Abstract
There is little field evidence of fruit-infesting insect species surviving passage through the digestive tract of frugivorous mammal species. In this study, we collected brushtail possum faecal pellets from the wild and demonstrated emergence of adult torymid wasps from Rosa seed without removing the seeds from the pellets. Nineteen percent of possum dung pellets were infested by adult wasps, and a large proportion (85 %) of wasps survived. Survival to emergence was high considering that gut passage time in possums averages 50 h. This period potentially allows greater dispersal distances of wasps than would occur naturally by their own means. This plant–insect-disperser triad was studied in New Zealand where all three species were introduced and did not co-evolve. An exaptation process might have occurred since introduction of these species 150–180 years ago.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fruit-infesting insect species that survive passage through the digestive tract of frugivorous vertebrate species are generally dispersed over greater distances. This process is not that common (Hernández and Falcó 2008). A recent review indicates such “plant–insect-disperser triads” (Herrera 1984) do not necessarily involve species that co-evolved (Hernández 2011). Most of the recorded cases are in captive conditions involving birds and mammals (Guix and Ruix 2000; Nalepa and Piper 1994) with only few cases reported in the wild (Hernández and Falcó 2008; Hernández 2009). Birds can swallow small fruits without damaging the seeds (Hernández 2009; Nalepa and Piper 1994). Recent studies have shown that generalist mammals are also efficient dispersers of seeds of fleshy fruits, as the seed damage produced by mastication is usually low (e.g. Koike et al. 2008). In this study, we show evidence of a plant–insect–disperser triad in the wild involving a rose species, a rose seed torymid wasp, and a medium-sized marsupial mammal—all introduced species in New Zealand.
Rosa rubiginosa L. (Rosaceae) is a woody shrub which has become a serious exotic weed in the drier areas of New Zealand’s South Island hill country. R. rubiginosa was recorded in New Zealand gardens as early as 1835 (Molloy 1964) and was planted extensively as a horticultural rose (Hunter 1983). It blooms from November to January and the fruits ripen and turn red in February–March, and persist until August–September. The larvae of the torymid wasp Megastigmus aculeatus (Swederus), that probably arrived to New Zealand with R. rubiginosa, feed on the endosperm of the rose seeds (Syrett 1990). Adult females oviposit when the fruit begins development just after petal-fall (late February−May). The wasps overwinter as mature larvae within the achene (seed), pupate in late September to November, and emerge as adults in January–February. It has been suggested that the wasp may survive gut passage through small- to medium-sized mammals (Hernández 2009), however this has not been recorded in the wild. We tested this in New Zealand, where the most abundant medium-sized arboreal mammal is the brushtail possum (Trichosurus vulpecula), introduced from Australia in 1858 (Clout and Ericksen 2000). In this study, brushtail possum faecal pellets were collected from the wild, and emergence of adult M. aculeatus was demonstrated without removing the seeds from the pellets. We report the survival to emergence of wasps in seeds that pass intact through the digestive system, and compare this to the rate of seed infestation and wasp survival in unconsumed fruits. Due to the length of the possum digestion processes, we hypothesised that survival of adult wasps in possum pellets would be lower than that in unconsumed fruits.
Materials and methods
The study was undertaken at Aldinga Conservation Area (420 ha) located in Central Otago in the southern South Island, New Zealand. The site consisted of highly modified semiarid grassland/shrubland habitat at a mean 500 m above sea level. A diverse array of habitat types is found in this area (e.g. rocky outcrop, open grass and dense shrub). Extensive infestations of R. rubiginosa occur at this site.
Possums defecate dung pellets in small groups. Fifty pellet groups, consisting of 700 individual pellets, were collected during five consecutive days in early August 2010 whilst slowly searching for pellets for 2 h each day. Only fresh groups of pellets were collected and placed separately in small (10 × 20 cm) paper bags. They were taken immediately to the laboratory and left to dry at room temperature (approximately 20 °C) for 1 week. Pellet groups were then placed separately in plastic bags (10 × 20 cm) which were tied off and left at room temperature. Pellet groups were inspected in late February 2011 to ensure enough time had elapsed for all wasps to emerge. The number of emerged adult wasps were counted and placed in small containers of 70 % ethyl alcohol for identification. Bags were carefully inspected to ensure no wasps had escaped by chewing a hole in the plastic. Some wasps were trapped inside a seed (Fig. 1). Therefore, to estimate survival rate of adult wasps, a total of 100 randomly-selected pellets (two from each pellet group) were broken apart by hand and inspected for seeds with exit holes to check whether adult wasps were dead inside. All of the intact rose seeds without exit holes, except for clearly aborted and undeveloped seeds, were opened using small pliers and were identified as: empty (developed but with no endosperm), filled (non-infested but with endosperm) or larva-infested (wasp larva or pupae inside), following Hernandez’s (2009) methodology. Adult wasp survival rate in seeds that passed intact through the digestive system was estimated using the formula:
where E the number of adult wasps emerged, derived from the number of seeds with exit holes and without presence of any stage of the wasp inside, T number of adult wasps trapped inside a seed, and L number of wasp larva or pupae inside a seed.
We also randomly collected a pool of 50 ripe fruit from nine mature Rosa bushes at the same time the possum pellets were collected (August 2010) to estimate the rate of wasp infestation and survival in unconsumed fruit. Fruits were treated in the same way as the pellets and opened in February 2011. Adult survival was measured as above.
Standard contingency table tests (Chi square) were used to test for differences in both rates of wasp infestation and adult wasp survival between unconsumed fruits and possum dung pellets.
Results
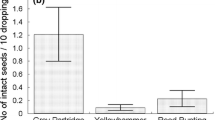
By February, 146 adult wasps had emerged from 700 possum dung pellets. A high proportion (88 %) of pellets contained Rose seeds [17.2 ± 2.0 (SE) seeds per pellet on average] and 19 % of pellets were infested by wasps. For unconsumed Rose fruits (containing 32.2 ± 1.0 seeds per fruit on average), 42 % were infested by wasps. Nearly seven percent of seeds within these fruits were infested, which was significantly higher than in seeds in possum dung pellets (5 %) (Yates corrected χ2 = 4.21, d f = 1, P < 0.05). However, survival of adult wasps in unconsumed fruit (85 %, Table 1) was no higher than in seeds that passed intact through the possums’ digestive system (85 %) (Yates corrected χ2 = 0.01, d f = 1, P > 0.05).
Discussion
The proportions of seeds infested by wasps were similar to those found by Syrett (1990) about 5 km from our study site (i.e. 5.6–6.3 %). Brushtail possums feed intensely on rose fruits during winter, including wasp-infested fruits. Survival of adults wasps through their digestive tract was similar to those recorded in late winter in Blackbird droppings in the wild (88 %, Hernández 2009), and higher than survival in captive Northern mockingbird droppings (53 %, Nalepa and Piper 1994). Contrary to expectations, survival of wasps through possums’ digestive tract was identical to survival in unconsumed fruits. Gut passage time in birds (e.g. blackbirds) is usually less than 1 h (Sorensen 1984), whereas passage time in brushtail possums is 50 h on average (Foley and Hume 1987). We expected this long passage time would compromise the viability of the seed and therefore the survival of the seed-infesting wasps.
We could not estimate absolute survival of wasps through the digestive system because it was not possible to measure seed intake by possums in the wild. Instead, we used Hernandez’s (2009) method to measure of survival to emergence in seeds that passed intact through the digestive system. This will necessarily overestimate the true rate of survival.
Several authors have stated that M. aculeatus has very limited flight capacity, therefore its dispersion and colonization of new areas could benefit from avian dispersion (Nalepa and Piper 1994). In New Zealand, Blackbirds are the most widely distributed avian seed disperser (Williams 2006), but dispersion by this species is likely to be mainly via short-distance “diffusion” dispersal of 50–100 m, and occasionally long-distance gap-crossing ‘saltation’ dispersal events (Davis and Thompson 2000). Brushtail possums on the other hand have home ranges up to 54 ha in our study area (Rouco et al. 2013), and would therefore potentially spread M. aculeatus much further than by birds. This demonstrates that mammals can facilitate the dispersion of this torymid species in the wild. Given the low prevalence of wasp-infested seeds in fruits and possum pellets, seed mortality from M. aculeatus would be low and unlikely to compromise the spread of R. rubiginosa.
From an ecological and evolutionary perspective, a better understanding of dispersal of seed-inhabiting insects by vertebrate frugivores advances our knowledge of species interactions (Hernández 2011), even more so when the players involved in the “plant–insect-disperser triad” are introduced species that have not co-evolved. In this sense, the mechanism of insect dispersal by frugivorous vertebrates is not an insect adaptation but an exaptation derived from the wasps’ pre-existing ability to conceal inside seeds and survive vertebrate ingestion and gut passage.
References
Clout M, Ericksen K (2000) Anatomy of a disastrous success: the brushtail possum as an invasive species. In: Montague T (ed) The brushtail possum: biology, impact and management of an introduced marsupial. Manaaki Whenua Press, Lincoln, pp 1–9
Davis MA, Thompson K (2000) Eight ways to be a colonizer; two ways to be an invader: a proposed nomenclature scheme for invasion ecology. Bull Ecol Soc Am 81:226–230
Foley WJ, Hume ID (1987) Passage of digesta markers in two species of arboreal folivorous marsupials—the greater gilder (Petauroides volans) and the brushtail possum (Trichosurus vulpecula). Physiol Zool 60:103–113
Guix JC, Ruiz X (2000) Plant-disperser-pest evolutionary triads: how widespread are they? Orsis 15:121–126
Hernández A (2009) Field-based evidence of rose seed infesting wasps Megastigmus aculeatus (Swederus) surviving bird gut passage. Anim Biol 59:189–199
Hernández A (2011) Internal dispersal of seed-inhabiting insects by vertebrate frugivores: a review and prospects. Integr Zool 6:213–221
Hernández A, Falcó J (2008) Frugivorous birds dispersing braconid parasitoids via endozoochory. Entomol Sci 11:323–326
Herrera CM (1984) Avian interference of insect frugivory: an exploration into the plant-bird-fruit pest evolutionary triad. Oikos 42:203–210
Hunter GG (1983) An assessment of the distribution of sweet brier (Rosa rubiginosa) in New Zealand. NZ J Exp Agr 11:181–188
Koike S, Morimoto H, Goto Y, Kozakai C, Yamazaki K (2008) Frugivory of carnivores and seed dispersal of fleshy fruits in cool-temperate deciduous forests. J Forest Res 13:215–222
Molloy BPJ (1964) Sweet brier—a vigorous woody weed in South Island tussock grassland. NZ J Agr 109:105–118
Nalepa CA, Piper WH (1994) Bird dispersal of the larval stage of a seed predator. Oecologia 100:200–202
Rouco C, Norbury G, Smith J, Byrom A, Pech R (2013) Population density estimates of brushtail possums (Trichosurus vulpecula) in dry grassland in New Zealand. NZ J Ecol 37 In press
Sorensen AE (1984) Energy and passage time: experiments with fruit preference in European blackbirds (Turdus merula). J Anim Ecol 53:545–557
Syrett P (1990) The rose seed chalcid Megastigmus aculeatus Swederus (Hymenoptera: torymidae) on sweet brier, Rosa rubiginosa, in the South Island tussock country. NZ Entomol 13:34–38
Williams PE (2006) The role of blackbirds (Turdus merula) in weed invasion in New Zealand. NZ J Ecol 3:285–291
Acknowledgments
We thank the Department of Conservation for allowing us to work on the study site. Special thanks to Dr Darren Ward, Invertebrate Ecology, Landcare Research-Auckland for wasp identification, Dr Roger Pech for his comments on previous versions of the manuscript and Jennifer Richardson for dissecting the seeds. The project was funded by the New Zealand Ministry for Science and Innovation, contract C09X0909.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rouco, C., Norbury, G. An introduced species helping another: dispersal of a rose seed infesting wasp by a marsupial in New Zealand. Biol Invasions 15, 1649–1652 (2013). https://doi.org/10.1007/s10530-013-0415-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-013-0415-1