Abstract
Frugivorous birds are important in the dispersal of many fleshy-fruited plant species, including invasive plants. Consequently, we investigated three native frugivorous avian species’ role in potential dispersal and germination success of the invasive American bramble (Rubus cuneifolius) in South Africa, particularly in terms of amount of fruit ingested, transit time, and their effects on seed germination. Three common species of frugivorous bird species were predicted to positively affect the spread of invasive R. cuneifolius. The bird species (speckled mousebirds Colius striatus, red-winged starlings Onychognathus morio and dark-capped bulbuls Pycnonotus tricolor) were fed R. cuneifolius fruit in captivity and amounts ingested were determined together with transit times. Seeds that were excreted and/or regurgitated by the three bird species, manually extracted seeds, and control whole fruit were then planted and their germination assessed daily. Although the three bird species varied in the amount of fruit consumed (~ 10–30 g), there was no significant difference in amount of R. cuneifolius fruit eaten per gram body mass among the species. Bird-ingested seeds emerged a mean 21–23 days after planting, while the seeds from the whole fruit took longer to emerge (mean 28 days). Germination of seeds ingested by the respective bird species was significantly higher (~ 60–75%) than seeds manually removed from fruits (~ 52%) or seeds in whole fruits (~ 7%). This suggests that removal of pulp and seed coat abrasion by the birds increased germination success. The three bird species all had R. cuneifolius seed transit times greater than 20 min, demonstrating their potential to disperse seeds a distance away from the parent plant. The results showed that the three bird species increased the germination success and suggests they are potentially important dispersers of the invasive R. cuneifolius.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An alien invasive plant is a species that is generally introduced, intentionally or unintentionally, to areas outside its native range, lacks natural predators and pathogens, and becomes a threat to local biodiversity and economic activity (Richardson et al. 2000; Ellstrand and Schierenbeck 2000; Richardson and Van Wilgen 2004; Pyšek and Hulme 2005; Kueffer et al. 2009). The major problem with invasive plants is that, with the lack of control mechanisms normally experienced in their native range, they often out-compete native plants (Parsons and Cuthbertson 1992). Some of these alien plants become successful invaders because of their mutualistic relationships with native frugivores (Richardson et al. 2000), [especially where native frugivores favour invasive species over native species and so positively affect the dispersal of the invasive species (Buckley et al. 2006)], or as a consequence exotic frugivores (Martin-Albarracin et al. 2018; Thibault et al. 2018).
Plants use a variety of mechanisms to ensure that seeds are dispersed away from the parent plant and movement of seeds generally occurs through wind or animal dispersal which has two forms: endozoochory and epizoochory (Dovrat et al. 2012; Hui and Richardson 2017). Endozoochory is achieved through the development of nutritious fleshy fruits that are attractive to a range of animals who ingest and then at a later stage disperse them when they excrete the seeds (Cypher and Cypher 1999). Frugivorous animals assist in the establishment of a variety of plant species through the moving of seeds away from the parent plant where the likelihood of safe germination and seedling growth is generally increased (Traveset and Willson 1997; Renne et al. 2000; D’Avila et al. 2010). Seed dispersal requires the ingestion of pulp and excretion of intact seeds (Traveset and Willson 1997; Renne et al. 2000; D’Avila et al. 2010). Successful avian seed dispersal is generally carried out by birds that consume the whole fruit including the seeds (Herrera 1981). Avian frugivores are capable of long-distance seed dispersal, which is critical to biodiversity when native species are dispersed (Cain et al. 2000; Pyšek and Hulme 2005). However, this same dispersal mechanism presents a major threat to biodiversity when invasive species are dispersed (Cain et al. 2000).
Generally, frugivorous avian and mammalian species promote both the successful dispersal and germination of invasive species (Traveset and Willson 1997; Hui and Richardson 2017). Dispersal is crucial as it assists seeds to germinate away from the parent plant where they are subject to less competition, predation and/or fungal attack (Chimera and Drake 2010; Jordaan et al. 2011a; Hui and Richardson 2017). Gut passage may enhance germination through thermal, chemical and mechanical removal of the seed coat (Clergeau 1992). However, when the seed coat is removed, germination success may be improved, or germination inhibitors may be activated (Barnea et al. 1991; Witmer and Cheke 1991). Many studies show that germination success increases after seed gut passage (Yagihashi et al. 1998; Traveset et al. 2001; Paulsen and Högstedt 2002; Thabethe et al. 2015); however, seed gut passage may also have no impact or even reduce germination success (Murray et al. 1994; Wilson and Downs 2012; Jordaan and Downs 2012; Thabethe et al. 2015). The size of the seed generally determines the effect gut passage has on germination. Small seeds may remain longer in the digestive tract and therefore have more chance of abrasion than large seeds; alternatively, they may be so badly damaged that they do not germinate (Traveset et al. 2001). Other factors may also influence gut passage: amount of fruit eaten, laxative compounds in fruits etc. Regardless, increased gut transit time generally increases the probability of increased dispersal distance (Murray et al. 1994).
South Africa has a high percentage of fleshy-fruited invasive species (Richardson and van Wilgen 2004). Studies have shown that native frugivorous avian and mammalian species are responsible for the dispersal and increased germination success of some of these invasive species (Jordaan et al. 2011a, 2012; Thabethe et al. 2015; Mokotjomela et al. 2015, 2016; Dlamini et al. 2018). American bramble (Rubus cuneifolius Pursh) is a fleshy-fruited species, native to North America but an invasive alien in South Africa, which is spread by frugivorous avian and mammalian species (Denny and Goodall 1991). This species has invaded the eastern portions of South Africa and is a threat to biodiversity, tourism and livestock production (Erasmus 1984; Morris et al. 1999; Ezemvelo KZN Wildlife 2016; Invasive Species South Africa 2017). In South Africa R. cuneifolius is categorised as category 1b invasive under the National Environmental Management: Biodiversity Act 2004 (Henderson 2011; Invasive Species South Africa 2017). The expanding invasion of R. cuneifolius may be affiliated to successful fruit set together with the ability of frugivorous birds to accomplish long-distance seed dispersal (Olckers 1999; Van Kleunen and Johnson 2007). This species’ characteristics that may promote whole fruit ingestion and preference by birds include having aggregate fruit with seeds small enough that it can be swallowed whole by most native avian frugivores (Downs 2008; Symes and Downs 2001; Jordaan et al. 2011a, b), and a fruit colour (purple-black) and nutritional composition that may further promote ingestion. It appears that birds consume several R. cuneifolius fruit and pass on many seeds in a single foraging event (Downs pers. obs.). Furthermore, R. cuneifolius thrives in parts of South Africa where the climate and landscapes favour its growth (Erasmus 1984). This species generally experiences little browsing pressure (Erasmus 1984). Rubus cuneifolius removal and control are difficult (Erasmus 1984) because of a range of factors including its coppicing, its thorny covered canes forming dense thickets, occurrence in inaccessible areas, no biological control agent, and cost of removal using pesticides.
Consequently, we investigated the role of three locally common, native frugivorous bird species in the potential dispersal and germination success of R. cuneifolius in South Africa, particularly in terms of the amount of fruit ingested, transit time, effects of gut passage on seed viability and germination, and overall potential seed dispersal. We hypothesised that the spread of the invasive R. cuneifolius in South Africa is facilitated by avian dispersers. We predicted that the three frugivorous bird species studied positively affect the potential dispersal of invasive R. cuneifolius, especially in terms of its potential dispersal and germination.
Materials and methods
Plant species
The taxonomy of Rubus in South Africa remains unresolved, complicated by hybridisation between introduced and indigenous species and unresolved taxonomy within the native range of introduced species (Henderson 2011; Sochor 2018). What has for many years been considered Rubus cuneifolius Pursh may be more correctly referred to as R. sect. cuneifolii L.H. Bailey, however, we have retained the original taxonomy R. cunifolius Pursh in this study and confirm that the species referred to and used in this study is encompassed within what Sochor (2018) has referred to as R. sect. cuneifolii L.H. Bailey and as R. cuneifolius (Taxon A) by Henderson (2011).
Rubus cuneifolius is a rambling, spiny shrub from the Rosaceae family originating from the south-eastern USA (Morris et al. 1999; Olckers 2004). In South Africa, it produces white flowers in September which develop into purple-black, fleshy fruits in December (Hummer 1996). The plant is perennial and long-lived, producing fertile stems which endure for a year and a half (Denny and Goodall 1991; Hummer 1996). In South Africa R. cuneifolius occurs in the Limpopo, Mpumalanga, Free State, KwaZulu-Natal and Eastern Cape Provinces, and is particularly abundant in the midlands and Drakensberg areas of KwaZulu-Natal (Morris et al. 1999; Ezemvelo KZN Wildlife 2016; SAPIA 2016; Invasive Species South Africa 2017). This species is a severe invasive problem in cool, moist areas of these provinces where it infests large areas with dense stands or thickets (Erasmus 1984). Rubus cuneifolius reproduces sexually and vegetatively and this efficient reproductive system further supports its spread (Erasmus 1984). The flowers give rise to berry-like aggregate fruits composed of 40–50 tightly packed single-seeded drupelets (Erasmus 1984). Hereafter these aggregate drupelets are referred to as fruits. The seeds are ~ 3 mm long with a hard, resistant seed-coat (Erasmus 1984).
Bird species
We chose three native frugivorous bird species that are locally common in the area where bramble occurs in KwaZulu-Natal (SABAP2 2019), and that had previously been observed feeding on R. cuneifolius in the wild (pers. obs.). These were red-winged starlings (Onychognathus morio) (± 130 g), dark-capped bulbuls (Pycnonotus tricolor) (± 38 g) and speckled mousebirds (Colius striatus) (± 58 g). Red-winged starlings belong to the family Sturnidae and are passerine birds that feed on a variety of seeds, nectar and berries (Craig 2005; Craig and Feare 2010). They have a mean body mass of 130 g (Craig 2005; Jordaan et al. 2011b). Dark-capped bulbuls are passerine birds from the family Pycnonotidae (Fishpool and Tobias 2005; Lloyd 2005). Although dark-capped bulbuls feed on fruit, their diet also includes insects, flower buds and nectar (Lloyd 2005; Symes et al. 2008). Dark-capped bulbuls have a mean body mass of 40 g (Lloyd 2005; Jordaan et al. 2011b). Speckled mousebirds are non-passerine birds from the family Coliidae (Dean 2005). Speckled mousebirds usually feed on leaves, flowers and fruit and have a mean body mass of 50 g (Downs et al. 2000; Dean 2005; Jordaan et al. 2011b). All three species are generally residents year-round and only move a few kilometres from their respective roosting sites to forage (Craig 2005; Dean 2005; Lloyd 2005; Craig and Feare 2010; Downs unpublished data).
Capture and maintenance of birds
We carried out the study at the Animal House, University of KwaZulu-Natal (UKZN), Pietermaritzburg, South Africa (29°37′34″S, 30°24′11.9″E). The dark-capped bulbuls (n = 7), and speckled mousebirds (n = 6) were captured locally during December 2015 using mist-nets, while the red-winged starlings (n = 5), originally captured in 2006, were caught using a padded bird net in the aviaries located outside the animal house at UKZN. Birds were ringed and housed in single-species groups in outside aviaries before being moved to experimental rooms inside the animal house and caged (42.7 × 43 × 59.3 cm) individually. The experimental rooms were set to 25 °C with 12L:12D. Birds were acclimatised for a week and fed a daily maintenance diet consisting of commercial mixed fruit. AviPlus Softbill pellets (Avi-products, Durban, South Africa) were added to the diet as a supplement. Water was provided ad libitum. Rubus cuneifolius fruits were incorporated into the maintenance diet 24 h before the experiment commenced. The evening before the experiment, all food was removed overnight as the birds only feed diurnally.
Feeding trials
We weighed each of the birds at 06h00 and 18h00 on each feeding trial day. Body mass was monitored to determine if birds gained weight while feeding on R. cuneifolius fruit and to determine the amount ingested per day per gram body mass. Rubus cuneifolius fruit was weighed (~ 100 g per bird) before (06h00) each trial and then the remaining fruit was weighed afterwards (18h00) so that the difference allowed determination of the quantity of fruit eaten by each bird. To correct for the moisture lost from uneaten fruit, a control of weighed R. cuneifolius fruit was placed in the experimental room and reweighed at 06h00 and 18h00. Seed transit time was observed at the beginning of the feeding trial by recording the time the first American bramble fruit was ingested by each bird and recording the time when the seeds were then first visible in the excreta of each individual. After transit times had been obtained for all individuals, birds were only monitored hourly until 18h00. Birds were monitored hourly to assess that they were feeding and that there was sufficient fruit available. Regurgitated seeds and seeds in excreta from each bird were collected in the evening (18h00).
Germination trials
As the regurgitated or ingested R. cuneifolius seeds from each bird’s excreta were difficult to separate, we removed all and planted a total of 160 seeds (~ 80 seeds per tray) in two separate trays (265 × 180 × 75 mm) for each bird. Manually removed seeds (n = 160 seeds planted in two separate trays), and whole fruit controls (n = 40, 20 per tray) were planted concurrently in separate trays. For the whole fruit germination trial, seeds from 10 whole fruits were counted and their average was used to determine germination percentage. Seeds and whole fruit were buried ~ 0.7 cm deep in standard potting soil and trays placed in a greenhouse at UKZN and watered daily. Plant germination was recorded daily (where germination was defined as evidence of stem emergence and development of the first true leaves) and R. cuneifolius seedlings were removed after counting. We observed the trays until there was no further germination at ~ 120 days after planting.
Data analyses
We calculated cumulative germination percentage for whole fruit, manually removed seeds and ingested seeds of R. cuneifolius. Non-parametric analysis, a Kruskal–Wallis ANOVA, was used for comparison of mean time to first germination among whole, manually removed and ingested R. cuneifolius seeds because the data were not normally distributed. One-way ANOVA was used for comparing the following datasets: seed transit time among bird species, the amount of fruit consumed by different bird species, germination percentages amongst whole fruit, manually removed and ingested seeds, and seedling emergence amongst whole fruit, manually removed seeds and ingested seeds. Where significant differences were observed, data were subsequently analysed using a Bonferroni post hoc test. A two-tailed t test was used to compare the initial and final mass of each bird species. All statistics were conducted using Statistica (Statsoft, Tulsa, OK).
Results
Bird body mass
The three bird species body mass (g) was significantly different before feeding on R. cuneifolius fruit (Kruskal–Wallis ANOVA H = 15.21, n = 18, p < 0.005, Fig. 1). Red-winged starling initial body mass was significantly higher than both dark-capped bulbuls (post hoc Bonferroni, p < 0.05) and speckled mousebirds (post hoc Bonferroni, p < 0.05). In addition, the various bird species body mass after feeding on R. cuneifolius fruit was significantly different (Kruskal–Wallis ANOVA, H = 15.20, n = 18, p < 0.005). Red-winged starlings had a greater final body mass than the other two species (Fig. 1). The initial and final body masses of red-winged starlings (t-test t = − 24.59, df = 4, p < 0.0001, Fig. 1) and speckled mousebirds (t-test t = − 4.38, df = 5, p < 0.05, Fig. 1) were significantly different, with their final body mass increased after feeding on R. cuneifolius. However, there was no significant difference in dark-capped bulbul body mass before and after feeding on R. cuneifolius fruit (t-test t = 0.54, df = 5, p = 0.61, Fig. 1).
Rubus cuneifolius fruit ingestion
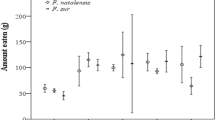
All three bird species ingested R. cuneifolius fruit. There was a significant difference in the mass of the fruit (g) of R. cuneifolius eaten among bird species (One-way ANOVA F2,14 = 16.83, p < 0.0001). The dark-capped bulbuls ate the least per day (10 g; ~ 3–4 fruits) while red-winged starlings ate the most per day (40 g; ~ 13–14 fruits; Fig. 2). However, the amount of R. cuneifolius fruit consumed by red-winged starlings and speckled mousebirds was not significantly different (Bonferronni, p = 0.2, Fig. 2a). When the amount of fruit eaten per gram body mass was considered, there was no significant difference in this (per g BM) among the three bird species (One-way ANOVA F2,14 = 3.50, df = 2, p = 0.059, Fig. 2b). The mean transit times showed no significant differences among species (One-way ANOVA F2,15 = 1.493, p = 0.26, Fig. 3) as there was individual variation with dark-capped bulbuls ~ 22 min red-winged starlings ~ 34 min and speckled mousebirds ~ 43 min
Germination of Rubus cuneifolius
Overall mean time for first seedling emergence of R. cuneifolius seeds regurgitated or ingested by the three avian frugivore species and the whole fruit were not significantly different (One-way ANOVA F2,15 = 0.31, df = 2, p = 0.644, Fig. 4). The first seedling emergence of ingested R. cuneifolius seeds of all bird species was from the 7th day but the mean first seedling emergence for all bird species were between 21 and 23 days, while for whole fruit the mean first seedling emergence was 28 days. Most manually removed seeds emerged 14 days after planting (Fig. 5). Rubus cuneifolius seeds ingested by dark-capped bulbuls, speckled mousebirds and red-winged starling started to emerge at a similar time and continued to emerge for several weeks (Fig. 5). Whole R. cuneifolius fruit took relatively longer for initial germination (Fig. 5).
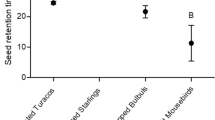
Germination percentages for ingested R. cuneifolius seeds across all frugivores were similar and relatively high (average across all three species ~ 69%) but there was no significant difference between species in terms of their effect on R. cuneifolius seed germination percentage (Fig. 6). Germination percentage of ingested seeds was significantly greater than the germination percentage for manually removed seeds (~ 52%) and whole fruit (~ 7%) (Fig. 6). Seeds ingested by frugivores were significantly more likely to germinate compared with both manually removed seeds (Bonferroni post hoc, p < 0.05) and whole R. cuneifolius fruit (Bonferroni post hoc, p < 0.05). Manually removed seeds had a significantly greater germination success than whole R. cuneifolius fruit (Bonferroni post hoc, p = 0.01).
Mean (± SE) germination percentage for Rubus cuneifolius fruit seeds ingested by three frugivorous bird species (dark-capped bulbuls, red-winged starlings, and speckled mousebirds), manually removed seeds and whole fruit. [Treatments with letters in common were not significantly different (p < 0.05)]
Discussion
Although all three bird species ingested R. cuneifolius fruit, the amounts varied with dark-capped bulbuls eating the least per day (10 g; ~ 3–4 fruits) while red-winged starlings ate the most per day (40 g; ~ 13–14 fruits). Germination success of R. cuneifolius was positively influenced by passage through the digestive tract of the three native frugivorous birds used in this study and all these species were shown to be the admissible seed dispersal agents of R. cuneifolius. The three bird species are generally resident but may show short-distance migration in response to food availability (Craig 2005; Dean 2005; Lloyd 2005; Craig and Feare 2010). All three bird species generally roost at different locations to where they forage, especially when feeding on R. cuneifolius fruit (pers. obs.), and in so doing may disperse the seed they ingest, including R. cuneifolius.
Our study showed that R. cuneifolius seeds do not necessarily have to pass through the digestive tract in order to germinate but that seed germination is significantly enhanced by gut passage. Consistent with studies on fruit of invasive plant species ingested by bird species (Panetta 2001; Day et al. 2003), gut transit passage and pulp removal increased the success of germination of R. cuneifolius with significantly more of the seeds germinating following bird ingestion. The effect gut passage has on germination success has frequently been affiliated with the intensity of seed coat abrasion, which is linked with morphological characteristics of the bird species’ digestive tracts (Barnea et al. 1991; Traveset and Willson 1997; Yagihashi et al. 1999). The removal of pulp by frugivores generally enhances germination because the pericarp may contain inhibitory substances (Witmer and Cheke 1991; Moore 2001; Panetta 2001). Moreover, certain seed predators utilise the pulp to determine the presence of seeds (Moles and Drake 1999), and the removal of pulp may minimise the probability of seed predation (Fricke et al. 2013). Seed ingestion is especially important for seeds that cannot grow and develop prior to the decomposition of the pulp (Panetta and McKee 1997; Yagihashi et al. 1998). One challenge is that certain seeds only germinate after the pulp has decomposed (Yagihashi et al. 1998, 1999). In this study, manually removed R. cuneifolius seeds germinated better than whole fruit and this may be an indication that effective outcomes of seed ingestion are made possible through the removal of the pulp. It also indicated that the frugivorous birds used in this study do not only enhance potential dispersal of seeds (mean gut transit times of 22–43 min depending on species) but germination success as well, as found in other studies of frugivory and seed dispersal (Jordano 1983; Charalambidou et al. 2003; Chimera and Drake 2010; Jordaan and Downs 2012).
With regard to seed germination, there are conflicting results explaining the impact of long and short seed transit times. Some studies have shown a higher rate of seed germination when the seed has been retained longer (Barnea et al. 1991), while in other studies there is evidence of a decreased rate (Murray et al. 1994; Charalambidou et al. 2003), or no impact (Barnea et al. 1990, 1991). Regardless of their effect on seed germination, overall the main function of bird frugivory is to disperse seeds away from the parent plant.
The three frugivorous bird species used in this study had R. cuneifolius mean seed transit times > ~ 20 min showing that transit times were long enough to allow seed dispersal away from the parent plant, and that bird species have potential to disperse R. cuneifolius. Furthermore, germination percentages for ingested R. cuneifolius seeds in all frugivores were similar and high (~ 75%). In particular, these were higher than R. cuneifolius whole fruit germination percentages (~ 10%) highlighting that bird ingestion was beneficial for germination and potentially important in this invasive weed’s success. Therefore, native avian frugivores have a role in both the potential dispersal and germination of R. cuneifolius. The data obtained on digestive tract passage rates and germination success in this study can be used, in conjunction with information on bird foraging behaviour, to model the spread of R. cuneifolius and design control strategies for this invasive species. However, it would be important to collect similar data on digestive tract passage time and germination success for other potential dispersers, especially mammalian species such as Chacma baboons (Papio ursinus) and vervet monkeys (Chlorocebus pygerythrus), that also consume R. cuneifolius fruits (pers. obs.).
In summary, our results suggest that dark-capped bulbuls, red-winged starlings and speckled mousebirds enhance the germination success and potentially facilitate the dispersal of seeds (sufficient retention time to allow dispersal away from the parent) so as to increase the establishment and spread of invasive R. cuneifolius, and potentially other fleshy-fruited alien species. Preliminary studies have shown that fire could be used to change the number of R. cuneifolius fruit produced per plant or per area (Pollard 2013). Consequently, effective and viable management strategies for R. cuneifolius should aim to control plants prior to fruiting or, where this is not possible, to reduce the number and/or availability of fruits to avian frugivores.
References
Barnea A, Yom-Tov Y, Friedman J (1990) Differential germination of two closely related species of Solanum in response to bird ingestion. Oikos 57:222–228
Barnea A, Yom-Tov Y, Friedman J (1991) Does ingestion by birds affect seed germination? Funct Ecol 5:394–402
Buckley YM, Anderson S, Catterall CP, Corlett RT, Engel T, Gosper CR, Nathan R, Richardson DM, Setter M, Spiegel O (2006) Management of plant invasions mediated by frugivore interactions. J Appl Ecol 43:848–857
Cain ML, Milligan BG, Strand AE (2000) Long-distance seed dispersal in plant populations. Am J Bot 87:1217–1227
Charalambidou I, Santamaria L, Langevoord O (2003) Effect of ingestion by five avian dispersers on the transit time, retrieval and germination of Ruppia maritima seeds. Funct Ecol 17:747–753
Chimera CG, Drake DR (2010) Effects of pulp removal on seed germination of five invasive plants in Hawaii. Plant Prot Q 25:137
Clergeau P (1992) The effect of birds on seed germination of fleshy-fruited plants in temperate farmland. Acta Oecol 13:679–686
Craig A (2005) Red-winged starling. In: Hockey P, Dean W, Ryan P (eds) Robert’s birds of Southern Africa, VII edn. Trustees of the John Voelcker Bird Book Fund, Cape Town, pp 961–962
Craig A, Feare C (2010) Starlings and Mynas. A&C Black, Barcelona
Cypher BL, Cypher EA (1999) Germination rates of tree seeds ingested by coyotes and raccoons. Am Midl Nat 142:71–76
D’Avila G, Gomes-Jr A, Canary AC, Bugoni L (2010) The role of avian frugivores on germination and potential seed dispersal of the Brazilian pepper Schinus terebinthifolius. Biota Neotrop 10:45–51
Day MD, Wiley CJ, Playford J, Zalucki MP (2003) Lantana: current management status and future prospects. ACIAR, Canberra
Dean WR (2005) Speckled mousebird. In: Hockey P, Dean W, Ryan P (eds) Robert’s birds of Southern Africa, VII edn. Trustees of the John Voelcker Bird Book Fund, Cape Town, pp 197–198
Denny R, Goodall J (1991) Variable effects of glyphosate and triclopyr used for the control of American bramble, Rubus cuneifolius Agg, in pine plantations. S Afr Forest J 159:11–15
Dlamini P, Zacharides C, Downs CT (2018) The effect of frugivorous birds on seed dispersal and germination of the invasive Brazilian pepper tree (Schinus terebinthifolius) and Indian laurel (Litsea glutinosa). S Afr J Botany 114:61–68
Dovrat G, Perevolotsky A, Ne’eman G (2012) Wild boars as seed dispersal agents of exotic plants from agricultural lands to conservation areas. J Arid Environ 78:49–54
Downs CT (2008) Aspects of diet choice and digestion in the dark-capped bulbul Pycnonotus barbatus. Ostrich 79:73–78
Downs CT, Wirminghaus JO, Lawes MJ (2000) Anatomical and nutritional adaptations of the speckled mousebird (Colius striatus). Auk 117:791–794
Ellstrand NC, Schierenbeck KA (2000) Hybridization as a stimulus for the evolution of invasiveness in plants? Proc Nat Acad Sci 97:7043–7050
Erasmus DJ (1984) A.3 Bramble. Weeds: Farming in South Africa. Department of Agriculture, Pretoria, South Africa
Ezemvelo KZN Wildlife (2016) Alien and invasive species management plan for the Maloti-Drakensberg Park World Heritage Site. Unpublished Management Plan, Pietermaritzburg
Fishpool L, Tobias J (2005) Family Pycnonotidae (Bulbuls). Handb Birds World 10:124–253
Fricke EC, Simon MJ, Reagan KM, Levey DJ, Riffell JA, Carlo TA, Tewksbury JJ (2013) When condition trumps location: seed consumption by fruit-eating birds removes pathogens and predator attractants. Ecol Lett 16:1031–1036
Henderson L (2011) Rubus species—morphology, taxonomy. Southern African plant Invader Atlas News No. 19, ARC-PPRI Weeds Research Programme, 9 pp
Herrera CM (1981) Are tropical fruits more rewarding to dispersers than temperature ones? Am Nat 118:896–907
Hui C, Richardson DM (2017) Invasion dynamics. Oxford University Press, Oxford
Hummer KE (1996) Rubus diversity. HortScience 31:182–183
Invasive Species South Africa (2017) http://www.invasives.org.za/legislation/item/337-american-bramble-rubus-cuneifolius. Downloaded June 2017
Jordaan LA, Downs CT (2012) Comparison of germination rates and fruit traits of indigenous Solanum giganteum and invasive Solanum mauritianum in South Africa. S Afr J Bot 80:13–20
Jordaan L, Johnson SD, Downs CT (2011a) The role of avian frugivores in germination of seeds of fleshy-fruited invasive alien plants. Biol Invasions 13:1917–1930
Jordaan LA, Johnson SD, Downs CT (2011b) Digestion of fruit of invasive alien plants by three southern African avian frugivores. Ibis 153:863–867
Jordaan LA, Johnson SD, Downs CT (2012) Wahlberg’s Epauletted Fruit Bat (Epomophorus wahlbergi) as a potential dispersal agent for fleshy-fruited invasive alien plants: effects of handling behaviour on seed germination. Biol Invasions 14:959–968
Jordano P (1983) Fig-seed predation and dispersal by birds. Biotropica 15:38–41
Kueffer C, Kronauer L, Edwards PJ (2009) Wider spectrum of fruit traits in invasive than native floras may increase the vulnerability of oceanic islands to plant invasions. Oikos 118:1327–1334
Lloyd P (2005) Dark-capped bulbul. In: Hockey P, Dean W, Ryan P (eds) Robert’s birds of Southern Africa, VII edn. Trustees of the John Voelcker Bird Book Fund, Cape Town, pp 766–767
Martin-Albarracin VL, Nuñez MA, Amico GC (2018) Non-redundancy in seed dispersal and germination by native and introduced frugivorous birds: implications of invasive bird impact on native plant communities. Biodivers Conserv 27:3793
Mokotjomela TM, Hoffmann JH, Downs CT (2015) The potential for birds to disperse the seeds of Acacia cyclops, an invasive alien plant in South Africa. Ibis 157:449–458
Mokotjomela T, Downs CT, Esler K, Knight J (2016) Seed dispersal effectiveness: a comparison of four bird species feeding on seeds of invasive Acacia cyclops in South Africa. S Afr J Botany 105:259–263
Moles AT, Drake DR (1999) Post-dispersal seed predation on eleven large-seeded species from the New Zealand flora: a preliminary study in secondary forest. New Zeal J Bot 37:679–685
Moore PD (2001) Ecology: the guts of seed dispersal. Nature 414:406–407
Morris M, Wood A, Den Breeÿen A (1999) Plant pathogens and biological control of weeds in South Africa: a review of projects and progress during the last decade. Afr Entom Mem 1:129–137
Murray KG, Russell S, Picone CM, Winnett-Murray K, Sherwood W, Kuhlmann ML (1994) Fruit laxatives and seed passage rates in frugivores: consequences for plant reproductive success. Ecology 75:989–994
Olckers T (1999) Biological control of Solanum mauritianum Scopoli (Solanaceae) in South Africa: a review of candidate agents, progress and future prospects. Afr Entom Mem 1:65–73
Olckers T (2004) Targeting emerging weeds for biological control in South Africa: the benefits of halting the spread of alien plants at an early stage of their invasion: working for water. S Afr J Sci 100:64–68
Panetta F (2001) Seedling emergence and seed longevity of the tree weeds Celtis sinensis and Cinnamomum camphora. Weed Res 41:83–95
Panetta F, McKee J (1997) Recruitment of the invasive ornamental, Schinus terebinthifolius, is dependent upon frugivores. Austr J Ecol 22:432–438
Parsons W, Cuthbertson E (1992) Noxious weeds of Australia. Inkata Press, Melbourne
Paulsen T, Högstedt G (2002) Passage through bird guts increases germination rate and seedling growth in Sorbus aucuparia. Funct Ecol 16:608–616
Pollard AD (2013) The potential use of fire as a means of large-scale control of American bramble (Rubus cuneifolius), in the uKhahlamba Drakensberg Park. Unpublished Honours thesis, University of Stellenbosch
Pyšek P, Hulme PE (2005) Spatio-temporal dynamics of plant invasions: linking pattern to process. Ecoscience 12:302–315
Renne IJ, Gauthreaux SA Jr, Gresham CA (2000) Seed dispersal of the Chinese tallow tree (Sapium sebiferum (L.) Roxb.) by birds in coastal South Carolina. Am Midl Nat 144:202–215
Richardson DM, Van Wilgen BW (2004) Invasive alien plants in South Africa: how well do we understand the ecological impacts? Working for Water. S Afr J Sci 100:45–52
Richardson DM, Allsopp N, D’antonio CM, Milton SJ, Rejmanek M (2000) Plant invasions—the role of mutualisms. Biol Rev 75:65–93
SABAP2 Southern African Bird Atlas Project (2019) Viewed 5 October 2019, http://sabap2.adu.org.za/
Sochor M (2018) Preliminary taxonomic treatment of eastern South African Rubus. Unpublished report, 44 pp
Southern African Plant Invaders Atlas (SAPIA) Database (2016) Plant Protection and Research Institute, Agricultural Research Council, viewed 16 August 2016, from http://www.arc.agric.za/arc-ppri/Pages/Weeds%20Research/Geographical-distribution-of-IAPs-in-southern-Africa-(SAPIA)-.aspx
Symes CT, Downs CT (2001) Feeding and energy intake in two avian frugivores, the blackeyed bulbul Pycnonotus barbartus and speckled mousebird Colius striatus. Durban Mus Novit 26:20–24
Symes CT, Nicolson SW, McKechnie AE (2008) Response of avian nectarivores to the flowering of Aloe marlothii: a nectar oasis during dry South African winters. J Ornith 149:13–22
Thabethe V, Wilson A-L, Hart L, Downs CT (2015) Ingestion by an invasive parakeet species reduces germination success of invasive alien plants relative to ingestion by indigenous turaco species in South Africa. Biol Invasions 17:1–11
Thibault M, Masse F, Pujapujane A, Lannuzel G, Bordez L, Potter MA, Fogliani B, Vidal E, Brescia F (2018) “Liaisons dangereuses”: the invasive red-vented bulbul (Pycnonotus cafer), a disperser of exotic plant species in New Caledonia. Ecol Evol 18:9259–9269
Traveset A, Willson MF (1997) Effect of birds and bears on seed germination of fleshy-fruited plants in temperate rainforests of southeast Alaska. Oikos 80:89–95
Traveset A, Riera N, Mas RE (2001) Ecology of fruit-colour polymorphism in Myrtus communis and differential effects of birds and mammals on seed germination and seedling growth. J Ecol 89:749–760
Van Kleunen M, Johnson SD (2007) South African Iridaceae with rapid and profuse seedling emergence are more likely to become naturalized in other regions. J Ecol 95:674–681
Wilson A-L, Downs CT (2012) Knysna Turacos (Tauraco corythaix) do not improve seed germination of ingested fruit of some indigenous South African tree species. S Afr J Bot 78:55–62
Witmer MC, Cheke AS (1991) The dodo and the tambalacoque tree: an obligate mutualism reconsidered. Oikos 61:133–137
Yagihashi T, Hayashida M, Miyamoto T (1998) Effects of bird ingestion on seed germination of Sorbus commixta. Oecologia 114:209–212
Yagihashi T, Hayashida M, Miyamoto T (1999) Effects of bird ingestion on seed germination of two Prunus species with different fruit-ripening seasons. Ecol Res 14:71–76
Acknowledgements
Special thanks to the Department of Water and Sanitation (ZA) for providing funding to KLM. We thank E. Ally and A. Young for technical assistance. We are grateful to P. Singh and C. Munyai for assisting with data analyses. We are grateful for financial support from the National Research Foundation (ZA) and DST-NRF Centre for Invasion Biology, University of Stellenbosch. Birds were caught and kept in captivity under Permit OP485-2016 issued by Ezemvelo KZN Wildlife, and experimental procedures approved by the UKZN Ethics Committee (Authorisation number 020/15/animal). We are most grateful for the constructive comments of the reviewers.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Molefe, K.L., Tedder, M.J., Thabethe, V. et al. Role of native avian frugivores in germination facilitation and potential dispersal of invasive American bramble (Rubus cuneifolius) in South Africa. Biol Invasions 22, 1109–1120 (2020). https://doi.org/10.1007/s10530-019-02164-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-019-02164-w