadian. Journal of Fisheries and Aquatic Sciences 49: 1018–1026. https://doi.org/10.1139/f92-114
Rubenson, E. S., D. J. Lawrence & J. D. Olden, 2020. Threats to rearing juvenile Chinook salmon from nonnative smallmouth bass inferred from stable isotope and fatty acid biomarkers. Transactions of the American Fisheries Society 149: 350–363. https://doi.org/10.1002/tafs.10237.
Sanderson, B. L., C. D. Tran, H. J. Coe, V. Pelekis, E. A. Steel & W. L. Reichert, 2009. Nonlethal sampling of fish caudal fins yields valuable stable isotope data for threatened and endangered fishes. Transactions of the American Fisheries Society 138: 1166–1177. https://doi.org/10.1577/T08-086.1.
Seddon, P. J., D. P. Armstrong & R. F. Maloney, 2007. Developing the science of reintroduction biology. Conservation Biology 21: 303–312. https://doi.org/10.1111/j.1523-1739.2006.00627.x.
Smith, T. W., B. W. Liermann & L. A. Eby, 2021. Ecological assessment and evaluation of potential eradication approaches for introduced redside shiners in a montane lake. North American Journal of Fisheries Management 41: 1473–1489. https://doi.org/10.1002/nafm.10668.
Sorel, M. H., A. G. Hansen, K. A. Connelly & D. A. Beauchamp, 2016a. Trophic feasibility of reintroducing anadromous salmonids in three reservoirs on the North Fork Lewis River, Washington: prey supply and consumption demand of resident fishes. Transactions of the American Fisheries Society 145: 1331–1347. https://doi.org/10.1080/00028487.2016.1219678.
Sorel, M. H., A. G. Hansen, K. A. Connelly, A. C. Wilson, E. D. Lowery & D. A. Beauchamp, 2016b. Predation by northern pikeminnow and tiger muskellunge on juvenile salmonids in a high-head reservoir: Implications for anadromous fish reintroductions. Transactions of the American Fisheries Society 145: 521–536. https://doi.org/10.1080/00028487.2015.1131746.
Stock, B. C., A. L. Jackson, E. J. Ward, A. C. Parnell, D. L. Phillips & B. X. Semmens, 2018. Analyzing mixing systems using a new generation of Bayesian tracer mixing models. PeerJ 6: e5096. https://doi.org/10.7717/peerj.5096.
Stockwell, J. D., K. L. Bonfantine & B. M. Johnson, 1999. Kokanee foraging: a Daphnia in the stomach is worth two in the lake. Transactions of the American Fisheries Society 128: 169–174. https://doi.org/10.1577/1548-8659(1999)128%3c0169:kfadit%3e2.0.co;2.
Tabor, R. A. & W. A. Wurtsbaugh, 1991. Predation risk and the importance of cover for juvenile rainbow trout in lentic systems. Transactions of the American Fisheries Society 120: 728–738. https://doi.org/10.1080/1548-8659(1991)120[0728:PRATIO]2.3.CO;2.
Tabor, R., C. Luecke & W. Wurtsbaugh, 1996. Effects of Daphnia availability on growth and food consumption of rainbow trout in two Utah reservoirs. North American Journal of Fisheries Management 16: 591–599. https://doi.org/10.1577/1548-8675(1996)016%3c0591:eodaog%3e2.3.co;2.
Seattle City Light, 1972. The aquatic environment, fishes and fishery: Ross Lake and the Canadian Skagit River (Interim report No. 1 Volume 1). City of Seattle, Washington. https://fercrelicensing.seattle.gov/library/skagit.
Seattle City Light, 1974. The aquatic environment, fishes and fishery: Ross Lake and the Canadian Skagit River (Interim report No. 3 Volume 1). City of Seattle, Washington. https://fercrelicensing.seattle.gov/library/skagit.
Seattle City Light, 2012. Biological Evaluation - supplement: Impacts of entrainment of bull trout Skagit River Hydroelectric Project License (FERC no. 553) amendment: Addition of a second power tunnel at the Gorge Development.
Seattle City Light, 2023. Final License Application to the Federal Energy Regulatory Committee. Exhibit E - Appendix G: Skagit project upstream and downstream fish passage program. https://www.seattle.gov/light/skagit/Relicensing/default.htm.
Thomsen, M. S., T. Wernberg, J. D. Olden, J. N. Griffin & B. R. Silliman, 2011. A framework to study the context-dependent impacts of marine invasions. Journal of Experimental Marine Biology and Ecology 400: 322–327. https://doi.org/10.1016/j.jembe.2011.02.033.
Trottier, G., H. Embke, K. Turgeon, C. Solomon, C. Nozais & I. Gregory-Eaves, 2019. Macroinvertebrate abundance is lower in temperate reservoirs with higher winter drawdown. Hydrobiologia 834: 199–211. https://doi.org/10.1007/s10750-019-3922-y.
Tunney, T. D., K. S. McCann, N. P. Lester & B. J. Shuter, 2012. Food web expansion and contraction in response to changing environmental conditions. Nature Communications 3: 1–9. https://doi.org/10.1038/ncomms2098.
Vander Zanden, M. J., M. K. Clayton, E. K. Moody, C. T. Solomon & B. C. Weidel, 2015. Stable isotope turnover and half-life in animal tissues: a literature synthesis. PLoS ONE 10: 1–16. https://doi.org/10.1371/journal.pone.0116182.
Weisel, G. F. & H. W. Newman, 1951. Breeding habits, development and early life history of Richardsonius balteatus, a Northwestern minnow. Copeia 1951: 187–194.
Welch, C. A., 2012. Seasonal and age-based aspects of diet of the introduced redside shiner (Richardsonius balteatus) in Ross Lake, Washington. Master’s thesis, Western Washington University, Bellingham.
Winder, M. & D. E. Schindler, 2004. Climate change uncouples trophic interactions in an aquatic ecosystem. Ecology 85: 2100–2106. https://doi.org/10.1890/04-0151.
Woodin, R. M., 1974. Age, growth, survival, and mortality of rainbow trout (Salmo gairdneri gairdneri, Richardson) from Ross Lake drainage. Master’s thesis, University of Washington, Seattle.
Woolway, R. I., S. Sharma, G. A. Weyhenmeyer, A. Debolskiy, M. Golub, D. Mercado-Bettín, M. Perroud, V. Stepanenko, Z. Tan, L. Grant, R. Ladwig, J. Mesman, T. N. Moore, T. Shatwell, I. Vanderkelen, J. A. Austin, C. L. DeGasperi, M. Dokulil, S. La Fuente, E. B. Mackay, S. G. Schladow, S. Watanabe, R. Marcé, D. C. Pierson, W. Thiery & E. Jennings, 2021. Phenological shifts in lake stratification under climate change. Nature Communications 12: 1–11. https://doi.org/10.1038/s41467-021-22657-4.
Acknowledgements
Funding and support for this study were provided by Seattle City Light—in particular, we thank Jeff Fisher, Erin Lowery, and the Diablo Lake boat house crew for critical support provided during our field operations. We would like to thank the National Park Service and Ross Lake Resort for their assistance with field sampling logistics. We also want to thank Tom Barnett in particular for his support, angling efforts, and knowledge of the Ross Lake fishery. We are grateful for Lisa Wetzel, Ella Wagner, Nancy Elder, and Jeff Duda for assistance in the field and laboratory, and Tom Quinn, Julian Olden, Jeff Duda, and two anonymous reviewers for providing valuable editorial comments that improved the quality of this manuscript.
Funding
This research was funded by Seattle City Light.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Handling of vertebrates was conducted under the auspices of the Institutional Animal Care and Use Committee of the U.S. Geological Survey, Western Fisheries Research Center IACUC protocol #2008-57.
Disclaimer
Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Additional information
Handling editor: Louise Chavarie
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Johnson, R.C., Code, T.J., Stenberg, K.D. et al. Change in growth and prey utilization for a native salmonid following invasion by an omnivorous minnow in an oligotrophic reservoir. Hydrobiologia 851, 3767–3785 (2024). https://doi.org/10.1007/s10750-024-05540-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-024-05540-3