Abstract
Lonicera maackii, a highly invasive species of riparian habitats, has the potential to substantially alter aquatic ecosystems. We investigated effects of this terrestrial invader on aquatic biota and ecosystem processes in three 3rd order headwater streams in southwestern Ohio. We assessed (1) in situ leaf breakdown and (2) aquatic macroinvertebrate colonization of leaf packs containing senesced foliage from either a) invasive (L. maackii), b) native (Fraxinus spp. and Platanus occidentalis), and c) a native-invasive species mix. Leaf breakdown rates and macroinvertebrate density, richness, and functional feeding group (FFG) relative abundances were measured for leaf packs deployed in the autumn of 2009. Invasive leaf breakdown rates were up to 4× faster than native leaves (F = 20.46, df = 2, P < 0.001), resulting in significantly less organic matter remaining compared to the other leaf pack types throughout the study (Friedman’s test = 8.00, P < 0.05). The gathering-collector FFG, represented by Chironomidae and Hydropsychidae, dominated the macroinvertebrate community in the invasive and mixed species leaf packs (F = 73, df = 4, P < 0.01) in all streams but were only dominant in native packs in one stream (F = 41.91, df = 4, P < 0.05). In summary, L. maackii leaf breakdown was significantly faster than native leaves in headwater streams, and colonization of macroinvertebrates was variable depending on leaf pack species composition. These results support the hypothesis that L. maackii can have direct and significant impacts on aquatic ecosystems by influencing organic matter availability and macroinvertebrate community dynamics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Riparian zone plant communities are extremely important to the ecology of headwater streams. Riparian vegetation influences water temperature (Clinton et al. 2010; Roth et al. 2010), light availability (Baxter et al. 2005), and nutrient fluxes into the stream (Harner et al. 2009; Polis and Strong 1996). Stream ecosystem metabolism (Tank et al. 2010), aquatic biota (Cummins et al. 1973; Cummins et al. 1989; Merritt and Cummins 2006), and overall stream health (Fellow et al. 2006; Young et al. 2008) have also been linked to the ecological integrity of riparian zones because aquatic food-webs are influenced by allochthonous organic matter (i.e., leaves, coarse woody debris) that enters the stream from the terrestrial environment (Bailey et al. 2001; Baxter et al. 2005; Gregory et al. 1991; Vannote et al. 1980; Webster et al. 1995). Aquatic macroinvertebrates utilize allochthonous leaf litter as a habitat and a nutrient resource and are thus sensitive to changes in riparian plant community composition (Bailey et al. 2001; Going and Dudley 2008).
Riparian zones are particularly vulnerable to colonization by invasive plants because they are typically areas of frequent disturbances and are associated with high nutrient and light availability (Daehler 2003; Futoshi et al. 2000; Nakamura et al. 2000; Stohlgren et al. 1998). These plant invasions can significantly alter the structure and function of aquatic ecosystems (Richardson et al. 2007). Studies have provided evidence that terrestrial invasive plants can impact aquatic systems in a variety of ways (Dudgeon 1994; Flory and Milner 1999; Stone et al. 2005). For example, Davies and Boulton (2009) reported that macroinvertebrate shredder density and growth were diminished when exposed to the invasive Cinnamomum camphora [L. (Sieb.)] in subtropical streams. Levin et al. (2006) demonstrated that invasion of coastal wetland habitats by Spartina spp. (Schreb.) drove a shift in macroinvertebrate community trophic structure by altering the dominant basal resource from algae to detritus. Further work in this field is needed as relatively few invasive species have been studied in this manner and the mechanism(s) of impact are not fully understood (Davies and Boulton 2009; Le Maitre et al. 1996; Moline and Poff 2008).
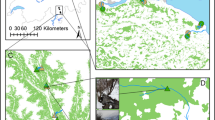
Lonicera maackii [Rupr. (Herder)] is an invasive shrub that has successfully invaded 27 states from New York to Texas since its introduction to the USA in 1896 (Luken and Thieret 1996; United States Department of Agriculture 2011); this shrub is an extremely successful invader of riparian corridors, especially in southwestern Ohio (McNeish, pers. observation). Lonicera maackii can be readily dispersed into riparian zones by birds (e.g., Turdus migratorius (L.); Bartuszevige and Gorchov 2006), exhibit rapid growth (Luken and Mattimiro 1991), and has a long growing season (McEwan et al. 2009a). The growth, survivorship, and reproduction of native plants have been shown to be negatively affected by L. maackii (Collier et al. 2002; Gorchov and Trisel 2003; Gould and Gorchov 2000; Hartman and McCarthy 2004; Miller and Gorchov 2004). One mechanism of impact is through the allelopathic suppression of potential plant competitors (Cipollini et al. 2008a; Cipollini et al. 2008b; Dorning and Cipollini 2006; McEwan et al. 2010) and insect herbivores (McEwan et al. 2009b). A number of studies have addressed the impacts of this species on terrestrial ecosystems (Deering and Vankat 1999; Demars and Boerner 1997; Hutchinson and Vankat 1997), but little is known about its potential effects on aquatic systems. Due to its large size and high population densities, the branches of L. maackii create an overarching canopy along colonized headwater streams (Fig. 1). In autumn, large amounts of L. maackii leaf litter enter the aquatic system (McNeish, unpublished data), creating the potential for significant alterations in aquatic ecosystem structure and function.
We are conducting a series of experiments to understand the effects of L. maackii on headwater stream ecosystems in southwestern Ohio, USA. Here we report results from an in-stream leaf breakdown experiment designed to determine how senesced L. maackii leaves (1) persist in headwater streams and (2) influence the colonizing macroinvertebrate community compared to native species and a native-invasive species mix. Lonicera maackii leaves decompose rapidly in terrestrial systems (Blair and Stowasser 2009; Trammell et al. 2011); therefore, we hypothesized that (H1) L. maackii in-stream leaf breakdown would be more rapid than that of native or mixed species leaf packs. Additionally, due to its known influence on terrestrial insects (McEwan et al. 2009b), we hypothesized that (H2) L. maackii leaf litter would support different densities and composition of aquatic macroinvertebrate taxa and functional feeding groups (FFG) compared to native and mixed species leaf litter.
Methods
Study sites
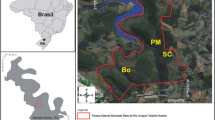
The study sites were located within the upper reaches of three 3rd order headwater streams located in the Centerville-Washington and Sugarcreek-Bellbrook park districts in southwestern Ohio (Fig. 2a). These sites were classified as headwater streams based on criteria described by Cushing and Allan (2001): (1) a relatively narrow (1.5–3.0 m width) streambed, (2) the surface is mostly shaded, and (3) the benthic substrate consists of sand, clay, and rocks. These streams do not have official names, and are hereafter referred to by the park associated with each site: Possum Run (PR), Fecher Park (FP), and Black Oak Park (BOP). Streams were similar in bank-full discharge, sinuosity, substrata, riparian vegetation, and abundance of riffle, run and pool habitats. All streams were formed on limestone geology (Schneider 1957), and had a substantial clay component in the streambed (McNeish, pers. observation). The riparian zones at all study sites were dominated by L. maackii, which formed a dense canopy over the stream (Fig. 1). The native tree composition was similar among the sites and was dominated by Platanus occidentalis (L.; sycamore), Fraxinus spp. (L.; ash), Populus deltoides (Bartram ex Marshall; cottonwood), and Ulmus spp (L.; elm). Black Oak Park and PR were located in suburban parks, while FP was about 0.25 km outside suburban development.
Possum Run, Fecher Park, and Black Oak Park stream sites located within the Little Sugar Creek (north) and Sugar Creek (south) sub-watersheds of the Little Miami watershed, in southwestern Ohio, USA (a). Schematic of the experimental design with 8 leaf packs/treatment (invasive, mix, native) randomly arranged on three rope transects within one riffle (n = 5 riffles per stream; b)
Leaf litter breakdown
Senesced leaves of Fraxinus spp., P. occidentalis and L. maackii were used in the in-stream leaf litter breakdown experiment. Fraxinus spp. and P. occidentalis were chosen because they were common native species present in the riparian zone at each stream and represented a range of native species breakdown rates, from the relatively rapid breakdown of Fraxinus spp. to the more recalcitrant of P. occidentalis (Reice 1978). Senesced leaves from all species were collected from late October to mid November 2009, air dried for 2 weeks, and oven dried to a constant mass at 50 °C. The leaf pack treatments consisted of invasive (10 g L. maackii), native (5 g each of Fraxinus spp. and P. occidentalis), or native-invasive mixed leaves (3.33 g each of the 3 species).
Leaf packs were randomly anchored within riffles (5 riffles/stream; Fig. 2b) on 15 December 2009, which coincided with the completion of natural L. maackii senescence. Riffle habitats were chosen because they generally have greater macroinvertebrate shredder biomass and higher concentrations of dissolved oxygen (Cummins et al. 1980). Fifteen leaf packs from each stream (1/treatment/riffle) were harvested weekly until breakdown of L. maackii leaf material was complete. Harvested leaf packs were preserved in 70 % denatured ethanol and processed within 3 months. Leaves were rinsed, sorted by species, oven dried to a constant mass at 50 °C, then combusted at 550° C for ash-free-dry-mass (AFDM) estimates as a measure of organic matter loss. For the duration of the experiment, micro-T temperature loggers (DS1921G) housed in waterproof underwater casings (DS9019 K; Nexsense Technology and Fondriest Environmental, Beavercreek, OH) were anchored in each stream and recorded the temperature every 15 min. These data were used to calculate accumulated degree-days (ADD) to account for temperature variation during the course of the study.
Macroinvertebrate community
All macroinvertebrates were collected from each harvested leaf pack, identified under a stereomicroscope to family level (except Gastropoda, Isopoda, Oligochaeta, and Amphipoda), and classified into FFG according to Merritt et al. (2008) and Thorp and Covich (2001). These data were used to determine total macroinvertebrate density, taxon richness, taxon density, and FFG relative abundance. Additionally, benthic macroinvertebrates were collected to compare the macroinvertebrate taxa colonizing the leaf packs to the source benthic community. Surber samples were randomly collected from each riffle by placing the frame along the stream bottom with water flowing into the net. All benthic substrata inside the Surber frame were scrubbed until clean, and all dislodged materials and macroinvertebrates were collected in the net and preserved in 70 % denatured ethanol.
Statistical analyses
Leaf pack decay coefficients (k) were calculated using linear regression models (Benfield 2007) and compared among treatments within each stream using one-way ANOVA (e.g., Whiles and Wallace 1997; Alonso et al. 2010). The decay coefficient was determined from the slope of the linear regression of the ln (%) organic matter remaining on ADD throughout the experiment (Benfield 2007). Normality tests for all macroinvertebrate data were inconclusive due to small sample size; therefore, both Friedman’s non-parametric test (with Dunn’s post-test) and parametric two-way ANOVA (with Bonferroni post-tests) were used to determine the effects of date and treatment on total leaf mass remaining, total macroinvertebrate density, individual taxon densities and FFG relative abundance. Macroinvertebrate results were presented with both non-parametric and parametric analyses to balance the interpretation of the lower power non-parametric tests with more powerful parametric analyses. All statistical analyses were conducted using GraphPad Prism version 5.0 (GraphPad Software, San Diego CA, USA, www.graphpad.com).
Results
Leaf litter breakdown
Leaf litter breakdown was influenced by species, day, and their interaction (FDay = 35.67, FTreatment = 25.92, FInteraction = 30.60, all P < 0.0001). In all streams, decay rates of L. maackii leaf material were significantly faster (2.0–5.5×) than native or mixed species leaf litter (Fig. 3). As a result of rapid breakdown, there was significantly less invasive species organic matter remaining compared to the other leaf species in all streams and on all dates (Fig. 4; all sites Friedman’s test = 8.00, P = 0.0046). For example, on day 7 the remaining invasive leaf organic matter was approximately 45 and 19 % less than that of native and mix species in PR, respectively (Fig. 4; all P < 0.05). At the conclusion of the experiment (day 53), there was approximately 68 and 76 % less organic matter of invasive leaf material compared to mix and native leaf packs, respectively (Fig. 4; all P < 0.05). Interestingly, in some instances, mix species leaf packs had significantly less organic matter remaining (approximately 20–25 %) than native packs, and on day 7 and 53 in PR, the remaining organic matter of the mix species was statistically intermediate between native and invasive leaf packs (Fig. 4; P < 0.05).
Linear regression models of the ln (%) leaf material remaining with accumulated degree days (ADD) to calculate processing coefficients (k) for invasive, mix species and native leaf packs in three, 3rd order headwater streams of southwestern Ohio, USA. Possum Run k = −0.01406 (invasive), −0.003537 (mix), and −0.0035171 (native). Fecher Park k = −0.01111 (invasive), −0.003660 (mix), and −0.002256 (native). Black Oak Park k = −0.0134 (invasive), −0.005677(mix), and −0.002451(native). Different letters (a, b) indicate significant differences between leaf pack breakdown rates (invasive, native, mix) determined by Dunn’s post-tests after one-way ANOVA
Mean (±SE) leaf litter remaining for invasive, mix species and native leaf packs in three, 3rd order headwater streams of southwestern Ohio, USA (Possum Run, Fecher Park, and Black Oak Park). Significant differences based on Bonferroni post-test (P < 0.05) after two-way ANOVA, and are represented with different letters between treatments within sampling day
Macroinvertebrate communities
Macroinvertebrate taxon richness was similar among streams (Table 1; 9 at PR; 10 at FP; 10 at BOP); however, there were compositional differences (Table 1). For instance, Planaridae and Amphipoda were only present within BOP, whereas Empididae and Acari were found at PR, and Hirudinea and Aeshnidae were only collected at FP. In contrast, Chironomidae was the dominant taxon present collectively across leaf packs and streams (Table 1). For the duration of the experiment, leaf packs supported all macroinvertebrate taxa present in the source benthic community, which indicated that the leaf pack mesh did not influence macroinvertebrate leaf pack colonization.
Invasive and mix species leaf packs generally supported greater macroinvertebrate densities compared to native leaf packs across all streams (Fig. 5); however, this pattern was not statistically significant. Invasive leaf pack macroinvertebrate densities were significantly greater than the other leaf pack types within PR on day 43 (Fig. 5; P < 0.05). Macroinvertebrate densities on leaf packs within FP and BOP were statistically indistinguishable between treatments and dates, and had lower densities than that of PR leaf packs (Fig. 5).
Mean (±SE) macroinvertebrate density supported by invasive, mix species and native leaf packs in three, 3rd order headwater streams of southwestern Ohio, USA (Possum Run, Fecher Park, and Black Oak Park) over 53 days. Significant differences were determined by Bonferroni post-tests (P < 0.05) after two-way ANOVA between treatments on a sampling day, and are represented by an asterisk
There were differences in taxon densities among leaf pack treatments over time (Fig. 6; Online Resource 1: electronic supplemental data). For example, on day 43, invasive leaf packs in PR and BOP supported significantly greater Chironomidae densities, while there was significantly greater Simuliidae density within PR invasive leaf packs (Fig. 6; all P < 0.01), compared to other treatments. Within FP, Oligochaeta densities were higher in mix species compared to invasive leaf packs on day 43 (Fig. 6; P < 0.01), but in PR, they were greater in native compared to invasive species leaf packs (Fig. 6; P = 0.01).
Mean (±SE) macroinvertebrate taxon density supported by invasive, mix species and native leaf packs in three, 3rd order headwater streams of southwestern Ohio, USA (Possum Run, Fecher Park, Black Oak Park) over 53 days. Only the dominant 4 taxa are shown as all other taxa were represented by low densities and were not significantly different among treatments. Bonferroni post-tests (P < 0.05) after two-way ANOVA were used to determine significant differences between taxa on the same day, and are represented by an asterisk
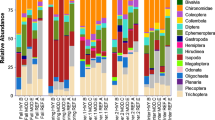
Leaf pack macroinvertebrate communities were dominated by gathering-collectors, and comprised 40–95 % of the macroinvertebrate community that had colonized invasive leaf packs across streams (Fig. 7; all P < 0.05). Mix species leaf pack communities were also dominated by gathering-collectors, although, there was significant temporal variation within and between streams (Online Resource 2: electronic supplemental data). For example, gathering-collectors were dominant on days 14 and 43 within PR (P < 0.001) and BOP (P < 0.05), while they were only dominant on day 14 in FP (Fig. 7; P < 0.001). The dominate FFGs that colonized native leaf packs were more variable compared to other leaf pack treatments between streams, although, gathering-collectors within FP and BOP made up from 40 to 50 % and 45–95 % within PR (Fig. 7).
Mean (±SE) macroinvertebrate relative functional feeding group abundances between invasive, mix species and native leaf packs in three, 3rd order headwater streams of southwestern Ohio, USA (Possum Run, Fecher Park, and Black Oak Park) over 53 days. Bonferroni post-test (P < 0.05) after a two-way ANOVA were used to determine significant differences between functional feeding groups (FFG) relative abundance on the same day (asterisks) and between days (open circles)
Discussion
Terrestrial leaf litter inputs are the main allochthonous organic energy resource for aquatic organisms living in headwater streams, and many aquatic macroinvertebrates rely on this organic matter for habitat and food resources (Benfield 2007; Giller and Malmqvist 1998; Minshall 1967; Vannote et al. 1980). Changes in riparian vegetation have the potential to cause significant alterations in the quality, quantity, and timing of organic matter inputs into stream ecosystems (Kominoski et al. 2011). In-stream processing of leaf litter organic matter is complex and influenced by variation in leaf characteristics (Ostrofsky 1997) and the benthic community composition (e.g., macroinvertebrate, microbial colonizers; Abelho 2001; Anderson and Sedell 1979; Gessner et al. 1999; Hieber and Gessner 2002; Short et al. 1980). Results from this experiment demonstrated that riparian L. maackii has substantial impacts on organic matter processing and aquatic macroinvertebrate communities in headwater streams.
Leaf litter breakdown
In this experiment, L. maackii leaf litter breakdown rates were significantly faster than native species, which supported our first hypothesis (H1) and was consistent with other recent work that has suggested that breakdown of L. maackii leaf material in terrestrial environments is significantly faster than that of native species (Blair and Stowasser 2009; Poulette and Arthur 2012; Trammell et al. 2011). Our study is the first to report that rapid breakdown of L. maackii leaf litter occurs in aquatic habitats and supports the general pattern that invasive species litter decays more rapidly than native species (Ashton et al. 2005; Grout et al. 1997; Swan et al. 2008). For instance, a leaf decomposition experiment conducted in a wet tropical forest in Hawai’i demonstrated that the leaf breakdown of six different invasive species was significantly faster than the litter of five native species (Allison and Vitousek 2004). Cameron and Spencer (1989) reported that leaf decomposition of Sapium sebiferum (L. (Roxb.)) was significantly faster than the native species Salix nigra (Marshall) in terrestrial habitats. In another study, the leaf litter of the invasive species Lythrum salicaria (L.) decayed 4 × faster than that of the native Carex lyngbyei (Hornem.) in the Fraser River estuary in British Columbia (Grout et al. 1997). Our work with L. maackii suggests that it is representative of many invasive species in that (1) it is a dominant invader of disturbed riparian zones and (2) that its breakdown is significantly faster than native species.
Leaf litter quality is an important determinant of leaf breakdown rates. Lignin content is a strong regulator of leaf decomposition processes (Melillo et al. 1982), with fast breakdown rates associated with low levels of lignin in the litter (Royer and Minshall 2001). Lignin content is approximately 21 % in Fraxinus spp. leaves (Hendriksen 1990) and 39 % in P. occidentalis leaves (Ostrofsky 1997), while L. maackii leaf lignin content has been reported to be 7.15 % (Trammell et al. 2011). Higher leaf nitrogen has also been reported with accelerated breakdown rates (Leroy and Marks 2006). Nitrogen is an important nutrient for macroinvertebrate growth and development (Tank et al. 2007) and is often present in higher concentrations in invasive species (Ashton et al. 2005) than native. Lonicera maackii leaves have a C:N ratio of 25 ± 1.6 (Blair and Stowasser 2009), suggesting higher relative nitrogen content compared to Fraxinus spp. (C:N 32.6 ± 0.38; Poulette and Arthur 2012) and P. occidentalis (C:N 50.5; Ostrofsky 1997); therefore, the native litter used in this experiment likely was lower in nitrogen than L. maackii litter. These results suggest that leaf litter quality mediated breakdown rates and macroinvertebrate colonization of our leaf packs.
Leaf litter decomposition is also affected by microbial communities. For instance, bacteria and aquatic hyphomycetes play a direct role in organic matter breakdown through enzymatic activities (Bärlocher 1982; Pascoal and Cassio 2004) and also increase leaf litter palatability for macroinvertebrates, facilitating colonization by macroinvertebrates and, therefore, affecting leaf breakdown rates (Kaushik and Hynes 1971). This microbial activity is facilitated by high nutrient levels (e.g., phosphorus, nitrogen) in the environment (Robinson and Gessner 2000). Microbial communities may have substantial impacts on L. maackii leaf litter breakdown in headwater streams and should be considered for future studies.
Macroinvertebrate community
We found significant differences in macroinvertebrate density, composition and FFG dominance among leaf pack types (invasive, mix, native), supporting our second hypothesis (H2). The presence of higher macroinvertebrate densities and gathering-collectors relative abundance within L. maackii leaf packs suggests this organic matter was more readily and quickly available as a habitat and/or food resource for macroinvertebrates compared to native leaves. It is possible that macroinvertebrates are primarily utilizing L. maackii as a habitat resource rather than for food. Robinson et al. (1998) suggested that microorganisms were primarily responsible for the breakdown of Alnus virdis [Chaix (Dc.)] leaves, while macroinvertebrates used these leaves primarily as a habitat resource. In our study, invasive leaf packs were dominated by the gathering-collectors that primarily utilize leaf packs for habitat (Tarrant et al. 2009). Further work is needed to identify patterns of macroinvertebrate utilization of invasive species leaf litter in headwater streams.
The phenology of L. maackii, particularly the fact that it drops its leaves later in the season than native species, may have implications for the life history timing and development of stream macroinvertebrates. McEwan et al. (2009a) demonstrated that leaf senescence of co-occurring native shrubs, Lindera benzoin (L.) and Asimina triloba (L. (Dunal)), ended mid-November, while L. maackii continued to senesce into December. Within BOP, P. occidentalis and Fraxinus spp. leaves collected from within the stream during natural senescence peaked on 7 and 14 November 2010, respectively (unpublished data). In contrast, L. maackii senescence peaked on 21 November and made up approximately 23 % of in-stream leaf litter on 5 December 2010. This temporal variation between native and invasive leaf senescence alters the quantity and quality of the allochthonous resources and, based on leaf breakdown rates, contributes to substantial effects on the native organic processing in these streams.
Studies that have focused on the influence of terrestrial invasive species report a variety of responses in aquatic organisms to leaf litter changes. One study demonstrated that the leaves of the riparian invasive F. japonica supported similar macroinvertebrates compared to native and mix species leaf packs (Braatne et al. 2007). In another study, Moline and Poff (2008) reported that Tipula [Diptera: Tipulidae (Latreille)] had higher growth rates on invasive Tamarix spp. compared to native Populus (L.) leaf litter food resources. Reinhart and VandeVoort (2006) found that riparian invasive species leaf litter supported 73 % greater density of macroinvertebrate predators compared to native leaves, which suggested invasive leaf litter can have bottom-up food-web impacts. From these studies, it is apparent that extensive riparian invasion of exotic species can influence macroinvertebrate food-web structure, and these effects are species and system specific. More work is needed to develop a trait-based and predictive framework for understanding the impacts of terrestrial invasive plants on aquatic ecosystems.
Conclusions
Differences in leaf breakdown rates among riparian plant species create a “leaf processing continuum” that supports aquatic food-webs (Peterson and Cummins 1974). When the native vegetation of a riparian zone is supplanted by an invasive species, particularly if that species has unique foliar traits, the “continuum” can be altered. Native flora replacement by invasives with rapid leaf breakdown can lead to reduced leaf litter availability and lower leaf pack habitat heterogeneity, impacting aquatic food-web dynamics. We found rapid leaf breakdown and changes in the macroinvertebrate community were associated with L. maackii invasion along headwater stream riparian areas. Lonicera maackii is an extremely successful invader across the Midwest; therefore, there is potential for this terrestrial invader to have cumulated effects on aquatic biota and ecosystem process at the local, regional, and large watershed scale.
References
Abelho M (2001) From litterfall to breakdown in streams: a review. Sci World J 1:656–680
Allison AD, Vitousek PM (2004) Rapid nutrient cycling in leaf litter from invasive plants in Hawai’i. Oecologia 141:612–619
Alonso A, Gonzalez-Munoz N, Castro-Diez P (2010) Comparison of leaf decomposition and macroinvertebrate colonization between exotic and native trees in a freshwater ecosystem. Ecol Res 25:647–653
Anderson NH, Sedell JR (1979) Detritus processing by macroinvertebrates in stream ecosystems. Ann Rev Entomol 24:351–377
Ashton IW, Hyatt LA, Howe KM, Gurevitch J, Lerdau MT (2005) Invasive species accelerate decomposition and litter nitrogen loss in a mixed deciduous forest. Ecol Appl 15:1263–1272
Bailey JK, Schweitzer JA, Whitham TG (2001) Salt cedar negatively affects biodiversity of aquatic macroinvertebrates. Wetlands 21:442–447
Bärlocher F (1982) On the ecology of Ingoldian fungi. BioSci 32:581–586
Bartuszevige AM, Gorchov DL (2006) Avian seed dispersal of an invasive shrub. Biol Invasions 8:1013–1022
Baxter CV, Fausch KD, Saunders WC (2005) Tangled webs: reciprocal flows of invertebrate prey link streams and riparian zones. Freshw Biol 50:20–220
Benfield EF (2007) Decomposition of leaf material. In: Hauer FR, Lamberti GA (eds) Methods in stream ecology, 2nd edn. Academic Press, San Diego, pp 711–720
Blair BC, Stowasser A (2009) Impact of Lonicera maackii on decomposition rates of native leaf litter in a southwestern Ohio woodland. Ohio J of Sci 109:43–47
Braatne JH, Sullivan SMP, Chamberlain E (2007) Leaf decomposition and stream macroinvertebrate colonisation of Japanese Knotweed, and invasive plant species. Int Rev Hydrobiol 92:656–665
Cameron GN, Spencer SR (1989) Rapid leaf decay and nutrient release in a Chinese tallow forest. Oecologia 80:222–228
Cipollini D, Stevenson R, Cipollini K (2008a) Contrasting effects of allelochemicals from two invasive plants on the performance of a nonmycorrhizal plant. Int J Plant Sci 169:371–375
Cipollini D, Stevenson R, Enright S, Eyles A, Bonello P (2008b) Phenolic metabolites in leaves of the invasive shrub, Lonicera maackii, and their potential phytotoxic and anti-herbivore effects. J Chem Ecol 34:144–152
Clinton BD, Vose JM, Fowler DL (2010) Flat Branch monitoring project: stream water temperature and sediment responses to forest cutting in the riparian zone. Res.Pap. SRS- 51. Asheville, NC: U.S. Department of Agriculture Forest Service, Southern Research Station. p 8
Collier MH, Vankat JL, Hughes MR (2002) Diminished plant richness and abundance below Lonicera maackii, and invasive shrub. Am Midl Nat 147:60–71
Cummins KW, Petersen RC, Howard FO, Wuycheck JC, Holt VI (1973) The utilization of leaf litter by stream detritivores. Ecology 54:336–345
Cummins KW, Spengler GL, Ward GM, Speaker RM, Ovink RW, Mahan DC, Mattingly RL (1980) Processing of confined and naturally entrained leaf litter in a woodland stream ecosystem. Limol Oceanogr 25:952–957
Cummins KW, Wilzbach MA, Gates DM, Perry JB, Taliaferro WB (1989) Shredders and riparian vegetation. Bioscience 39:24–30
Cushing CE, Allan JD (2001) Streams: their ecology and life. Academic Press, California
Daehler CC (2003) Performance comparisons of co-occurring native and alien invasive plants: implications for conservation and restoration. Annu Rev Evol Syst 34:183–211
Davies JN, Boulton AJ (2009) Great house, poor food: effects of exotic leaf litter on shredder densities and caddisfly growth in 6 subtropical Australian streams. J N Am Benthol Soc 28:491–503
Deering RH, Vankat JL (1999) Forest colonization and developmental growth of the invasive shrub Lonicera maackii. Am Midl Nat 141:43–50
Demars BG, Boerner REJ (1997) Foliar nutrient dynamics and resorption in naturalized Lonicera maackii (Caprifoliaceae) population in Ohio, USA. Am J of Bot 84:112–117
Dorning M, Cipollini D (2006) Leaf and root extracts of the invasive shrub, Lonicera maackii, inhibit seed germination of three herbs with no autotoxic effects. Plant Ecol 184:287–296
Dudgeon D (1994) The influence of riparian vegetation on macroinvertebrate community structure and functional organization in six New Guinea streams. Hydrobiol 294:65–85
Fellow CS, Calpcott JE, Udy JW, Bunn SE, Harch BD, Smith MJ, Davies PM (2006) Benthic metabolism as an indicator of stream ecosystem health. Hydrobiol 572:71–87
Flory EA, Milner AM (1999) Influence of riparian vegetation on invertebrate assemblages in a recently formed stream in glacier bay national park, Alaska. J N Am Benthol Soc 18:261–273
Futoshi N, Swanson FJ, Wondzell SM (2000) Disturbance regimes of stream and riparian systems—a disturbance-cascade perspective. Hydrol Process 14:2849–2860
Gessner MO, Chauvet E, Dobson M (1999) A perspective on leaf litter breakdown in streams. Oikos 85:377–384
Giller SG, Malmqvist B (1998) The biology of streams and rivers. Oxford University Press, New York
Going BM, Dudley TL (2008) Invasive riparian plant litter alters aquatic insect growth. Biol Invasions 10:1041–1051
Gorchov DL, Trisel DE (2003) Competitive effects of the invasive shrub, Lonicera maackii (Rupr.) Herder (Caprifoliaceae), on the growth and survival of native tree seedlings. Plant Ecol 166:13–24
Gould AMA, Gorchov DL (2000) Effects of the exotic invasive shrub Lonicera maackii on the survival and fecundity of three species of native annuals. Am Midl Nat 144:36–50
Gregory SV, Swanson FJ, McKee W, Cummins KW (1991) An ecosystem perspective of riparian zones. Bioscience 41:540–551
Grout JA, Levings CD, Richardson JS (1997) Decomposition rates of purple loosestrife (Lythrum salicaria) and Lyngbyei’s sedge (Carex lyngbyei) in the Fraser river estuary. Estuaries 20:96–102
Harner MJ, Crenshae CL, Abelho M, Sursova M, Shah JJF, Sinsabaugh RL (2009) Decomposition of leaf litter from a native tree and an actinorhizal invasive across riparian habitats. Ecol Appl 19:1135–1146
Hartman KM, McCarthy BC (2004) Restoration of a forest understory after the removal of an invasive shrub, Amur honeysuckle (Lonicera maackii). Restor Ecol 12:154–165
Hendriksen NB (1990) Leaf litter selection by detritivore and geophagous earthworms. Biol Fertil Soils 10:17–21
Hieber M, Gessner MO (2002) Contribution of stream detritivores, fungi, and bacteria to leaf breakdown based on biomass estimates. Ecology 83:1026–1038
Hutchinson TF, Vankat JL (1997) Invasibility and effects of Amur honeysuckle in southwestern Ohio forests. Conserv Biol 11:1117–1124
Kaushik NK, Hynes HBN (1971) The fate of the dead leaves that fall into stream. Arch Hydrobiol 68:465–515
Kominoski JS, Marczak LB, Richardson JS (2011) Riparian forest composition affects stream litter decomposition despite similar microbial and invertebrate communities. Ecology 92:151–159
Le Maitre DC, van Wilgen BW, Chapman HA, McKelly Dh (1996) Invasive plants and water resources in the western cape province, south Africa: modeling the consequences of a lack of management. J Appl Ecol 33:161–172
Leroy CJ, Marks JC (2006) Litter quality, stream characteristics and litter diversity influence decomposition rates and macroinvertebrates. Freshw Biol 51:605–617
Levin LA, Neira C, Grosholz EC (2006) Invasive cordgrass modifies wetland and trophic function. Ecology 87:419–432
Luken JO, Mattimiro DT (1991) Habitat specific resilience of the invasive shrub Amur honeysuckle (Lonicera maackii) during repeated clipping. Ecol Appl 1:104–109
Luken JO, Thieret JW (1996) Amur honeysuckle, its fall from grace. Bioscience 46:18–24
McEwan RW, Birchfield MK, Schoergendorfer A, Arthur MA (2009a) Leaf phenology and freeze tolerance of the invasive shrub Amur honeysuckle and potential native competitors. J of the Torrey Bot Soc 136:212–220
McEwan RW, Rieske LK, Arthur MA (2009b) Potential interaction between invasive woody shrubs and the gypsy moth (Lymantria dispar), an invasive insect herbivore. Biol Invasions 11:1053–1058
McEwan RW, Arthur-Paratley LG, Rieske LK, Arthur MA (2010) A multi-assay comparison of seed germination inhibition by Lonicera maackii and co-occurring native shrubs. Flora 205:475–483
Melillo JM, Aber JD, Muratore JF (1982) Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 63:621–626
Merritt RW, Cummins KW (2006) Trophic relationships of macroinvertebrates. In: Hauer FR, Lamberti GA (eds) Methods in stream ecology, 2nd edn. Academic Press, New York, pp 585–609
Merritt RW, Cummins KW, Berg MB (2008) An introduction to the aquatic insects of North America, 4th edn. Kendall/Hunt, Iowa
Miller KE, Gorchov DL (2004) The invasive shrub, Lonicera maackii, reduces growth and fecundity of perennial forest herbs. Oecologia 139:359–375
Minshall GW (1967) Role of allochthonous detritus in the trophic structure of a woodland spring brook community. Ecology 48:139–149
Moline AB, Poff NL (2008) Growth of an invertebrate shredder on native (Populus) and non- native (Tamarix, Elaeagnus) leaf litter. Freshw Biol 53:1012–1020
Nakamura F, Swanson FJ, Wondzell SM (2000) Disturbance regimes of stream and riparian systems—a disturbance-cascade perspective. Hydrol Process 14:2849–2860
Ostrofsky ML (1997) Relationship between chemical characteristics of autumn-shed leaves and aquatic processing rates. J N Am Benthol Soc 16:750–759
Pascoal C, Cassio F (2004) Contribution of fungi and bacteria to leaf litter decomposition in a polluted river. Appl and Environ Microbiol 70:5266–5273
Peterson RC, Cummins KW (1974) Leaf processing in a woodland stream. Freshwat Biol 4:345–368
Polis GA, Strong DR (1996) Food web complexity and community dynamics. The Am Nat 147:813–846
Poulette MM, Arthur MA (2012) The impact of the invasive shrub Lonicera maackii on the decomposition dynamics of a native plant community. Ecol Appl
Reice SR (1978) Role of detritivore selectivity in species-specific litter decomposition in a woodland stream. Verh Int Ver Theor Angew Limnol 23:1224–1231
Reinhart KO, VandeVoort R (2006) Effect of native and exotic litter on macroinvertebrate communities and decomposition in a western Montana stream. Divers and Distrib 12:776–781
Richardson DM, Holmes PA, Esler KJ, Galatowitsch SM, Stromberg JC, Kirkman SP, Pysek P, Hobbs RJ (2007) Riparian vegetation: degradation, alien plant invasions, and restoration prospects. Diversity Distrib 13:126–139
Robinson CT, Gessner MO (2000) Nutrient addition accelerates leaf breakdown in an alpine spring brook. Oecologia 122:258–263
Robinson CT, Gessner MO, Ward JV (1998) Leaf breakdown and associated macroinvertebrates in alpine glacial streams. Freshw Biol 40:215–228
Roth TR, Westhoff MC, Huwald H, Huff JA, Rubin JF, Barrenetxea G, Vetterli M, Parriaux A, Selker JS, Parlange MB (2010) Stream temperature response to three riparian vegetation scenarios by use of a distributed temperature validated model. Environ Sci Technol 44:2071–2078
Royer TV, Minshall GW (2001) Effects of nutrient enrichment and leaf quality on the breakdown of leaves in a hard water stream. Freshw Biol 46:603–610
Schneider WJ (1957) Relation of geology to stream flow in the upper little Miami basin. The Ohio J of Sci 57:11–14
Short RA, Canton SP, Ward JV (1980) Detrital processing and associated macroinvertebrates in a Colorado mountain stream. Ecology 61:727–732
Stohlgren TJ, Bull KA, Otsuki Y, Villa CA, Lee M (1998) Riparian zones as havens for exotic plant species in the central grasslands. Plant Ecol 138:113–125
Stone ML, Whiles MR, Webber JA, Williard KWJ, Reeve JD (2005) Macroinvertebrate communities in agriculturally impacted southern Illinois streams: patterns with riparian vegetation, water quality, and in-stream habitat quality. J Environ Qual 34:907–917
Swan CM, Healey B, Richardson DC (2008) The role of native riparian tree species in decomposition of invasive tree of heaven (Ailanthus altissima) leaf litter in an urban stream. Ecoscience 15:27–35
Tank JL, Bernot MJ, Rosi-Marshall EJ (2007) Nitrogen limitation and uptake. In: Hauer FR, Lamberti GA (eds) Methods in stream ecology, 2nd edn. Academic Press, San Diego, pp 213–238
Tank JL, Rosi-Marshall EJ, Griffiths NA, Entrekin SA, Stephen ML (2010) A review of allochthonous organic matter dynamics and metabolism in streams. J N Am Benthol Soc 29:118–146
Tarrant E, Nine A, Powers L, Heth RK (2009) Decomposition rate and community structure of leaf-packs in an urban and rural stream in southwestern Missouri. Trans Mo Acad Sci 43:39–45
Thorp JW, Covich AP (2001) Ecology and classification of North American freshwater invertebrates, 2nd edn. Academic Press, San Diego
Trammell TL, Ralston HA, Scroggins SA, Carreiro MM (2011) Foliar production and decomposition rates in urban forests invaded by the exotic invasive shrub, Lonicera maackii. Biol Invasions. doi:10.1007/s10530-011-0093-9
United States Department of Agriculture (2011) Plants Profile: Lonicera maackii (Rupr.) Herder Amur honeysuckle. http://plants.usda.gov/java/profile?symbol=LOMA6. Accessed 4 Oct 2011
Vannote RL, Minshall GW, Cummins KW, Sedell JR, Cushing CE (1980) The river continuum concept. Can J Fish Aquat Sci 37:130–137
Webster JR, Wallace JB, Benfield EF (1995) Organic processes in streams of the eastern United States. In: Cushing CE, Cummins KW, Minshall GW (eds) River and stream ecosystems. Elsevier, Amsterdam, pp 117–187
Whiles MR, Wallace JB (1997) Leaf litter decomposition and macroinvertebrate communities in headwater streams draining pine and hardwood catchments. Hydrobiologia 353:107–119
Young RG, Matthaei CD, Townsend CR (2008) Organic matter breakdown and ecosystem metabolism: functional indicators for assessing river ecosystem health. J N Am Benthol Soc 27:605–662
Acknowledgments
We would like to thank the Centerville-Washington and Sugarcreek-Bellbrook Park Districts for allowing us to conduct this research on their property. We also thank Tiffany Blair, Sarah Alverson, and Lucy Siefker, and all the undergraduate volunteers who have been involved in this project, for their help and support. We are also grateful to P. K. Williams and J. Robinson for helpful discussions during project development. The University of Dayton’s Graduate Summer Fellowship program and Department of Biology provided financial support for this research project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McNeish, R.E., Benbow, M.E. & McEwan, R.W. Riparian forest invasion by a terrestrial shrub (Lonicera maackii) impacts aquatic biota and organic matter processing in headwater streams. Biol Invasions 14, 1881–1893 (2012). https://doi.org/10.1007/s10530-012-0199-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-012-0199-8