Abstract
The exotic macrophyte species Myriophyllum aquaticum (Vell.) Verdc. is widely dispersed, mainly through stem fragments and rhizomes, in shallow littoral zones in which uniform canopy conditions are created by the pre-existing resident wetland plant species. Therefore, interactions between these two types of asexual propagules and the resident species may occur during the early establishment and growth phases of M. aquaticum. We tested the hypothesis that the establishment and growth of M. aquaticum are affected by three factors: the number of propagules supplied, the presence of a standing biomass of Carex japonica Thunb. and the presence of thick litter layers in the littoral zone. M. aquaticum rhizomes and stem fragments were introduced into a standing biomass of C. japonica in mesocosms using two types of sediments (littoral and sand-clay) and two propagule supply levels. After 4 months, the survival rates of both the rhizomes and stem fragments were high under all of the experimental treatments. The propagule supply positively affected the survival rates and growth of M. aquaticum. The survival of the rhizomes was unaffected by the presence of either C. japonica or littoral sediments, whereas the survival of the stem fragments was reduced by the presence of C. japonica. The presence of litter layers is a primary factor facilitating the growth of M. aquaticum propagules because of the high nutrient content of the litter. In addition, the presence of the standing C. japonica biomass and newly growing Eleocharis yokoscensis (Franch.et Sav.) Tang et Wang (a small ruderal species that, unexpectedly, grew rapidly following the removal of the standing biomass of C. japonica) reduced the growth of the M. aquaticum stem fragments and rhizomes, respectively. Our findings suggest that the loss of vegetative cover resulting from intense cattle herbivory and other factors in littoral zones may accelerate the invasion of M. aquaticum. An effective approach for preventing M. aquaticum invasion is to reduce the propagule supply and prevent propagules from dispersing.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The biological invasion of exotic species has caused increasing damage to ecosystems and the environment and has become a global ecological problem (Williamson 1996; Vitousek et al. 1997). In the case of plants, the invasion of exotic species has decreased the survival rates of local species and significantly affected their diversity and distribution (Funk and Vitousek 2007; Pyšek and Richardson 2007). Because asexual reproduction is more common in aquatic plants than in terrestrial plants, asexual propagules, such as woody cuttings, leaf sections or stem sections, play an important role in terms of plant invasion in aquatic habitats (Santamaría 2002; Wright and Davis 2006; Silvertown 2008; Dong et al. 2010b; Xie et al. 2010). Recently studies revealed extremely limited genotypic variation among populations of aquatic invading species, indicating that these species invade and become established in new habitats via asexual propagules (Okada et al. 2009; Zhang et al. 2010). Indeed, certain asexual propagules, such as stem fragments, are highly suited for dispersal in aquatic habitats (Dong et al. 2010a; Xie et al. 2010). Compared with other types of asexual propagation, stem fragments have the advantages of easy production and dispersal, long periods of survival and high colonization and regeneration abilities (Riis and Sand-Jensen 2006). For example, Myriophyllum spicatum L. are invading 45 states in the US and also in the Canadian provinces of British Columbia, Ontario and Quebec via fragment dispersal (Jacono and Richerson 2008). In southern China, fragments of Alternanthera philoxeroides (Mart.) Griseb. are also widespread in water bodies and irrigation ditches, which causes the invasion of this species (Dong et al. 2010a, 2012). Once established in favorable environments, invasive species may grow fast and produce large number of branches, which would increase the propagule pool (stem fragments) and potentially increase invasion success in both local habitat and surrounding habitats (Didham et al. 2007; Ehrenfeld 2010; Xie et al. 2010). However, compared with numerous studies in terrestrial habitats, fewer studies have focused on the invasions in aquatic habitat. Therefore, a better understanding of the determinants of the survival and growth of aquatic asexual propagules is of both scientific and practical interest.
Previous studies have shown that plant invasion is the outcome of complicated interactions that involve many biotic and abiotic factors (Davis et al. 2000; Daehler 2003; Lockwood et al. 2005; Melbourne et al. 2007; Chun et al. 2010), which can be divided into three broad categories: propagule supply, resource availability, and ecological resistance (Davis et al. 2000; Daehler 2003; Sanders et al. 2007; Simberloff 2009; Ehrenfeld 2010). Invasion dynamics can be altered by increases in resource availability due to resource enrichment or frequent disturbances within ecosystems, such as eutrophication and grazing (Davis et al. 2000). For instance, there are several types of globally distributed submersed weeds (e.g., Hydrilla and Myriophyllum) that are typically better adapted and competitive than native species under eutrophic conditions, conferring advantages over the native species in terms of resource uptake, often resulting in invasions (Ruiz et al. 1999; Chase and Knight 2006). However, increased availability of resources are not the only cause of invasion and must also coincide with the sustained arrival of new propagules (Davis et al. 2000; Melbourne et al. 2007). As more propagules arrive (because of either increased propagule numbers or an increased frequency of arrival events), the probability of successful invasion increases (Lockwood et al. 2005; Simberloff 2009). Although propagule supply is an obvious and likely factor in plant invasions, relatively few experimental studies have addressed the role of propagule supply in shaping this process, particularly with regard to asexual propagules of aquatic plants (Von Holle and Simberloff 2005; Simberloff 2009).
According to the “ecological resistance hypothesis”, resident native communities may indirectly control invasion success by reducing the input of exotic propagules and resource availability (e.g., acting as a barrier, Levine et al. 2004), thereby inhibiting the establishment and spreading phases of the invaders (Elton 1958; Levine et al. 2004). These indirect mechanisms are helpful in predicting invasion dynamics and controlling the invasive species and to elucidate the interaction between invasive and native species in ecosystems (Levine et al. 2004). However, most studies to date have focused on the direct competition between the resident and exotic plant species and have not include observations of such phases (Vilà and Weiner 2004; Tanentzap and Bazely 2009). Furthermore, for aquatic plants, buoyant asexual propagules (e.g., stem fragments) are more likely to be trapped in shallow water or riparian vegetation than seeds and may establish fast through rapid adventitious roots production under such relatively stable environments (Boedeltje et al. 2003; Riis and Sand-Jensen 2006). Thus, the complicated interactions among the resource availability, propagule supply and resident species may affect the establishment phase and growth of exotic species. Our understanding of the factors contributing to the process of invasion in freshwater habitats remains limited.
Myriophyllum aquaticum (Vell.) Verdc. is a semi-submersed aquatic macrophyte that is native to the Amazon River in South America, and has become a harmful weed species in North America since it was introduced circa the late 1800s (Sutton 1985). During the twentieth century, this species colonized areas in South Africa, East Asia, Europe, New Zealand and Australia (Sheppard et al. 2005; Thiébaut 2007). Once M. aquaticum reaches the water surface, extensive vertical shoots occur as the growth of horizontal stem, which facilitates the rapid covering of the water surface (Wersal and Madsen 2011a). This species can cause many localized problems, such as forming thick monospecific stands that reduces the relative abundance and richness of other local species, impeding water movement and increasing the eggs and larvae of mosquito in streams and ditches in its invasion regions (Sytsma and Anderson 1993a; Wersal et al. 2011). After being introduced into East Asia as an ornamental species, it escaped into the wild and became widely distributed in aqueducts in Japan, Taiwan and mainland China (Xie et al. 2001). M. aquaticum is dioecious, but staminate plants are rare, even in its native range, and seed production is, therefore, limited in natural habitats (Sutton 1985). Previous study reported that M. aquaticum can tolerate frost out of its native range (Aiken 1981; Thiébaut 2007), suggesting that this species may have the ability to invade more northern places. The plants lack structures for storage, dispersal, and perennation (e.g., tubers or turions), and the stem fragments and rhizomes (older submersed stems without leaves) are believed to serve all of these functions in both native and invasive ranges (Sytsma and Anderson 1993a). These asexual propagules are able to float for a long time (six months) and disperse over great distances (several kilometers) between water bodies through the production of adventitious roots which take up nutrients from the water column (Boedeltje et al. 2003; Wersal and Madsen 2011b; Sarneel 2012). Once these roots attach to the sediment, M. aquaticum typically displays high colonization and regeneration abilities (Xie et al. 2010).
M. aquaticum usually invades shallow aquatic habitats, such as wetlands, littoral zones of lakes, ponds, sloughs, and backwaters (Wersal and Madsen 2011b). When propagules of M. aquaticum arrive in such habitats, the nutrient-rich sediments arise from the long decomposition of aboveground plant tissues often support the nuisance growth of this species (Wersal and Madsen 2011b). The pre-existing littoral vegetation may also provide strong resistance (e.g., shading or competition for nutrients) against establishment of the propagules. However, it remains unclear how these factors interact with each other and influence the establishment and growth of M. aquaticum. In this study, the following hypotheses were examined: (1) an increase in the propagule supply will increase the establishment and growth of M. aquaticum, even under conditions of heavy resistance; (2) littoral layers will facilitate the establishment and growth of M. aquaticum through high nutrient availability; and (3) the removal of pre-existing native species (e.g., Carex species) will increase the establishment and growth of M. aquaticum.
Materials and methods
Study-site
The study was conducted at the National Field Station of Freshwater Ecosystem of Liangzi Lake, China (30º5′–30º18′N, 114º21′–114º39′E). In the littoral zone of Liangzi Lake, the vegetative cover was dominated by Carex japonica Thunb. (Yu et al., unpublished data), accounting for approximately 90 % of the standing biomass at the study site. The water depth at the site was 5–10 cm.
Experimental design
A mesocosm study was conducted during a 4-month period from May to September 2009. In April 2009, 48 mesocosms (40 × 30 × 20 cm) were constructed, each containing one patch (40 × 30 × 15 cm) of C. japonica dug from the littoral zone. An additional 48 mesocosms were filled with local sediment (a sand-clay mixture taken from beneath the littoral layer, 15 cm deep). We hypothesize that survival and growth of M. aquaticum should be lower in this sediment than in the littoral layer due to lack of nutrients. The 96 mesocosms were left in situ until the experiment began.
The experimental treatments were performed using a randomized block design to separate microsite effects from the treatment effects. A factorial experimental design was employed (Fig. 1) that included two types of sediment (littoral and sand-clay mixture), two levels of light treatment (unshaded and shaded), and two propagule supply levels [10 propagules per mesocosm (approximately 80 propagules m−2) and 5 propagules per mesocosm (approximately 40 propagules m−2)] in 6 blocks, with one replicate per block. The low propagule supply density approximated the density observed in the natural populations of M. aquaticum in Liangzi Lake. The littoral sediment was obtained from the littoral layer (with few decomposing litter on the surface and we did not cleared them away during the experimental period) in which C. japonica occurs and was mainly a mud substratum (mostly composed of the decomposed shoot biomass of C. japonica); the mixed sediment was a mixture of the sand and clay substrata located below the littoral layer inhabited by C. japonica. In the unshaded treatment with littoral sediment, the standing biomass of C. japonica was removed by cutting with scissors once a week; approximately 1 cm of C. japonica was retained because complete removal would have damaged the sediment surface. In the mixture treatment, black plastic nets were used for shading to achieve a light intensity similar to that was observed under C. japonica canopies. Detailed information on the treatments is provided in Table 1.
Experimental design in one block. Rhizomes and stem fragments of M. aquaticum were placed in mesocosms that were subjected to different treatments. Two propagule supply levels were introduced to littoral and mixed sediments, with or without standing C. japonica biomass. To achieve a similar light intensity under the C. japonica canopies, black plastic nets were used for shading in the mixed-substrate treatment. In the experiment, there were 6 total blocks for rhizomes (48 mesocosms total, with 3 factors of 2 levels each), and another 6 blocks for stem fragments (48 mesocosms total, with 3 factors of 2 levels each)
A total of 360 rhizomes (in 48 mesocosms total, with 3 factors of 2 levels each) and 360 stem fragments (also in 48 mesocosms total, with 3 factors of 2 levels each) of M. aquaticum were used as propagules in this study and were collected from 4 different populations (locations) in Liangzi Lake. For rhizomes/stem fragments, 5 propagules were placed into each of 24 mesocosms to achieve the low propagule supply treatment, and 10 propagules were placed into each of 24 mesocosms to achieve the high propagule supply treatment. The rhizomes and stem fragments were cut into 7-cm long pieces (with at least 2 internodes), their fresh weights were recorded, and they were placed randomly (in terms of both their location within mesocosm and the population of origin) into the mesocosms. A total of 48 sediment cores (radius of 1 cm, depth of 5 cm) for each light treatment and sediment type were sampled and digested with a K-435 digestion unit (Büchi® Labortechnik AG, Flawil Switzerland) for total nitrogen (TN) and total phosphorus (TP) analysis. For TP analysis, the samples were digested with a HCLO4-H2SO4 method (Bray and Kurz 1945). All samples were analyzed for the TN concentration using a KjelFlex B-324 nitrogen analyzer (Büchi® Labortechnik AG, Flawil Switzerland) and for the TP concentration using a IL-500P phosphorus analyzer (Hach® Company, Loveland, USA) (Table 1).
During the experimental period, dead rhizomes and stem fragments were removed from the mesocosms. All of the rhizomes and stem fragments were planted on 10 May 2009 and harvested on 10 September 2009. Lake water [TN 0.33–0.57 mg L−1, TP 0.03–0.11 mg L−1 during the experimental period; water samples were analyzed once a week using a IL-500 N nitrogen analyzer and a IL-500P phosphorus analyzer (Hach® Company, Loveland, USA)] was added to the mesocosms every day to compensate for evaporation and to maintain a constant water level (5 cm) during the experiment. The sediment surface temperature and light intensity were recorded everyday at noon using temperature and external quantum sensors (LI-6400, Li-Cor Inc., Lincoln, Nebraska, USA) (Table 1). The following characteristics were measured at the time of harvest: propagule survival, number of new shoots and stem length. The propagules were divided into roots, leaves, and stems, dried at 70 °C, and weighed. Following the removal of the standing biomass of C. japonica, we observed the sprouting of a small ruderal species Eleocharis yokoscensis (Franch.et Sav.) Tang et Wang; we did not remove these plants because they grew rapidly and their complete removal might have damaged the sediment surface. Instead, the E. yokoscensis present were also harvested at the end of the experiment (Table 1).
Statistical analyses
The data including the survival rate (expressed as the percent of surviving propagules), plant total biomass, root:shoot ratio, number of new apical shoots and the total stem length per mesocosm, were analyzed using three-way ANOVA, with the sediment type, light intensity and propagule supply as the fixed factors and the block as the random factor, all the interactions among the fixed factors were tested. Duncan tests were used to compare the levels within factors for significance (p < 0.05). All of the experimental data were transformed using a log(x) function to meet the homogeneity of variance or a normal distribution of residuals and then analyzed. All of the data were analyzed using the SPSS 19.0 program (SPSS, Chicago, IL, USA).
Results
High survival rates were observed for both rhizomes and stem fragments of M. aquaticum during the 4 months of the study. The propagule supply had a significant effect on the survival of both the rhizomes and stem fragments (Table 2): larger propagule supplies were associated with higher survival rates (Fig. 2a, f). The propagule supply showed a significant positive relationship with all of the growth traits of the M. aquaticum propagules, with the exception of the root:shoot ratio (Fig. 2; Table 2).
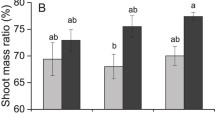
Comparison of the survival (a, f), total plant biomass (b, g), root: shoot ratio (c, h), number of new shoots (d, i) and total stem length of the rhizomes and stem fragments (e, j) of M. aquaticum among the treatments. The data are presented as the mean ± s.e. (n = 6). Bars having different letters indicate significant differences among the treatments (p < 0.05, three-way ANOVA with Duncan correction). The data were transformed using the log (x) function
Neither the survival of rhizome nor the survival of stem fragments was significantly affected by the sediment type (Table 2). The sediment type significantly affected the total biomass, root:shoot rations, the number of new shoots production and the total stem lengths of rhizomes and stem fragments (Table 2). When growing on littoral sediments, both of these propagules accumulated more biomass than those grown on the mixed sediment (Fig. 2b, g), and the propagules grown on littoral sediments had lower root:shoot ratios than those grown on the mixed sediment (Fig. 2c, h). In addition, the propagules grown on the littoral sediments produced more new shoots and exhibited a greater total stem length compared with those grown on the mixed sediments (Fig. 2d, e, i, j).
Shading (the presence of standing C. japonica biomass or plastic nets) only had a significant effect on stem fragment survival (Table 2). The shading treatment significantly reduced the total biomass of the stem fragments in the littoral sediments, but had no significant effect on the total biomass of the stem fragments in the mixed sediment (Fig. 2 g), whereas shading had no significant effect on the total biomass of the rhizomes in either the littoral or mixed sediment (Table 2; Fig. 2b). The root:shoot ratio of the stem fragments grown under low light conditions was lower than the stem fragments grown under high light conditions (Table 2; Fig. 2c), and shading caused a significant decrease in the root:shoot ratio only in the littoral sediments at a low supply of rhizome (Table 2; Fig. 2h). Shading significantly reduced the production of new shoots for both types of propagules and the total stem length of the stem fragments in the littoral sediments, but had no significant effect on either of these parameters for either types of propagule in the mixed sediments (Fig. 2d, e, i, j).
Discussion
Although previous studies have indicated the importance of asexual propagules in the invasion and establishment of populations by exotic species in freshwater habitats, few of these studies have focused on the mechanisms whereby pre-existing native plant species and local environmental factors influence the establishment and growth of exotic species (Lozon and MacIsaac 1997; Dong et al. 2010b, 2012). In the present study, a high survival rate was observed for both rhizomes and stem fragments of M. aquaticum, indicating that these two types of propagules are well adapted for propagation (Sytsma and Anderson 1993a; Hussner 2009; Wersal and Madsen 2011b). A greater propagule supply was associated with the higher survival and growth parameters (e.g., total biomass, number of new shoots, and total stem length) of both types of propagules, supporting the concept that as the release of more individuals into a community is expected to buffer demographic decreases in survival or reproduction in a population after its initial introduction, even when environmental negative influences are high (Lockwood et al. 2005; Von Holle and Simberloff 2005; Melbourne et al. 2007; Simberloff 2009). Our results may seem counterintuitive as the lack of density-dependent effect on survival and growth. This may due to the following reasons: first, the horizontal and vertical growth of M. aquaticum shoots could mitigate the space limitation, coinciding with results in marine ecosystems that more space will lead to high survival of invaders and low intraspecific/interspecific competitions (Britton-Simmons and Abbott 2008; Clark and Johnston 2009); second, our density of M. aquaticum biomass was lower than the biomass in its invasive regions (e.g., 2 kg dry weight m−2 in Germany, with sediment TN 0.007 mg g−1 and TP 0.35 mg g−1) (Hussner 2009). Therefore, if our initial propagule density is larger or experiment period lasts longer, the density-dependent effect would emerge. Moreover, our results also suggest that clonal invading species (e.g., M. aquaticum) have great ecological impacts on native communities (Liu et al. 2006; Jia et al. 2009), not only because the clonal species have adaptive advantages in heterogeneous environments (Hood and Naiman 2000), but more importantly, multiple types of asexual propagule may cause species-specific invasions (e.g., M. spicatum and A. philoxeroides) (Smith et al. 2002; Liu and Yu 2009). However, there have been few studies to date on the impact of asexual propagule pressure on native communities, and further investigation is needed.
The survival of rhizomes and stem fragments was not significantly affected by the sediment type, consistent with our previous study on stem fragments of M. aquaticum (Xie et al. 2010). A probable explanation for this finding is that both of these propagule types contain large amounts of storage resources, helping M. aquaticum adapt to and survive in heterogeneous environments (Sytsma and Anderson 1993a; Wersal et al. 2011). Although the growth on littoral sediments did not increase the survival rates of either of these two types of propagules, it did significantly increase their growth parameters because of the higher nutrient availability. Both the total biomass and aboveground biomass (low root:shoot ratios) of these propagules in the littoral sediments were higher than those of the propagules in the mixed sediments, and similar results were obtained for the number of new shoots and the total stem length. These results are consistent with those of previous studies showing that M. aquaticum prefers nutrient-rich sites (Sytsma and Anderson 1993b; Hussner et al. 2009), and plants tend to accumulate more shoot biomass under nutrient-rich conditions (Hermans et al. 2006). The M. aquaticum plants used in the present study, which produced more apical shoots and longer stems in littoral sediments, may have larger propagule pools and potentially threaten both local and surrounding habitats because M. aquaticum stems are brittle and easily fragmented (Xie et al. 2010; Wersal et al. 2011). Therefore, the eutrophication currently occurring in many bodies of water in China may favor the establishment of M. aquaticum (Xie et al. 2010), similar to the manner in which eutrophication processes allowed M. aquaticum to become the dominant species in the lakes of Europe (Sheppard et al. 2005) and North America (Sytsma and Anderson 1993a). In the present study, the low root:shoot ratios (mostly significant in stem fragments) associated with the decreased number of new shoot, is thought to be a relevant strategy for macrophytes under poor light conditions (Aiken 1981; Barko and Smart 1981). However, the values of the growth parameters (e.g., total biomass, number of new shoot and total stem length) measured were relatively low and were not significantly affected by high vs. low light intensity for the M. aquaticum growing on mixed sediments. Consistent with previous findings for M. aquaticum and M. spicatum (Xie et al. 2007; Hussner et al. 2009), we suggest that a low nutrient availability rather than a low light intensity caused the decreased growth of M. aquaticum in mixed sediments.
In the present study, the pre-existing populations of C. japonica in the littoral sediments caused significant reduction of the survival and growth rates of the M. aquaticum stem fragments, a finding that is in contrast to previous studies in which the M. aquaticum stem fragments display strong regeneration and colonization abilities, particularly in nutrient-rich habitats (Sytsma and Anderson 1993b; Xie et al. 2010). Our findings are consistent with those other studies indicating that M. aquaticum populations are less competitive and show poor growth under a sustained low light intensity (e.g., when shaded by taller and more robust emergent macrophytes or under deep flood conditions) (Sytsma and Anderson 1993b; Wersal and Madsen 2011a). M. aquaticum has a relatively high light saturation point with regard to gas exchange (>2,000 μmol photons m−2 s−1) and prefers a high light intensity (Hussner 2009). Therefore, disturbances that reduce the C. japonica cover in the littoral zone, such as increasing levels of cattle herbivory (Kreyling et al. 2011), are likely to facilitate the invasion of M. aquaticum stem fragments.
The total biomass and total stem length of the rhizomes were not increased by a high light intensity in the littoral sediments, indicating that other factors affect rhizome growth. We hypothesize that the aboveground biomass of C. japonica remaining after cutting and the new E. yokoscensis growth may have decreased the rhizome growth in the littoral sediments under high light conditions. Because the remaining C. japonica and the newly growing E. yokoscensis are short and robust, they can impose a physical barrier that decreases the probability of rooting for those rhizomes that reach the sediment surface. Also, the unclipped C. japonica we used as the shade treatment in the littoral mesocosms could potentially have imposed a physical barrier on rooting M. aquaticum propagules. Similar results were obtained in H. verticillata fragments invading V. americana populations in North America (Chadwell and Engelhardt 2008). Moreover, the number of newly produced shoots of rhizomes growing in littoral sediments were higher than in mixed sediment, suggesting that these rhizomes still present a threat to the local aquatic habitats, if the environmental conditions are favorable (e.g., higher nutrient availability and light intensity) (Barrat-Segretain 2005). Overall, it seems that M. aquaticum stem fragments are subject to resistance by C. japonica mainly through shading (or possible physical barrier), whereas M. aquaticum rhizomes are subject to resistance by remaining C. japonica aboveground biomass and new E. yokoscensis growth mainly through nutrient competition and physical capture.
Stem fragments grew more than rhizomes, supporting the concept that stem fragments with leaves are more invasive than rhizomes in aquatic habitats (Hussner 2009; Dong et al. 2010a). For instance, the number of new shoots produced by M. aquaticum stem fragments was 5 fold higher than that produced by the rhizomes over a period of 8 weeks (Hussner 2009). The leaves of aquatic plants can have a short-term storage function (Sytsma and Anderson 1993a; Wersal et al. 2011), and also act as primary carbohydrate producers through photosynthesis (Dong et al. 2010a). For these reasons, stem fragments with more leaves may withstand adverse environments more successfully.
Conclusions
In China, M. aquaticum is now considered to be an introduced macrophyte species that is capable of invading aquatic habitats. The present study revealed that rhizomes and stem fragments are important means of dispersal for this species. Both of these types of asexual propagules can survive for a long period (4 months) during dispersal. Increasing the propagule supply can increase the rhizome and stem fragment survival rates in M. aquaticum. Due to its high nutrient content, the aboveground C. japonica litter is a primary factor facilitating the establishment of M. aquaticum. The presence of standing C. japonica biomass and newly growing E. yokoscensis reduced the growth of M. aquaticum stem fragments and rhizomes, respectively. Thus, disturbances that reduce the vegetative cover in the littoral zone, such as increasing levels of cattle herbivory, are likely to encourage M. aquaticum invasion if stem fragments of this species are present. M. aquaticum rhizome growth was not increased by decreasing the cover of C. japonica, most likely because the remaining C. japonica and newly growing E. yokoscensis imposed a physical barrier to such growth. Our study underscore how propagule supply, resource availability and resident vegetation interact and shape the invasion dynamics of exotic macrophyte, which could help predict the spread and potential impact of exotic macrophytes on natural aquatic ecosystems.
References
Aiken SG (1981) A conspectus of Myriophyllum (Haloragaceae) in North America. Brittonia 33:57–69
Barko JW, Smart RM (1981) Comparative influences of light and temperature on the growth and metabolism of selected submersed freshwater macrophytes. Ecol Monogr 51:219–236
Barrat-Segretain MH (2005) Competition between invasive and indigenous species: impact of spatial pattern and developmental stage. Plant Ecol 180:153–160
Boedeltje G, Bakker JP, Bekker RM, Van Groenendael JM, Soesbergen M (2003) Plant dispersal in a lowland stream in relation to occurrence and three specific life-history traits of the species in the species pool. J Ecol 91:855–866
Bray RH, Kurz LT (1945) Determination of total organic and available forms of phosphorus in soils. Soil Sci 59:39–45
Britton-Simmons KH, Abbott KC (2008) Short- and long-term effects of disturbance and propagule pressure on a biological invasion. J Ecol 96:68–77
Chadwell TB, Engelhardt KAM (2008) Effects of pre-existing submersed vegetation and propagule pressure on the invasion success of Hydrilla verticillata. J Appl Ecol 45:515–523
Chase JM, Knight TM (2006) Effects of eutrophication and snails on Eurasian watermilfoil (Myriophyllum spicatum) invasion. Biol Invasions 8:1643–1649
Chun YJ, van Kleunen M, Dawson W (2010) The role of enemy release, tolerance and resistance in plant invasions: linking damage to performance. Ecol Lett 13:937–946
Clark GF, Johnston EL (2009) Propagule pressure and disturbance interact to overcome biotic resistance of marine invertebrate communities. Oikos 118:1679–1686
Daehler C (2003) Performance comparisons of co-occurring native and alien invasive plants: implications for conservation and restoration. Annu Rev Ecol Evol Syst 34:183–211
Davis MA, Grime JP, Thompson K (2000) Fluctuating resources in plant communities: a general theory of invasibility. J Ecol 88:528–534
Didham RK, Tylianakis JM, Gemmell NJ, Rand TA, Ewers RM (2007) Interactive effects of habitat modification and species invasion on native species decline. Trends Ecol Evol 22:489–496
Dong BC, Yu GL, Guo W, Zhang MX, Dong M, Yu FH (2010a) How internode length, position and presence of leaves affect survival and growth of Alternanthera philoxeroides after fragmentation? Evol Ecol 24:1447–1461
Dong BC, Zhang MX, Alpert P, Lei GC, Yu FH (2010b) Effects of orientation on survival and growth of small fragments of the invasive, clonal plant Alternanthera philoxeroides. PLoS One 5:e13631
Dong BC, Alpert P, Guo W, Yu FH (2012) Effects of fragmentation on the survival and growth of the invasive, clonal plant Alternanthera philoxeroides. Biol Invasions 14:1101–1110
Ehrenfeld JG (2010) Ecosystem consequences of biological invasions. Annu Rev Ecol Evol Syst 41:59–80
Elton CS (1958) The ecology of invasions by animals and plants. Wiley, New York, USA
Funk J, Vitousek P (2007) Resource-use efficiency and plant invasion in low-resource systems. Nature 446:1079–1081
Hermans C, Hammond JP, White PJ, Verbruggen N (2006) How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci 11:610–617
Hood WG, Naiman RJ (2000) Vulnerability of riparian zones to invasion by exotic vascular plants. Plant Ecol 148:105–114
Hussner A (2009) Growth and photosynthesis of four invasive aquatic plant species in Europe. Weed Res 49:506–515
Hussner A, Meyer C, Busch J (2009) The influence of water level and nutrient availability on growth and root system development of Myriophyllum aquaticum. Weed Res 49:73–80
Jacono CC, Richerson MM (2008) Myriophyllum spicatum. In: USGS nonindigenous aquatic species database, Gainesville, FL. http://nas.er.usgs.gov/queries/factsheet.aspx?SpeciesID=237. Accessed: 26 April 2012
Jia X, Pan XY, Li B, Chen JK, Yang XZ (2009) Allometric growth, disturbance regime, and dilemmas of controlling in invasive plants: a model analysis. Biol Invasions 11:743–752
Kreyling J, Jentsch A, Beierkuhnlein C (2011) Stochastic trajectories of succession initiated by extreme climatic events. Ecol Lett 14:758–764
Levine JM, Adler PB, Yelenik SG (2004) A meta-analysis of biotic resistance to exotic plant invasions. Ecol Lett 7:975–989
Liu C, Yu D (2009) The bud and root sprouting capacity of Alternanthera philoxeroides after over-wintering on sediments of a drained canal. Hydrobiologia 623:251–256
Liu J, Dong M, Miao SL, Li ZY, Song MH, Wang RQ (2006) Invasive alien plants in China: role of clonality and geographical origin. Biol Invasions 8:1461–1470
Lockwood JL, Cassey P, Blackburn T (2005) The role of propagule pressure in explaining species invasions. Trends Ecol Evol 20:223–228
Lozon JD, MacIsaac HJ (1997) Biological invasions: are they dependent on disturbance? Environ Rev 5:131–144
Melbourne BA, Cornell HV, Davies KF, Dugaw CJ, Elmendorf S, Freestone AL, Hall RJ, Harrison S, Hastings A, Holland M (2007) Invasion in a heterogeneous world: resistance, coexistence or hostile takeover? Ecol Lett 10:77–94
Okada M, Grewell BJ, Jasieniuk M (2009) Clonal spread of invasive Ludwigia hexapetala and L. grandiflora in freshwater wetlands of California. Aquat Bot 91:123–129
Pyšek P, Richardson D (2007) Traits associated with invasiveness in alien plants: where do we stand? In: Nentwig W (ed) Biol invasions. Springer, Heidelberg, Berlin, pp 97–125
Riis T, Sand-Jensen K (2006) Dispersal of plant fragments in small streams. Freshw Biol 51:274–286
Ruiz GM, Fofonoff P, Hines AH, Grosholz ED (1999) Non-indigenous species as stressors in estuarine and marine communities: assessing invasion impacts and interactions. Limnol Oceanogr 44:950–972
Sanders NJ, Weltzin JF, Crutsinger GM, Fitzpatrick MC, Nuñez MA, Oswalt CM, Lane KE (2007) Insects mediate the effects of propagule supply and resource availability on a plant invasion. Ecology 88:2383–2391
Santamaría L (2002) Why are most aquatic plants widely distributed? Dispersal, clonal growth and small-scale heterogeneity in a stressful environment. Acta Oecol 23:137–154
Sarneel J (2012) The dispersal capacity of vegetative propagules of riparian fen species. Hydrobiologia. doi:10.1007/s10750-012-1022-3
Sheppard AW, Shaw RH, Sforza R (2005) Top 20 environmental weeds for classical biological control in Europe: a review of opportunities regulations and other barriers to adoption. Weed Res 46:93–117
Silvertown J (2008) The evolutionary maintenance of sexual reproduction: evidence from the ecological distribution of asexual reproduction in clonal plants. Int J Plant Sci 169:157–168
Simberloff D (2009) The role of propagule pressure in biological invasions. Annu Rev Ecol Evol Syst 40:81–102
Smith DH, Madsen JD, Dickson KL, Beitinger TL (2002) Nutrient effects on autofragmentation of Myriophyllum spicatum. Aquat Bot 74:1–17
Sutton DL (1985) Biology and ecology of Myriophyllum aquaticum. In: Proceeding, 1st international symposium on watermilfoil (Myriophyllum spicatum) and related haloragaceae species. Vancouver, BC, pp 59–71
Sytsma MD, Anderson LWJ (1993a) Biomass, nitrogen, and phosphorus allocation in parrotfeather (Myriophyllum aquaticum). J Aquat Plant Manag 31:244–248
Sytsma MD, Anderson LWJ (1993b) Transpiration by an emergent macrophyte: source of water and implications for nutrient supply. Hydrobiologia 271:97–108
Tanentzap AJ, Bazely DR (2009) Propagule pressure and resource availability determine plant community invisibility in a temperate forest understorey. Oikos 118:300–308
Thiébaut G (2007) Invasion success of non-indigenous aquatic and semi-aquatic plants in their native and introduced ranges. A comparison between their invasiveness in North America and in France. Biol Invasions 9:1–12
Vilà M, Weiner J (2004) Are invasive plant species better competitors than native plant species?—Evidence from pair-wise experiments. Oikos 105:229–238
Vitousek PM, Mooney HA, Lubchenco J, Melillo JM (1997) Human domination of Earth’s ecosystems. Science 277:494–499
Von Holle B, Simberloff D (2005) Ecological resistance to biological invasion overwhelmed by propagule pressure. Ecology 86:3212–3218
Wersal RM, Madsen JD (2011a) Comparative effects of water level variations on growth characteristics of Myriophyllum aquaticum. Weed Res 51:386–393
Wersal RM, Madsen JD (2011b) Influences of water column nutrient loading on growth characteristics of the invasive aquatic macrophyte Myriophyllum aquaticum (Vell.) Verdc. Hydrobiologia 665:93–105
Wersal RM, Cheshier JC, Madsen JD, Gerard PD (2011) Phenology, starch allocation, and environmental effects on Myriophyllum aquaticum. Aquat Bot 95:194–199
Williamson M (1996) Biological invasions. Chapman & Hall, London
Wright JT, Davis AR (2006) Demographic feedback between clonal growth and fragmentation in an invasive seaweed. Ecology 87:1744–1754
Xie Y, Li Z, Gregg WP, Li D (2001) Invasive species in China-an overview. Biodivers Conserv 10:1317–1341
Xie Y, Luo W, Ren B, Li F (2007) Morphological and physiological responses to sediment type and light availability in roots of the submerged plant Myriophyllum spicatum. Ann Bot 100:1517–1523
Xie D, Yu D, Yu LF, Liu CH (2010) Asexual propagations of introduced exotic macrophytes Elodea nuttallii, Myriophyllum aquaticum, and M. propinquum are improved by nutrient-rich sediments in China. Hydrobiologia 655:37–47
Zhang Y–Y, Zhang D-Y, Barrett SCH (2010) Genetic uniformity characterizes the invasive spread of water hyacinth (Eichhornia crassipes), a clonal aquatic plant. Mol Ecol 19:1774–1786
Acknowledgments
We thank Dr. Ling-fei Yu, Hui Wang, Shu-feng Fan and Jin-ning Zhu for laboratory/field assistance and helpful discussions. We also greatly appreciate two anonymous reviewers for valuable comments on an early version of the manuscript. This research was supported by the National Natural Science Foundation of China (30930011 and 31170339).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xie, D., Yu, D., You, WH. et al. The propagule supply, litter layers and canopy shade in the littoral community influence the establishment and growth of Myriophyllum aquaticum . Biol Invasions 15, 113–123 (2013). https://doi.org/10.1007/s10530-012-0272-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-012-0272-3