Abstract
Some species of avian malaria parasites are invaders and responsible for diversity losses worldwide. Here we analyze the prevalence and genetic characterization of avian malaria and related haemosporidian parasites in Neotropical birds from two different regions of Peru. We detected an overall prevalence of 32.4 % comprising 12 infected bird species. The pathogen Plasmodium relictum SGS1 was widespread and the most prevalent parasite found in our study (39 % of the total infections), infecting 8 host species in both localities. To the best of our knowledge, this is the first report of this invasive pathogen in the mainland Americas, thus representing a possible menace to over one-third of all bird species in the world.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many Emerging Infectious Diseases (EID) are a result of the increasing incidence and impact on host fitness by biological invasions of parasites that have “jumped ship” to novel host species (Hatcher et al. 2012). Malaria parasites (genus Plasmodium) are globally distributed, including several hundred species infecting birds of most species. Plasmodium relictum is among the most pathogenic species of avian malaria, being responsible for mass mortality, population declines and even extinctions of many bird species worldwide after its introduction outside its native range (Van Riper et al. 1986; Valkiūnas 2005). P. relictum lineage pSGS1 is a widespread and actively transmitted parasite lineage in Europe, Africa and Asia (Palinauskas et al. 2007), although recently it has also been recorded infecting native and indigenous birds in Oceania (Howe et al. 2012). P. relictum lineage pSGS1 has been reported in over 60 species of birds, but as of yet, this invasive lineage has not been reported in the mainland Americas (Beadell et al. 2006; Durrant et al. 2006; Merino et al. 2008; Marzal et al. 2011; Lacorte et al. 2013). It is closely related to P. relictum lineage pGRW4 (where lineages are based on partial sequences of the cytochrome b gene), which also has a broad geographical range including New Zealand, Africa, Asia and the Americas (Beadell et al. 2006; Marzal et al. 2011). Both parasites lineages might easily switch to new hosts as they spread into new areas (Beadell et al. 2006; Hellgren et al. 2009). For all these reasons, the International Union for Conservation of Nature (IUCN) classifies avian malaria P. relictum to be among the 100 of the world’s worst invasive alien species (Lowe et al. 2000). Hence, the identification of the geographical distribution of P. relictum lineages and their infection prevalences in birds has become essential in order to develop appropriate management strategies to facilitate biodiversity conservation efforts worldwide. Here we analyze the presence of pSGS1 in Neotropical birds from two different areas of Peru; Lima and Huanuco. This system in Peru will allow us to test how the arrival of an introduced Plasmodium species affects birds that have already been exposed to other endemic (native) malaria parasites.
Methods
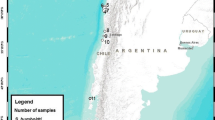
The study was carried out in two different areas of Peru: Pantanos de Villa wetland Reserve, a RAMSAR protected area in the south of Lima including a complex of lagoons, pools and marsh areas of Pacific coast (12°12′S, 76°59′W; 10 masl), and Huánuco region, located between the eastern slope of the Andes Mountain Range and the Amazon plain (9°55′S, 76°14′W; 1894 masl) (Fig. 1). In June 2012, 102 individuals from 18 native bird species were caught using mist-nets. Different morphometric characters were measured by the same observer (AM). We captured individuals at each site and recorded their body weight with a Pesola spring balance to the nearest 0.5 g. We measured tarsus length with a digital caliper to the nearest 0.01 mm. We calculated body mass index as the residuals from an ordinary least squares linear regression of body weight against tarsus length (Jakob et al. 1996).
One microcapillary of blood (70 µl) was obtained from the brachial vein of each individual and stored in 500 μl SET buffer (0.15 M NaCl, 0.05 M Tris, 0.001 M EDTA, pH 8.0) until analyses. Blood films were fixed with methanol and stained with Giemsa. Plasmodium and Haemoproteus infections were detected from blood samples using molecular methods (see Hellgren et al. 2004 for detailed description). The obtained sequences of 478 bp of the cyt b were edited, aligned and compared in a sequence identity matrix using the program BioEdit (Hall 1999). P. relictum SGS1 and new lineages were also sequenced from the reverse end using the primer HaemR2.
These molecular analyses were repeated in three independent laboratories to verify the validity and reproducibility of detection of the SGS1 lineage. The obtained results in all the cases were the same. Moreover, we also amplified specific nuclear genes of pSGS1 (García-Longoria, unpublished) to confirm that this finding was not resulting from sporadic lab contamination. The obtained sequences match SGS1 for this fast evolving gene which easily separates SGS1 from other closely related lineages (e.g. GRW4). Up to 40,000 erythrocytes were microscopically examined on each PCR-positive blood films for P. relictum SGS1. Malaria infection was confirmed microscopically in 61.5 % of individuals as early trophozoites and meronts of Plasmodium spp (Fig. 2). Unfortunately, most likely due to low parasitemia, we were not able to identify any gametocytes in the blood films. Blood smears were deposited in the museum collection of Museo de Historia Natural (Universidad Ricardo Palma, Lima, Peru) under the accession numbers PROT-MHN-001–PROT-MHN-007. Intensities of infection were low with parasitemias that did not exceed 1 parasites/10,000 red blood cells. Using the information on the host species and geographical distribution of 1338 parasite lineages provided by MalAvi database (Version 2.0.5 June 2013, Bensch et al. 2009), we studied the host range and geographical distribution of parasites found in our study. We classified as exotic parasite species those lineages that have been previously reported infecting wild birds from other zoogeographical regions (i.e. pSGS1), whereas parasites linages reported in previous studies infecting wild birds from North or South America, but not found in other zoogeographical regions, were considered as native parasite species. New parasite lineages that were found for the first time in the present study were excluded from this categorization. All new DNA sequences have been deposited in GenBank under the accession numbers (KF482344, KF482356, KF482358).
Results and discussion
A total of 102 bird blood samples were examined for haemosporidian infection. We detected an overall prevalence of 32.35 % (33 positive samples) (Table 1). We found 5 Haemoproteus lineages and 5 Plasmodium lineages infecting 12 different bird species (Table 1). Plasmodium relictum SGS1 was widespread and the most prevalent parasite lineage found in our study, infecting 13 individuals from 8 host species in both localities (39.40 % of the total infections) (Table 1). Although the fauna of haemosporidians have been studied irregularly in South America, this parasite lineage may be a recent invader because it has not been previously reported in any of the 39 studies analyzing avian malaria and related haemosporidian parasites from 213 native and introduced bird species from 17 orders covering almost the entire geographical range from North and South America (MalAvi database, version 2.0.5 June 2013, Bensch et al. 2009). Therefore, to the best of our knowledge, this is the first report of this invasive pathogen in the mainland Americas.
Several characteristics are predicted of invasive parasite species to enable their successful establishment into a new range area, such as to be a host generalist and to be cosmopolitan in their distribution (Ewen et al. 2012). Here we found that pSGS1 infects 8 different host species from 2 orders of Peruvian birds, being the most host generalist parasite lineage in our study (Table 1). Moreover, pSGS1 was also the most geographically widespread parasite, being the only Plasmodium lineage infecting birds in both study areas (Table 1). These data agree with previous records on the host range and geographical distribution of generalist pSGS1 (Bensch et al. 2009), suggesting that pSGS1 may be a recent invader and also showing the invasive potential of this species. This invasive parasite, however, could have not become so widely established without the presence of a suitable vector. Unfortunately we are not able to confirm the origin of this emerging disease and we can only speculate about the mechanisms that may have facilitated its spread to South America. Previous reports of avian malaria outbreaks have shown that the combination of naïve native birds, infected non-native birds, and abundant competent mosquito vectors has expedited the rapid establishment and spread of avian malaria (Atkinson et al. 1995; Tompkins and Gleeson 2006). Hence, once a population of a suitable vector has been fully established, the introduction of chronic avian malaria through exotic birds (e.g. pets) and/or migratory birds may provoke an outbreak of avian malaria in endemic birds (Van Riper et al. 1986; Warner 1968). Future studies examining blood parasites from non-native birds and mosquitoes in both study areas will help to elucidate when and where infection was most likely acquired by the Peruvian native birds.
Evolutionary theory predicts that virulence of parasites will be low in hosts that have evolved with the parasite (Schmid-Hempel 2011). In contrast, exotic parasites should be highly virulent to their new hosts because the lack of evolved immunological resistance (Schmid-Hempel 2011). In this sense, invasive parasite species often cause extreme morbidity and mortality in novel hosts because naive host populations usually lacks protective immunity, resulting in high mortality. For example, as an outcome of the fatal introduction of exotic P. relictum GRW4 lineage and its competent vector in Hawaii in the 19th century, the mortality of resident birds increased up to 90 % and many native species went extinct (Atkinson et al. 1995; Beadell et al. 2006; Lapointe et al. 2012). A similar situation has been reported in New Zealand, where different exotic avian malaria parasites lineages have recently arrived in multiple independent events along with their primary vector Culex quinquefasciatus and may have an impact on New Zealand native birds (Ewen et al. 2012; Howe et al. 2012). Here we did not find that birds infected with exotic avian malaria pSGS1 had lower body mass scores than birds infected with native parasite lineages when introducing bird host species ID in the model (mean body mass index (SD): infected with exotic malaria parasite = −1.761 (3.763); infected with endemic malaria parasite =2.226 (5.728); GLMM: estimate = −0.391, P = 0.622). However, the effects of pSGS1 on Neotropical birds could be underestimated due to the use of mist-nets, which tend to under-sample highly infected birds exhibiting high morbidity (Valkiūnas 2005). Experimental studies have shown that avian haemosporidians provoke detrimental effects on their hosts by decreasing body condition (Atkinson et al. 2000; Valkiūnas et al. 2006), which in turn may reduce adult bird survival (Valkiūnas 2005). Hence, the presence of this exotic Plasmodium lineage in birds of South America may represent a serious risk to this avifauna. Ornithological fauna from Peru represents 20 % of bird diversity of the world and more than 62 % of bird species richness of South America. Many of these species of birds are considered a priority for conservation because of their high degree of endemism or their risk of extinction (Schulenberg et al. 2010). Moreover, Peru has the second largest portion of the Amazon rain forest after the Brazilian Amazon, being one of the most biologically diverse areas on Earth. The high prevalence of this invasive parasite in the limit with Peruvian Amazon (34.6 % of the overall infection in Huanuco) should warn us about the potential threat to over one-third of all bird species in the world (Da Silva et al. 2005).
Summarizing, we show for first time the presence of invasive avian malaria P. relictum SGS1 infecting birds in the mainland Americas. Our intention is to underscore the conservation implications of invasive avian malaria and to elucidate the impact of exotic avian malaria parasites on individuals, populations and species of Neotropical birds.
References
Atkinson CT, Woods KL, Dusek RJ et al (1995) Wildlife disease and conservation in Hawaii: pathogenicity of avian malaria (Plasmodium relictum) in experimentally infected Iiwi (Vestiaria coccinea). Parasitology 111:S59–S69
Atkinson CT, Dusek RJ, Woods KL et al (2000) Pathogenicity of avian malaria in experimentally-infected Hawaii amakihi. J Wild Dis 36:197–204
Beadell JS, Ishtiaq F, Covas R et al (2006) Global phylogeographic limits of Hawaii’s avian malaria. Proc R Soc Lond B Biol Sci 273:2935–2944. doi:10.1098/rspb.2006.3671
Bensch S, Hellgren O, Pérez-Tris J (2009) MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol Ecol Res 9:1353–1358. doi:10.1111/j.1755-0998.2009.02692.x
Da Silva JMC, Rylands AB, Da Fonseca GAB (2005) The fate of the Amazonian areas of endemism. Conserv Biol 19:689–694. doi:10.1111/j.1523-1739.2005.00705.x
Durrant KL, Beadell JS, Ishtiaq F et al (2006) Avian malaria in South America: a comparison of temperate and tropical zones. Ornithol Monogr 60:98–111
Ewen JG, Bensch S, Blackburn TM et al (2012) Establishment of exotic parasites: the origins and characteristics of an avian malaria community in an isolated island avifauna. Ecol Lett 15:1112–1119. doi:10.1111/j.1461-0248.2012.01833.x
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acid Symp Ser 41:95–98
Hatcher MJ, Dick JTA, Dunn AM (2012) Disease emergence and invasions. Funct Ecol 26:1275–1287. doi:10.1111/j.1365-2435.2012.02031.x
Hellgren O, Waldenström J, Bensch S (2004) A new PCR assay for simultaneous studies of leucocytozoon, plasmodium and haemoproteus from avian blood. J Parasitol 90:797–802
Hellgren O, Peréz-Tris J, Bensch S (2009) A jack-of-all-trades and still a master of some: prevalence and host range in avian malaria and related blood parasites. Ecology 90:2840–2849. doi:10.1890/08-1059.1
Howe L, Castro IC, Schoener ER et al (2012) Malaria parasites (Plasmodium spp.) infecting introduced, native and endemic New Zealand birds. Parasitol Res 110:913–923. doi:10.1007/s00436-011-2577-z
Jakob EM, Marshall SD, Uetz GW (1996) Estimating fitness: a comparison of body condition indices. Oikos 77:61–67
Lacorte GA, Félix GMF, Pinheiro RRB et al (2013) Exploring the diversity and distribution of neotropical avian malaria parasites—a molecular survey from Southeast Brazil. PLoS One 8(3):e57770. doi:10.1371/journal.pone.0057770
Lapointe DA, Atkinson CT, Samuel MD (2012) Ecology and conservation biology of avian malaria. Ann NY Acad Sci 1249:211–226. doi:10.1111/j.1749-6632.2011.06431.x
Lowe S, Browne M, Boudjelas S et al (2000) 100 of the world’s worst invasive alien species a selection from the global invasive species database. IUCN/SSC Invasive Species Specialist Group (ISSG), Auckland
Marzal A, Ricklefs RE, Valkiūnas G et al (2011) Diversity, loss, and gain of malaria parasites in a globally invasive bird. PLoS One 6(7):e21905. doi:10.1371/journal.pone.0021905
Merino S, Moreno J, Vásquez RA et al (2008) Haematozoa in forest birds from southern Chile: latitudinal gradients in prevalence and parasite lineage richness. Austral Ecol 33:329–340. doi:10.1111/j.1442-9993.2008.01820.x
Palinauskas V, Kosarev V, Shapoval A et al (2007) Comparison of mitochondrial cytochrome b lineages and morphospecies of two avian malaria parasites of the subgenera Haemamoeba and Giovannolaia (Haemosporida: plasmodiidae). Zootaxa 1626:39–50
Schmid-Hempel P (2011) Evolutionary parasitology: the integrated study of infections, immunology, ecology, and genetics. Oxford University Press, Oxford
Schulenberg TS, Stotz DF, Lane DF et al (2010) Birds of Peru, revised and updated. Princeton University Press, Princeton
Tompkins DM, Gleeson DM (2006) Relationship between avian malaria distribution and an exotic invasive mosquito in New Zealand. J Roy Soc New Zeal 36:51–62
Valkiūnas G (2005) Avian malaria parasites and other haemosporidia. CRC Press, Boca Raton
Valkiūnas G, Zickus T, Shapoval AP et al (2006) Effect of Haemoproteus belopolskyi (Haemosporida: haemoproteidae) on body mass of the blackcap Sylvia atricapilla. J Parasitol 92:1123–1125
Van Riper C, van Riper SG, Goff ML et al (1986) The epizootiology and ecological significance of malaria in Hawaiian land birds. Ecol Monogr 56:327–344. doi:10.2307/1942550
Warner RE (1968) The role of introduced diseases in the extinction of the endemic Hawaiian avifauna. Condor 70:101–120
Acknowledgments
We are grateful to many students and researchers for collaboration in collecting samples during avian malaria training workshops in Peru. Two anonymous reviewers provided suggestions to improve the manuscript. This study was funded in part by the US National Science Foundation sponsored Research Coordination Network for Haemosporida of Terrestrial Vertebrates (malariarch.org, NSF 0954891), and the Spanish Ministry of Education and Science (CGL2012-36665). AM and LGL were supported by grants from Spanish Ministry of Education and Science (JC2011-0405 and BES-2010-030295, respectively). Technical and human support provided by Facility of Bioscience Applied Techniques of SAIUEx (financed by UEX, Junta de Extremadura, MICINN, FEDER and FSE). The authors declare that they have no conflict of interest. All the experiments comply with the current laws of Peru, where the experiments were performed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marzal, A., García-Longoria, L., Cárdenas Callirgos, J.M. et al. Invasive avian malaria as an emerging parasitic disease in native birds of Peru. Biol Invasions 17, 39–45 (2015). https://doi.org/10.1007/s10530-014-0718-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-014-0718-x