Abstract
The occurrence of non-native species at high densities may generate competition for resources and possibly exclude native species in various environments. We evaluated the effects of increased densities of the non-native invasive macrophyte Hydrilla verticillata on the growth of the native species Egeria najas in different sediment types and with only root interactions or root + shoot interactions. We tested the hypothesis that the effect of the invasive on the native species is density dependent and that it is greater when competition for light and nutrients occurs (root + shoot interactions). The results of these experiments demonstrated that increased density of the invasive species H. verticillata significantly decreased the growth of the native species independent of sediment type (sand or mud sediments). When plants competed for water and sediment resources (root + shoot interactions), the native species was more impacted by the invasive than when they competed only for water resources (only shoots interacting). Our results show that E. najas is probably unable to colonize sites highly colonized by hydrilla, and this applies to both sand and mud sediments. This outcome suggests that H. verticillata is a threat for E. najas and likely other native submerged species in South America.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Non-native invasive species have attracted great attention due to their potential impacts on natural ecosystems (Mack et al. 2000; Tassin et al. 2011). Several species of aquatic macrophytes are currently spreading rapidly, and many new areas have become invaded by these species (Brundu 2015; MacIsaac et al. 2016; Wang et al. 2016). Invasive aquatic plants are already problematic in many freshwater ecosystems worldwide, where they have caused severe impacts on biodiversity and ecological functions (Clayton and Edwards 2006; Carey et al. 2016; Cuassolo et al. 2016).
Invasive plants that attain success in introduced ranges usually have high growth rates and are very efficient in using resources (Richardson and Rejmanék 2011), which make them competitively superior to native species mainly when resources are abundant (Engelhardt and Anderson 2011). As a result of competition, invasive macrophytes may negatively affect native species (Schultz and Dibble 2012; Zefferman 2015) or even suppress many native species, reducing macrophyte diversity (Madsen et al. 1991; Santos et al. 2011). The inter-specific competition in submerged macrophytes involves mainly the acquisition of light, inorganic carbon (aboveground competition) and nutrients (belowground competition). For example, one experiment that isolated roots and leaves of Hydrilla verticillata (L.f.) Royle (hereafter “hydrilla”) showed that this species outcompetes another submerged species through both aboveground and belowground competition (Wang et al. 2008).
Submerged aquatic macrophytes obtain their nutrient requirements from both water and sediment, but the sediment has been considered the most important source of nutrients for these plants (Carignan and Kalff 1980; Chambers and Kalff 1985; Barko and Smart 1986; Barko et al. 1986; Bolpagni and Pino 2017). Because the abiotic features of water and sediment determine plant growth, the competitive ability of macrophytes is expected to change in different habitats. This expectation was confirmed by several experiments that showed that the degree of inter-specific competition between macrophytes depends on water features and sediment type (e.g., Martin and Coetzee 2014; Shields and Moore 2016) and that the interaction between competitive ability and habitat characteristics may explain the distribution of macrophyte species in environmental gradients (Crain et al. 2004).
In addition to the importance of habitat characteristics, the outcome of inter-specific competition is also affected by plant densities. The occurrence of non-native plant species at high densities may generate more competition for resources (Ren and Zhang 2009) and growth inhibition of other plants (Wang et al. 2008). In turn, the impacts caused by invasive plants on natives also depend on invasive abundance, and the greatest negative effects on native biota occur at higher invasive biomass (e.g., Capers et al. 2007). Although some investigations infer the impacts of invasive macrophytes measuring their effects only at high plant densities, such inferences would become more realistic if a gradient of plant densities is taken into account (e.g., Mony et al. 2007; Awan et al. 2015).
The factors highlighted above, however, do not influence the outcome of competition in isolation. In fact, the impacts of invasive competition on native macrophytes depend on below- and aboveground interactions and how these interactions are affected by habitat characteristics and invasive densities. For example, there is evidence that the competitive ability of invasive macrophytes depends on the interactions between soil fertility and invasive density (e.g., Van et al. 1999; Mony et al. 2007; Martin and Coetzee 2014; Li et al. 2015). There are also studies on competition between invasive and native submerged macrophytes that show the isolated interactions of below and aboveground parts but keeping plant densities fixed (e.g., Wang et al. 2008). However, to our knowledge, the investigation of below versus aboveground competition of hydrilla with a morphologically similar native macrophyte and of how competition is affected by plant density in different types of sediment has not been conducted. Studies employing this strategy should attain the attention of scientists and managers because sediment features (in terms of organic matter and nutrients) are greatly variable and because hydrilla is widely distributed around the world (Langeland 1996; Carey et al. 2016).
The non-native species hydrilla is considered one of the most troublesome submersed species in the world (Langeland 1996). The first record of this species in Brazil dates from 2005, in the Upper River Paraná (Sousa 2011). Hydrilla is highly competitive and may affect other native species (Langeland 1996; Sousa 2011). For example, it can compete successfully with native species such as Egeria najas Planch. (hereafter “egeria”) in the Upper Paraná River Floodplain (Sousa et al. 2010) and Ceratophyllum demersum L. in an experimental study in New Zealand (Hosfra et al. 1999). Given the global threat of this plant, it is of interest to determine whether its impacts on native species occur through below- or aboveground interactions and how its competitive ability is affected by sediment features and plant density.
In this study, we investigated the effects of increasing densities of the invasive species hydrilla on the growth of the native species egeria in two sediment types (sand and mud). These two species have very similar morphologies (Fig. 1) and ecological features, and this niche overlap can potentially cause intense competitive interactions between them (Sousa et al. 2009). Our hypotheses were that (1) an increase in the density of the invasive species would decrease the growth of the native egeria in different types of sediment and (2) the negative effects of the invasive species on egeria are greater when these species compete for both light and nutrients (below- and aboveground structures in contact) than when they compete only for light (only aboveground structures in contact). These hypotheses are based on the broad ranges of habitats where hydrilla succeeds (Sousa 2011) and on its fast growth rates (Bianchini et al. 2010), which imply that it uses both water and sediment resources with high efficiency.
Methods
Our experiments were conducted from April to June 2009 on the campus of the University of Maringá (South Brazil). Apical portions of healthy macrophytes (10 cm long) were collected from the Upper Paraná River floodplain (22°45′S, 53°15′W and 22°45′S, 53°30′W). The apical portions of both species were washed to remove the attached material (e.g., periphyton, algae and invertebrates) and planted in the different treatments (see below). Fragments were planted in pots that remained in tanks with water for 1 week for acclimatization (approx. 20 °C) before the start of the experiment.
The experiment followed an additive design (Gibson et al. 1999), which is based on combinations between a target species with constant density (in our case, the native egeria) and an associated species with increasing densities (in this experiment, the non-native hydrilla). The additive design is appropriate to accomplish our objectives because it allows the identification of the effects of one species (in our case, the invasive hydrilla) on the growth of another species (in our case, the native egeria) and the determination of whether the intensity of competition depends on the invasive density (see Gibson et al. 1999).
The sediment was obtained in two environments of the Upper Paraná River floodplain. The first sediment type was sampled in the river (coarse sand with a predominance of grain size > 0.5 mm), and the second sediment type was sampled in a floodplain lake (mud sediment composed mainly of silt + clay and with a predominance of grain size < 0.062 mm; Rosin et al. 2010; Ragonha et al. 2013). Sediment type (mud × sand) was used as a co-variate. We used these two types of sediment because they occur in habitats colonized by both species of macrophytes (Sousa et al. 2009; Silveira 2015) and because these habitats are colonized at different degrees by the native and invasive species (Sousa et al. 2010; Sousa 2011).
To test the effects of belowground (hereafter root) and aboveground (hereafter shoot) competition, we conducted two sets of treatments (hereafter “sediment” and “cultures”). The first set of treatments was carried out to assess the effects of competition between shoots of both species, and it was conducted from April to May. First, we added 5 cm of sediment (sand or mud) to 0.5 L pots. Then, 10 cm apical fragments without adventitious roots or branches of both species were planted in pots containing sand or mud. This procedure guaranteed that roots were separated, and thus, any measured effect was derived from competition between shoot (stems + leaves) structures. For both sand and mud treatments, there was a control containing only egeria (our target species; treatment 3 egeria:0 hydrilla). In the other treatments, we maintained the same number of egeria (3 individuals) and increased the density of hydrilla—3:3, 3:6, 3:12 and 3:18. Each density treatment was replicated three times in different sediment types (sand or mud). All pots, including the control treatment, were randomized and allocated in 15 tanks (220 cm × 60 cm × 80 cm). Each tank was divided into two compartments with a perforated sheet of Plexiglas that allowed water to circulate but prevented contact between plants in the two treatments (sand and mud); three replicates were established in each compartments) and were finally filled with tap water. Each tank contained one treatment with sand and another with mud sediment. Pots remained close to increase the interactions between the aboveground plant structures. The second set of treatments (from May to June) followed the same procedures as the first set, but plant fragments were planted in trays (60 cm × 8 cm × 30 cm), i.e., roots of the two species were not isolated. Thus, the results of this experiment were derived from the interaction between roots and shoots in conjunction.
In both sets of treatments, all tanks remained outdoors; thus, the incidence of light was similar to that experienced by plants grown in natural ecosystems. The first set of treatments (in which there were only shoot interactions) and the second set (in which both roots and shoots interacted) were incubated separately because we did not have enough tanks to run the experiment simultaneously. Thus, we used an analytical protocol to account for possible effects derived from incubations at different times (see “Data analyses”).
At the start and end of the experiment, we measured the water temperature, alkalinity (Gran titration; Carmouze 1994), underwater PAR—Photosynthetically active radiation (LI-189, LI-COR, U.S.A), and the available nitrogen and phosphorus in the sediment. Available nitrogen and phosphorus in the sediment were extracted with solutions of KCl and NaOH-NaCl (16 h), and concentrations were determined in a spectrophotometer as NH4+ and PO43−, following Bremner (1965).
Before starting the experiment, sediments were inspected, and all fragments of plants found were removed. Moreover, we inspected the experiment every 5 days and removed plants that did not belong to our experiment but that were occasionally growing.
The experiment ended when the plants reached the water surface and formed canopies (45 days). Plants were removed, separated by species, washed, and dried at 80 °C to constant weight. Data obtained in situ in the River Paraná showed that hydrilla takes 30–60 days to reach 50–70 cm after recovering from a flood disturbance (Sousa et al. 2010). However, hydrilla and egeria grow much more in situ, reaching ca. 1.0–2.2 m (Sousa et al. 2010); thus, we highlight that our experiment simulated the competition occurring during the early stages of plant recovery from natural disturbances.
Data analyses
We used the final biomass of hydrilla as the predictor variable, while the length relative growth rate (RGR) and the total biomass of the egeria (aboveground + belowground biomass) were used as response variables. The RGR was calculated according to the following equation: RGR = (ln L2 – ln L1)/(t2 − t1), where L1 and L2 refer to plant length at times 1 and 2, and t2 − t1 is 45 days (Barrat-Segretain and Elger 2004).
To test the effects of hydrilla density on egeria growth, the data (egeria biomass and RGR as dependent variables) were modelled using mixed models. The random effects were considered in the intercept within a nested tank structure within the type of culture (pots = only shoot interactions; trays = both root + shoot interactions), controlling for any possible dependency structure in the experimental design. The approach used to test the hypotheses was the model selection, with the backward strategy, starting from the complete model and by means of the likelihood ratio test. The predictive variables, with fixed effect, contained in the complete model were the density of hydrilla (measured by dry mass), sediment type (sand or mud) and the interaction between the two (density × sediment). To verify whether the type of culture (shoot or root + shoot interactions) interferes with the density effect, the following interactions were added: density × culture type and density × sediment × culture type. These interactions were added to the model to test the hypothesis that there is interaction between the shoots and roots + shoots in the competition between the species. Thus, it is expected that the slope of the line (effect of density of hydrilla) would differ between sediment types and the types of culture (pots, where only shoots interact, versus trays, where roots and shoots interact). The main effect on the intercept of the culture type was not considered because it is expected that, in the absence of the invader (effect on the intercept), the growth of the native is the same regardless of the type of culture. However, any variability regarding the shape of the crop or experimental design in the egeria biomass and egeria RGR should be captured by the random effect of the type of culture.
Finally, the temperature and alkalinity of the water, underwater PAR—photosynthetically active radiation and nitrogen and phosphorus of the sediments were considered in the complete model. Although these latter variables are not of interest for the hypotheses tested in the work, they were included because they may be important for the growth of the native species and especially to control for any uncontrolled variation of these co-variables on the dependent variables, providing greater robustness to the tested hypotheses. To eliminate possible correlations of these co-variables with the variables of interest, such as sediment type, they were centralized based on the means of each sediment type × type of culture. All analyses were carried out in R software (R Core Team 2016) with the package nlme (Pinheiro et al. 2016).
Results
The values of the abiotic variables were relatively constant over time and very close between sand and mud treatments (see SD values in Table 1). However, levels of available nitrogen and phosphorus in sediment were higher in the mud than in the sand treatments (Table 1).
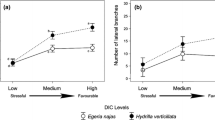
The model selection showed that the density of the invasive species is significantly associated with the growth of the native egeria species, independent of the sediment type (Table 2 and S1). However, when the growth of egeria is evaluated based on the increase in biomass, the type of culture [pots (shoot interactions) × trays (root + shoot interactions)] also interacted with the coefficient of regression (slope) of the density of the invasive species (Tables 2 and S1), resulting in two slopes, one for each culture type (Fig. 2a). Based on estimates of the egeria biomass model, it is possible to state that when both species grew in trays in which both roots and shoots interacted, the negative effect of the invasive species density on native biomass growth was increased (P < 0.001, Table 2). Thus, the influence of the density of the invasive species on the growth in biomass of the native depends on whether there were only shoot (pot treatment) or root + shoot (tray treatments) interactions. On the other hand, when the plants grew in the pots, which restrict the competitive interaction to aboveground parts, the increase in the invasive density did not significantly affect the native biomass (P = 0.139, Table 2) (Fig. 2a).
Egeria najas growth [a biomass (DW—dry weight); b length RGR—relative growth rates] produced in different densities of Hydrilla verticillata growing in different sediment (mud and sand) and culture (pots: aboveground plant structures (shoot) interacted; trays: above and belowground plant (shoot + root) structures interacted) types. These lines represent the expected average growth according to the selected final models, considering the average values of the covariates nitrogen and/or alkalinity in each stratum (sediment and culture type), according to Table 2. a Note that only the interaction between the type of culture (shoots × roots + shoots) and density was significant. Thus, there are four lines combining two different slopes, one for each culture type (roots + shoots: solid lines; shoots: dashed lines) and two intercepts, one for each sediment type (mud: black lines; sand: grey lines). b Note that we fitted lines that represent sand and mud individually because only the effects of the invasive density and type of sediment were significant, whereas their interactions were not; thus, there are two lines (mud: black line; sand: grey line) with a single slope
For growth in length (egeria RGR), the density of the invasive species negatively affected the growth of the native, independent of whether it was growing in trays or pots (P < 0.001, Tables 2 and S1) (Fig. 2b). Thus, although native species elongation decreases with increasing native density, this decrease does not depend on whether there was only shoot competition or both root and shoot competition.
Although the sediment type did not affect the association between hydrilla density and native growth (i.e., slopes were similar in mud and sand sediments), sediment type had a significant effect on intercept (Tables 2 and S1; Fig. 2a, b). This indicates that egeria accumulates more biomass and elongates more when it grows in mud sediment.
It is important to note that the nitrogen concentration in the sediment also played an important role in significantly favouring the growth of the native species, both in biomass and in length (Tables 2 and S1). The alkalinity also contributed significantly to the biomass increase in the native species (Tables 2 and S1). Finally, considering that (1) nitrogen was included in the centralized model in the mean of each stratum (sediment type and culture type), (2) this nutrient was positively associated with egeria growth and (3) this nutrient had higher concentrations in the mud sediment (see Table 1), it is plausible to infer that the higher growth of egeria in this sediment can be explained by nitrogen.
Discussion
Our experiment showed that hydrilla has great competitive ability and that increasing its density significantly reduced the length and biomass of the native egeria in both mud and sand sediments, which corroborates our first hypothesis. In addition, the biomass of the native species decreased faster with increasing invasive densities when both roots and shoots were interacting than when roots were isolated. These results indicate the importance of root competition between these two submerged macrophytes, corroborating our second hypothesis. The significant effects of sediment nitrogen and alkalinity (an indicator of carbon availability) on egeria growth indicate that these are likely important limiting factors for egeria growth. Although biomass accumulation in the invasive density gradient differed between distinct culture types, i.e., shoot interactions (pots) and root + shoot interactions (trays), plant length RGR did not differ when roots or shoots interacted, indicating that this trait does not depend on root interactions.
Although facilitation is an important interaction that can influence the co-existence between invasive and native species (Martin-Fores et al. 2017), the significant decreases in biomass and length RGR obtained in our experiment indicate that the interaction between egeria and hydrilla was predominantly competition rather than facilitation. The increase in hydrilla biomass was determinant even in treatments with greater nutrient concentrations (mud sediment), in which we believed that both species could coexist due to the higher resource availability (in this case, available nitrogen and phosphorus in the sediment).
Plant density manipulations are important to test inter-specific competition because plant growth and nutrient uptake are density dependent (Creed et al. 1997; Xie et al. 2006). For example, some studies have demonstrated that the occurrence of non-native plant species in high densities may generate competition for resources (Ren and Zhang 2009), which can lead to the growth inhibition of other plants (Wang et al. 2008), as we showed for the native egeria, which co-occurs with hydrilla in the field.
Some studies have demonstrated that hydrilla shows greater and faster growth than other species (Bianchini et al. 2010; Sousa 2011), and consequently, this invasive species has the potential to exclude native species in their natural habitats. Previous studies demonstrated a competitive advantage of hydrilla over Egeria densa (Mony et al. 2007) and E. najas (Sousa et al. 2010) as well as other aquatic macrophytes with lower biomass (Wang et al. 2008). Thus, our results corroborate these previous investigations and confirm that hydrilla has a substantial capacity to affect the native flora.
In our experiment, hydrilla formed dense mats reaching the tank surface, which likely reduced light penetration and space, which, in turn, has the potential to reduce the growth of egeria, mainly in the treatments with high densities of invasive plants. However, because the effects of hydrilla density on egeria growth were not significant in the treatments in which only shoots were interacting, and, the underwater PAR variable was not incorporated in the final model, it seems that the influence of light and space on egeria growth was minor in our experiment (see Table 2 and Fig. 2a). In contrast to our results, the role of underwater light and space in the competitive outcome of submerged species has been recognized in other experiments that tested the effects of canopy-forming species (such as hydrilla) on other submerged macrophytes (Wang et al. 2008; Richter and Gross 2013).
It is also important to emphasize that with the high root production and root interactions (as occurred in the tray treatment in our experiment), there is an increase in belowground competition, mainly in sites with high plant densities. Apart from its ability to produce adventitious roots, which can grow from stem nodes, hydrilla has a well-developed root system with stolons over the water–sediment interface and within the sediment (Cook and Luond 1982; Madsen and Smith 1999). In contrast, egeria has only simple root systems (Cook and Urmi-Köning 1984). Thus, in addition to an increase in root density along with plant density, root morphology may also account for the strong competitive ability of hydrilla on egeria when belowground structures interacted in our experiments. Previous investigations have demonstrated that belowground competition is more important than aboveground plant competition (Von Felten and Schmid 2008) and can help structure plant communities (Cahill 1999; Weigelt et al. 2007).
Sites with high concentrations of nitrogen and phosphorus are more prone to invasion by non-native species (González et al. 2010). According to our results, it seems that hydrilla is able to invade sites independently of their nitrogen or phosphorus concentrations, at least for the two extreme conditions we tested (sand and mud sediments). In contrast, data obtained in the field in the Upper Paraná River (Brazil) indicate that hydrilla is not able to invade lakes with mud sediments (Sousa 2011). Thus, the growth and high competitive ability identified in our experiment, even in mud sediment, indicates that factors other than sediment characteristics may be involved in the lack of hydrilla in the lakes studied by Sousa (2011). Underwater light limitation and biotic resistance (e.g., Ribas et al. 2017), which were not taken into account in our experiment, might explain why hydrilla succeeds in mud sediments in our tanks but does not colonize the same types of sediment in the field.
Experiments carried out in the field and greenhouse showed that nutrients in sediment affect the cover, production, competitive ability and composition of submersed macrophyte assemblages (Barko and Smart 1986; Zhou et al. 2017). Effects of nutrients were also detected in our experiment (see the effects of the intercept for both egeria biomass and length RGR; Table 2), indicating that native species growth is enhanced in mud sediments. Other factors not measured in our experiment, such as the metabolism of microbial communities in sediments, likely explain the differences in egeria responses. For example, the biomass and activity of bacteria related to P cycling (Torres et al. 2017) and the abundance of different groups of bacteria related to N cycling (Veraart et al. 2017) differ between sediments with different organic matter contents, such as the ones we used in our experiments. However, despite the enhanced growth of egeria in the mud sediment, the negative effects of hydrilla on the native species did not depend on sediment type, demonstrating that this invasive species poses a threat to the native species in multiple habitats. This threat is even more important because the native species is not able to escape from light limitation by etiolation (plant elongation), as indicated by the significant negative effects of hydrilla density on egeria length RGR (see Fig. 2b). In fact, hydrilla at high densities almost completely suppressed the growth of egeria in sand and mud treatments (see Fig. 2a, b).
In summary, the results of our experiment showed that the increase in densities of the invasive species hydrilla decreases the growth of the native species egeria in different sediment types, and competition was stronger when roots and shoots interacted then when only shoots interacted, demonstrating the importance of competition for nutrients in sediment. Thus, we suspect that egeria is not able to establish and succeed in sites dominated by hydrilla (a condition simulated in the higher densities in our experiment). On the other hand, the co-existence of the two species in the field (e.g., Sousa 2011) can be explained by priority effects (egeria colonizing a site first; see, for example, Zefferman 2015) or disturbances that can displace hydrilla in the field (e.g., herbivory; e.g., Ribas et al. 2017). Therefore, future studies to assess the effects of hydrilla (or other invasive species) on native species should manipulate other organisms involved in biotic resistance (e.g., herbivorous) and priority effects (colonization order).
References
Awan TH, Sta Cruz PC, Chauhan BS (2015) Growth analysis and biomass partitioning of Cyperus iria in response to rice planting density and nitrogen rate. Crop Prot 74:92–102
Barko JW, Smart RM (1986) Sediment-related mechanisms of growth limitation in submersed macrophytes. Ecology 67:1328–1340
Barko JW, Adms MS, Clesceri NL (1986) Environmental-factors and their consideration in the management of submersed aquatic vegetation —a review. J Aquat Plant Manag 24:1–10
Barrat-segretain MH, Elger A (2004) Experiments on growth interactions between two invasive macrophytes species. J Veg Sci 15:109–114
Bianchini I Jr, Cunha Santino MB, Milan JAM, Rodrigues CJ, Dias JH (2010) Growth of Hydrilla verticillata (L.f.) Royle under controlled conditions. Hydrobiologia 644:301–312
Bolpagni R, Pino F (2017) Sediment nutrient drivers of the growth dynamics of the rare fern Marsilea quadrifolia. Hydrobiologia 792(1):303–314
Bremner JM (1965) Inorganic forms of nitrogen. In: Black CA (ed) Methods of soil analysis. American Society of Agronomy, Madison
Brundu G (2015) Plant invaders in European and Mediterranean inland waters: profiles, distribution, and threats. Hydrobiologia 746(1):61–79
Cahill JF (1999) Fertilization effects on interactions between above - and belowground competition in an old field. Ecology 80:466–480
Capers RS, Selsky R, Bugbee GJ, White JC (2007) Aquatic plant community invasibility and scale dependent patterns in native and invasive species richness. Ecology 88:3135–3143
Carey MP, Sethi SA, Larsen SJ, Rich CF (2016) A primer on potential impacts, management priorities, and future directions for Elodea spp. in high latitude systems: learning from the Alaskan experience. Hydrobiologia 777:1–19
Carignan R, Kalff J (1980) Phosphorus sources for aquatic weeds water or sediments. Science 207:987–989
Carmouze JP (1994) Metabolismo dos ecossistemas aquáticos: fundamentos teóricos, métodos de estudo e análises químicas. Ed: Edgard Blücher: FAPESP
Chambers PA, Kalff J (1985) Depth distribution and biomass of submersed aquatic macrophyte communities in relation to Secchi depth. Can J Fish Aquat Sci 42:701–709
Clayton J, Edwards T (2006) Aquatic plants as environmental indicators of ecological condition in New Zealand lakes. Hydrobiologia 570:147–151
Cook CDK, Luond R (1982) A revision of the genus Hydrilla (Hydrocharitaceae). Aquat Bot 13:485–504
Cook CDK, Urmi-Köning K (1984) A revision of the genus Egeria (Hydrocharitaceae). Aquat Bot 19:73–96
Crain CM, Silliman BR, Bertness SL, Bertness MD (2004) Physical and biotic drivers of plant distribution across estuarine salinity gradients. Ecology 85:2539–2549
Creed JC, Norton TA, Kain JM (1997) Intraspecific competition in Fucus serratus germlings: the interaction of light, nutrient and density. J Exp Mar Biol Ecol 212:211–223
Cuassolo F, Bastidas NB, Balseiro E, Modenutti B (2016) Effect of light on particulate and dissolved organic matter production of native and exotic macrophyte species in Patagonia. Hydrobiologia 766:29–42
Engelhardt MJ, Anderson RC (2011) Phenological niche separation from native species increases reproductive success of an invasive species: Alliaria petiolata (Brassicaceae)—garlic mustard. J Torrey Bot Soc 138:418–433
Gibson DJ, Connolly J, Hartnett DC, Widenhamer JD (1999) Designs for greenhouse studies of interactions between plants. J Ecol 87:1–16
González AL, Kominoski JS, Danger M, Ishida S, Iwai M, Rubach A (2010) Can ecological stoichiometry help explain patterns of biological invasions? Oikos 119:779–790
Hosfra DE, Clayton J, Green JD, Auger M (1999) Competitive performance of Hydrilla verticillata in New Zealand. Aquat Bot 63:305–324
Langeland KA (1996) Hydrilla tuber formation in response to single and sequential bensulfuron methyl exposures at different times. Hydrobiologia 340:247–251
Li YP, Feng YL, Chen YJ, Tian YH (2015) Soil microbes alleviate allelopathy of invasive plants. Sci Bull 60:1083–1091
MacIsaac HJ, Eyraud AP, Beric B, Ghabooli SJ (2016) Can tropical macrophytes establish in the Laurentian Great Lakes? Hydrobiologia 767:165–174
Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz FA (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl 10:689–710
Madsen JD, Smith DH (1999) Vegetative spread of dioecious Hydrilla colonies in experimental ponds. J Aquat Plant Manag 37:25–29
Madsen JD, Sutherland JW, Bloomfield JA, Eichler LW, Boylen CW (1991) The decline of native vegetation under dense eurasian watermilfoil canopies. J Aquat Plant Manag 29:94–99
Martin GD, Coetzee JA (2014) Competition between two aquatic macrophytes, Lagarosiphon major (Ridley) Moss (Hydrocharitaceae) and Myriophyllum spicatum Linnaeus (Haloragaceae) as influenced by substrate sediment and nutrients. Aquat Bot 114:1–11
Martin-Fores I, Guerin GR, Lowe AJ (2017) Weed abundance is positively correlated with native plant diversity in grasslands of Southern Australia. PLoS ONE 5:e0178681
Mony C, Koschnick TJ, Haller WT, Muller S (2007) Competition between two invasive Hydrocharitaceae (Hydrilla verticillata (L. f.) (Royle) and Egeria densa (Planch) as influenced by sediment fertility and season. Aquat Bot 86:236–242
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2016) nlme: Linear and nonlinear mixed effects models. R package version 3.1-125. http://CRAN.R-project.org/package=nlme
Ragonha FH, Chiaramonte JB, Junior Fontes HM, Cunha ER, Benedito E, Takeda AM (2013) Spatial distribution of aquatic Oligochaeta in Ilha Grande National Park, Brazil. Acta Sci Biol Sci 35:63–70
R Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Ren MX, Zhang QG (2009) The relative generality of plant invasion mechanisms and predicting future invasive plants. Weed Res 49:449–460
Ribas LGD, Cunha ER, Vitule JRS, Mormul RP, Thomaz SM, Padial AA (2017) Biotic resistance by snails and fish to an exotic invasive aquatic plant. Fresh Biol 62:1266–1275
Richardson DM, Rejmanék M (2011) Trees and shrubs as invasive alien species—a global review. Divers Distrib 17:788–809
Richter D, Gross EM (2013) Chara can outcompete Myriophyllum under low phosphorus supply. Aquat Sci 75:457–467
Rosin GC, Mangarotti DPO, Takeda AM (2010) Chironomidae (Diptera) community structure in two subsystems with different states of conservation in a floodplain of southern Brazil. Acta Limnol Bras 22:276–286
Santos JS, Anderson LW, Ustin SL (2011) Effects of invasive species on plant communities: an example using submersed aquatic plants at the regional scale. Biol Invasions 13:443–457
Schultz R, Dibble E (2012) Effects of invasive macrophytes on freshwater fish and macroinvertebrate communities: the role of invasive plant traits. Hydrobiologia 684:1–14
Shields EC, Moore KA (2016) Effects of sediment and salinity on the growth and competitive abilities of three submerged macrophytes. Aquat Bot 132:24–29
Silveira MJ (2015) The effect of habitat and sediment type on the occurrence of non-native and native species of aquatic macrophytes in subtropical regions. Biosci J 31:268–274
Sousa WTZ (2011) Hydrilla verticillata (Hydrocharitaceae), a recent invader threatening Brazil’s freshwater environments: a review of the extent of the problem. Hydrobiologia 669:1–20
Sousa WTZ, Thomaz SM, Murphy KJ, Silveira MJ, Mormul RP (2009) Environmental predictors of exotic Hydrilla verticillata L.f. Royle and a native Egeria najas Planch. occurrence in a sub-tropical river floodplain: the upper River Paraná, Brazil. Hydrobiologia 632:65–78
Sousa WTZ, Thomaz SM, Murphy KJ (2010) Response of native Egeria najas Planch. and invasive Hydrilla verticillata (L.f.) Royle to altered hydroecological regime in a subtropical river. Aquat Bot 92:40–48
Tassin J, Thiébaut G, Dutartre A (2011) Toward an objective perception of biological invasions. Rev Ecol Terre Vie 66:195–198
Torres IC, Turner BL, Reddy KR (2017) Phosphatase activities in sediments of subtropical lakes with different trophic states. Hydrobiologia 788:305–318
Van TK, Wheeler GS, Center TD (1999) Competition between Hydrilla verticillata and Vallisneria americana as influenced by soil fertility. Aquat Bot 62:225–233
Veraart AJ, Dimitrov MR, Schrier-Uijl AP, Smidt H, de Klein JJM (2017) Abundance, activity and community structure of denitrifiers in drainage ditches in relation to sediment characteristics, vegetation and land-use. Ecosystems 20:928–943
Von Felten S, Schmid B (2008) Complementarity among species in horizontal versus vertical rooting space. J Plant Ecol 1:33–41
Wang JW, Yu D, Xiong W, Han YQ (2008) Above- and belowground competition between two submersed macrophytes. Hydrobiologia 607:113–122
Wang H, Wang Q, Bowler PA, Xiong W (2016) Invasive aquatic plants in China. Aquat Invasions 607:113–122
Weigelt A, Schumacher J, Walther T, Bartelheimer M, Steinlein T, Beyschlag W (2007) Identifying mechanisms of competition in multi-species communities. J Ecol 95:53–64
Xie YH, An SQ, Wu BF, Wang WW (2006) Density-dependent root morphology and root distribution in the submerged plant Vallisneria natans. Environ Exp Bot 57:195–200
Zefferman EP (2015) Experimental tests of priority effects and light availability on relative performance of Myriophyllum spicatum and Elodea nuttallii propagules in artificial stream channels. PLoS ONE 10:e0120248
Zhou J, Pan X, Xu H, Wang Q, Cui LJ (2017) Invasive Eichhornia crassipes affects the capacity of submerged macrophytes to utilize nutrients. Sustainability 9:565
Acknowledgements
We acknowledge with appreciation the comments provided by two reviewers and the handling editor, which improved the quality of our manuscript. We also acknowledge the “Parque Tecnológico da Itaipu” (PDTA/FPTI-BR) for providing a scholarship to MJ Silviera. SM Thomaz is especially thankful to the National Council for Scientific and Technological Development (CNPq) for providing continuous funding through a Research Productivity Grant. Finally, we thank Roberta Becker Rodrigues for helping in laboratory analyses. This research was partially funded by Itaipu Binacional.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Silveira, M.J., Alves, D.C. & Thomaz, S.M. Effects of the density of the invasive macrophyte Hydrilla verticillata and root competition on growth of one native macrophyte in different sediment fertilities. Ecol Res 33, 927–934 (2018). https://doi.org/10.1007/s11284-018-1602-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-018-1602-4