Abstract
Stream functioning is affected by allochthonous and autochthonous energy sources, organic matter decomposition and the structure and composition of the aquatic community. The presence of non-native tree species in the riparian zone may affect stream functioning. Thus, we quantified the allochthonous organic matter input to streams from native tree species and Hovenia dulcis, a non-native species, over a year, and we evaluated litter colonization and decomposition by aquatic invertebrates. The input of native organic matter was greater in Winter and Spring. On the other hand, the input of H. dulcis was higher in Autumn. The annual contribution of native organic matter was twofold greater than that of H. dulcis and was correlated with rainfall. H. dulcis leaf litter had decomposition rates that were three- to fourfold greater than those of native leaf litter. The invertebrate abundance and richness, and functional feeding groups did not vary between native and non-native leaf litter. We conclude that the presence of H. dulcis in the riparian zone changed the input patterns of allochthonous organic matter into streams. Furthermore, H. dulcis litter broke down faster than that of native species and did not directly affect the associated invertebrate community. However, the dominance of this species in riparian zones causes homogenisation of environment, resulting in changes in the composition of other biological communities (e.g., fungi and fish).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Stream ecosystem functioning depends on complex process that include (1) allochthonous and/or autochthonous organic matter production (matter and energy source) (Vannote et al. 1980; Rezende et al. 2017), (2) plant litter decomposition (nutrient cycling) (Chauvet et al. 2016) and (3) the structure and composition of aquatic communities, which facilitate the energy incorporation into the trophic web (Wallace and Webster 1996). In forested streams, primary productivity is limited by shading, making the input of allochthonous organic matter essential for the maintenance of aquatic communities (Vannote et al. 1980; Graça 2001; Neres-Lima et al. 2017). Microorganisms and aquatic invertebrates colonize and consume allochthonous organic matter. Microorganisms degrade plant tissue structures, increasing its nutritional richness (e.g., increased nitrogen concentrations) and making it palatable to shredders (Graça 2001; Taylor and Andrushchenko 2014; Biasi et al. 2019). Shredders directly consume leaf litter by converting coarse particulate organic matter into fine particulate organic matter, which is a food resource for other aquatic invertebrates including collectors and filterers (Graça 1993, 2001; Marks 2019). Therefore, they contribute to decomposition processes, nutrient cycling and the incorporation of matter and energy in the trophic web (Graça 1993; Abelho 2001; Cummins 2002; Graça et al. 2015).
The input of allochthonous organic matter to streams can occur through several pathways. Organic matter can enter vertically (directly from trees), laterally (from the riparian zone) or from upstream (Gonçalves et al. 2006; Gonçalves and Callisto 2013). In the neotropics, allochthonous material comprises leaves (~ 70%), branches (~ 30%) and reproductive materials (i.e., flowers, fruits and seeds) (Gonçalves et al. 2014; Rezende et al. 2017).
The processing of organic matter is directly affected by the chemical and structural characteristics of the matter varying between species (König et al. 2014; Medina-Villar et al. 2015). Thus, leaf litter of high quality (high N concentrations and lowest C:N ratio) (Canhoto and Graça 1996), low concentrations of polyphenols (Tonin et al. 2014a) and low toughness (Biasi et al. 2016) tends to be colonized and consumed more efficiently by detritivores.
Monocultures (e.g., Eucalyptus sp. and Pinus sp.) (Juvenal and Matos 2002) or the presence of invasive non-native species (e.g., Hovenia dulcis) (Dechoum et al. 2015) can negatively affect the dynamics of allochthonous organic matter in streams. For example, the litter fall of H. dulcis increases in Autumn and substantially changes the dynamics of stream organic matter (Carvalho 1994). Consequently, riparian zones dominated by such species can have energy deficits in certain periods of the year. Some non-native species may also reduce the rate of the decomposition process (Casas et al. 2013; Martínez et al. 2013a, b). Further, some non-native species can alter the structure and composition of microorganism (Bärlocher and Graça 2002; Menéndez et al. 2013; Ferreira et al. 2017; Biasi et al. 2020) and invertebrate assemblages (Albariño and Balseiro 2002; Gonçalves et al. 2012; Martínez et al. 2013a; Tonin et al. 2014b). The negative effects of non-native species favor functional redundancy (Ferreira et al., 2019) and reduce beta diversity (Biasi et al., 2020) among microbial communities. In addition, non-native species can affect detritivore communities by inducing changes in nutrient availability, solar irradiation or bedding characteristics. The presence of non-native species results in the reduction of the diversity of aquatic invertebrates and influences the activity of shredders (Tonello et al. 2014; Seeney et al. 2019). More specifically, H. dulcis has litter with a high nutritional quality and is therefore rapidly decomposed (König et al. 2014; Biasi et al. 2020). However, H. dulcis may affect the structure and composition of the aquatic hyphomycete community, also decreasing its reproductive activity (Biasi et al. 2020).
Hovenia dulcis Thunb. (Rhamnaceae) is a deciduous zoochoric tree, originally from Asia, with a high invasive capacity in native forest remnants (Carvalho 1994). In subtropical region of the Atlantic Forest biome, H. dulcis is considered extremely aggressive, promoting homogenization of native forests and changes in ecosystems functioning (Padilha et al. 2015; Schmidt et al. 2020). This study was carried out in a remnant of Atlantic Forest biome in southern Brazil, where the biological invasion by Hovenia dulcis has become an increasing problem for native forest systems (Padilha et al. 2015) and for aquatic ecosystems (Biasi et al. 2020). The objective of this study was to understand the effects of this on the functioning of small streams ecosystems. We quantified the allochthonous organic matter input to streams from native tree species and Hovenia dulcis, over a year, and evaluated leaf litter colonization and decomposition by aquatic invertebrates. Our premises were: (1) the organic matter input in the streams can be influenced by climatic factors (e.g., temperature and rainfall) (Tonin et al. 2017); (2) the most nutritive and less tough leaf litter will be more easily colonized by detritivores (König et al. 2014); and, (3) resource homogeneity reduces the abundance and diversity of organisms (Biasi et al. 2020). Thus, our first hypothesis was that allochthonous organic matter input would be affected by climatic factors. Second, we hypothesized that H. dulcis leaf litter would exhibit higher decomposition rates than native plants due to its lower leaf toughness. Our third hypothesis was that there would be higher variability of abundance, richness and feeding functional groups of invertebrates on the litter of native species than that of H. dulcis.
Materials and methods
Study area
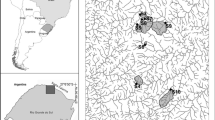
This study was carried out in a forest remnant (between − 27.4713 and − 27.5161 S; − 51.9208 and − 51.9616 W) located in a conservation unit (Municipal Park Mata do Rio Uruguai Teixeira Soares) in southern Brazil (Fig. 1). Geologically, the region is located around the Serra Geral formation and the climate is classified as subtropical humid (Köppen classification), with an annual mean temperature of 18.7 °C and mean rainfall of 1700 mm (Alvares et al. 2013). This area is located in the Atlantic Forest biome, which consists of a transition zone between the Semideciduous Seasonal Forest and the Araucaria Atlantic Forest (Oliveira-Filho et al. 2015). In addition, the non-native species Hovenia dulcis (Thunb.) Rhamnaceae occurs in this region (Padilha et al. 2015; Schmidt et al. 2020).
We selected three morphologically similar streams (2nd order; named PM, SC and BO; Fig. 1) with different densities of H. dulcis ((individuals per hectare: 20 in PM; 460 in SC; 720 in BO). We measured stream water physical and chemical characteristics during the experimental period. The water from all streams was similar, with good levels of oxygenation (> 11 mg L−1), slightly acidic pH (~ 6.4), and low phosphorus (< 60 µg L−1) and nitrite concentrations (< 6 µg L−1) (Supplementary Material, Table S1). Rainfall data were obtained during the months in which experiments were performed (June 2016–May 2017) from a weather station installed 8 km from the study site (Supplementary Material, Figure S1).
Allochthonous organic matter dynamics
In each stream, we quantified allochthonous organic matter input from plants over the course of 1 year (June 2016–May 2017) using a methodology adapted from Gonçalves and Callisto (2013). We collected allochthonous organic matter with buckets (area 0.04 m2 per bucket), which were suspended about 1 m from the streambed in three stretches that were about 15–20 m (15 buckets were included in each stretch, and a total of 45 buckets per stream). We drilled holes in the bottoms of the buckets to drain rainfall. Monthly, we collected all allochthonous organic matter retained within the buckets, packed materials in plastic bags and transported them to the laboratory to be dried (40 ± 5 °C for 72 h), identified and weighed. From the plant allochthonous organic matter collected, we included all types of organic matter in our analyses (leaves, branches, fruits and flowers and seeds). We identified non-native H. dulcis plant allochthonous organic matter and weighed it separately, while plant material from native species was weighed collectively (called ‘native’). We define the seasons in accordance with the austral system, where Summer months extended from January to March, Autumn from April to June, Winter from July to September and Spring from October to December.
Litter decomposition
We used senescent leaves of non-native H. dulcis and native tree species commonly found in the study region. The senescent leaves were previously dried at ambient temperature (~ 20 °C/15 days). We organized two treatments, one composed of H. dulcis leaves exclusively and the other composed of a leaf mixture containing Nectandra megapotamica (Spreng.) Mez (70% of mixture), Cryptocarya aschersoniana Mez, Luehea divaricata Mart. & Zucc., Ilex paraguariensis A. St.-Hil., Campomanesia xanthocarpa O. Berg and Ocotea puberula (Rich.) Nees. (30% of mixture). These plant species are common in the forest remnants of the study region, including in the riparian zones of many streams (Leyser et al. 2012; Mélo et al. 2013). We chose to use a large proportion of N. megapotamica in the native group because this species was often dominant in the native forest remnants of the study region while the other species occurred less frequently (Leyser et al. 2012; Mélo et al. 2013). We performed a preliminary analysis to characterize the H. dulcis and native litter (Supplementary Material, Table S2). Only the toughness of the leaf tissues of treatments differed. It was higher (t = 5.5; df = 5; p = 0.01) in the group comprised of native plants (258.6 ± 41.3 g) than in the non-native treatment (109.5 ± 8.6 g).
We undertook leaf litter decomposition experiments in the Spring of 2016 (November) and Autumn (April) of 2017. In each experiment, we used 54 fine mesh litter bags (10 × 20 cm; 0.5 mm mesh) and 54 coarse mesh litter bags (10 × 20 cm; 10 mm mesh), with 3.0 ± 0.1 g of leaves each. In each stream, we incubated 36 litter bags (18 of each mesh), which contained a subsample of native mix leaves and 36 litter bags with exclusively H. dulcis leaves (total = 108 litter bags). After 3, 15 and 28 immersion days, 6 litter bags of each mesh type (3 litter bags with native and non-native leaves) were randomly removed from each stream for analysis. We packed the litter bags in plastic bags and transported them to the laboratory in an ice box. In the laboratory, we washed leaves to remove sediment and associated invertebrates. We dried the leaf litter in an oven at 40 ± 5 °C/72 h. After this period, we weighed the leaves to determine the remaining mass and decomposition rates.
Associated invertebrates
We washed the leaf litter collected from the coarse mesh litter bags to remove associated invertebrates. We passed water through 250 μm mesh to retain invertebrates. We fixed the invertebrates in 70% ethanol for screening and identification using a stereomicroscope (40× magnification). We identified organisms until the family level according to the taxonomic key proposed by Mugnai et al. (2010). After identification, we classified invertebrates into functional feeding groups (FFG) according to Cummins et al. (2005), Tomanova et al. (2006) and Ramírez and Gutiérrez-Fonseca (2014).
Data analysis
We assessed data normality using the Shapiro–Wilk test and ln(x + 1) transformed data that were not normally distributed. To assess the differences in allochthonous organic matter input between seasons and organic matter origin types (H. dulcis and native), we used a two-way ANOVA. We verified the relationship between monthly rainfall with the native and H. dulcis organic matter input using a Pearson linear correlation.
We determined decomposition rates using the negative exponential model Wt = W0 × e−kt, where Wt is the remaining weight at time t (in days), W0 is the initial weight and k is the decomposition rate (Webster and Benfield 1986). We evaluated the native and non-native leaf decomposition rates in the fine and coarse mesh separately using a covariance analysis (ANCOVA; time in days as co-variable).
To determine invertebrates associated in leaf litter, we tested data normality using the Shapiro–Wilk test and homoscedasticity was assessed using the Bartlett test. We transformed (ln(x + 1)) the data that were not normally distributed. We used rarefied richness to eliminate the effect of varied abundance of organisms on invertebrate richness (Gotelli and Colwell 2001). We evaluated variation of abundance, rarefied richness and abundance of each functional feeding group between native and non-native leaf litter using a t test. In addition, we used a multivariate permutational variance analysis (PerMANOVA; 999 permutations) to evaluate the invertebrates’ taxonomic and functional composition for each leaf litter type and season. We performed statistical analyses using the R statistical software with the “vegan” package (R Core Team 2017).
Results
Stream organic matter dynamics
The annual native organic matter input (181 g m−2) was twofold that of H. dulcis (97 g m−2). The highest H. dulcis organic matter input was in Autumn (65 g m−2), while for native organic matter the highest input was in Winter (55 g m−2) (Fig. 2). The organic matter input varied between the litter types and throughout the seasons (winter and spring; Fig. 2; Table 1). The native organic matter input was higher than the H. dulcis in Winter and Spring (Fig. 2). Only the native organic matter input was positively associated with rainfall (r = 0.60; df = 10; p = 0.04).
Leaf litter decomposition
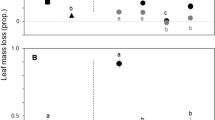
Hovenia dulcis litter decomposed faster than native litter in fine and coarse mesh in both seasons (Fig. 3). In the fine mesh, the H. dulcis leaf decomposition was approximately twofold higher than in the native litter. The accelerated mass loss in the H. dulcis litter leaf resulted in higher decomposition rates compared to the native litter (Fig. 3). The H. dulcis fine mesh decomposition rates (k = − 0.033 ± 0.006 day−1) was twice as high as in the native leaf litter (k = − 0.033 ± 0.006 day−1) (F(1;33) = 73.8; p < 0.001). Likewise, the H. dulcis decomposition rates in the coarse mesh (k = − 0.089 ± 0.023 day−1) was four times higher than native litter decomposition rates (k = − 0.023 ± 0.002 day−1) (F(1;33) = 68.1; p < 0.001).
Associated invertebrates
We identified a total of 9119 invertebrates associated with native and H. dulcis leaf litter in streams throughout the experiments (Supplementary Material, Table S3). Gastropoda and Chironomidae were the most abundant (~ 46 and ~ 44%, respectively), followed by Calamoceratidae (~ 3.6%), Leptoceridae (~ 1.5%), Baetidae (~ 1%) and Leptophlebiidae (~ 1%). Other invertebrate taxa made up 5% of the total identified. Invertebrate abundance was similar between native and H. dulcis leaf litter in both seasons (t = 0.2, df = 16, p = 0.82 and t = 0.8, df = 16, p = 0.40, Autumn and Spring, respectively) (Fig. 4). In addition, we observed similar pattern for invertebrate richness in both leaf litter (t = 1.1, df = 16, p = 0.27 and t = 0.6, df = 16, p = 0.54, Autumn and Spring, respectively) (Fig. 4).
The most abundant FFG were scrapers (~ 46% of total), collectors (~ 46%) and shredders (~ 5%) (Fig. 4a–d). Gastropoda was the most abundant taxa among scrapers, and Chironomidae among collectors, whereas Calamoceratidae (genus Phylloicus sp.) and Leptoceridae (Nectopsyche sp. and Triplectides sp.) were the most abundant shredders. However, the relative abundance of FFG did not vary between leaf litter types (Fig. 4c, f). The invertebrate taxonomic composition was similar between the native and non-native leaf litter (F(1; 32) = 0.02; p = 0.80), but differed between Spring and Autumn (F(1; 32) = 5.9; p = 0.001).
Discussion
In this study, we evaluated the effects of the invasion of the non-native species Hovenia dulcis on the functioning of small streams in the Brazilian Atlantic Forest biome. We observed that the stream allochthonous input of organic matter to streams was significantly altered with the presence of H. dulcis in the riparian zone and that decomposition of the H. dulcis leaf litter was higher than that of native litter. However, we found no clear evidence of differences in the structure and composition of the aquatic invertebrate community associated with native and non-native leaf litter.
During the study period, the native organic matter input was twofold higher than H. dulcis input. However, in Autumn, with the highest H. dulcis input, the leaf litter input in streams was higher. The high levels of native organic matter in the Winter were due to leaf renewal, where plant species eliminated old leaves to grow new ones (Turchetto and Fortes 2014). We also observed that native organic matter input was related to rainfall, in accordance with other studies (Dick et al. 2015; Tonin et al. 2017). The average rainfall during the study period was slightly lower than the average for last 10 years. Thus, we can expect that this rainfall-organic matter relationship is common throughout the year in the region under study.
Hovenia dulcis leaf fall is dependent on lower temperatures (Schumacher et al. 2008), occurring from beginning of Autumn until mid-Winter. Other non-natives’ (e.g., Eucalyptus) peak of organic matter input occurs normally in Summer months (Abelho and Graça 1996; Pozo et al. 1997), demonstrating that species identity is important in determining organic matter dynamics. In this study, this was clear when we observed that the input of native organic matter was higher than that of H. dulcis, again indicating the importance of identity for litter dynamics (Capellesso et al. 2016; Rezende et al. 2017).
The Atlantic Forest is characterized by the presence of tree species with specific phenological characteristics and different litterfall periods (Ruschel et al. 2005) leading to a consistency of energy and material input (Lisboa et al. 2015). In riparian vegetation in which H. dulcis predominates, vegetal allochthonous resources are limited due to restricted leaf fall patterns that occur at specific periods throughout the year (i.e., Autumn).
The leaf decomposition rates of the H. dulcis leaf litter were higher than those of native leaf litter, possibly because of its low toughness. Studies indicate that shredders abundance accelerates leaf decomposition (Tonin et al. 2014b; Tonello et al. 2016). However, in the present study, the accelerated decomposition of H. dulcis was not a direct effect of shredders that presented similar abundances between both leaf litters.
Leaf litter decomposition rates are determined by its structural and chemical characteristics and not by its origin (Zhang et al. 2019). Thus, we have shown that decomposition is faster in non-native than in native species, as seen in other studies (König et al. 2014; Medina-Villar et al. 2015). In some cases, the structure of the aquatic invertebrate community that colonizes the non-native litter may alter the aquatic invertebrate community (see Castro-Díez and Alonso 2017), possibly due to reduce nutritional quality, but we found no difference in the community between the native and non-native litter (König et al. 2014). This result was contrary to our expectations, since it is apparent that greater plant diversity typically sustains a greater diversity of consumers (Abelho 2009). It is possible that the lower toughness of the non-native species countered any litter diversity effect. We observed that litter-dwelling invertebrate communities varied between seasons. Similarly, invertebrate assemblages have been shown to be structured by season rather than the type of litter available (Larrañaga et al. 2014).
Conclusion
Our main conclusion was that the establishment of H. dulcis in riparian vegetation altered the organic matter dynamics and litter decomposition in the streams. While native organic matter input was relatively constant throughout the year, varying with rainfall intensity, the input of H. dulcis was seasonal. Although we found no differences in invertebrate communities between litter types, the changes in organic matter dynamics may affect ecosystem functioning, although we have no evidence of this. The H. dulcis input in a single period of year and its rapid decomposition can alter the availability of allochthonous resources, affecting the use of this resource by stream organisms. In addition, when H. dulcis is dominant in riparian zones, it can potentially decrease the diversity of allochthonous plant resource in streams.
References
Abelho M (2001) From litterfall to breakdown in streams: a review. Sci World J 1:656–680. https://doi.org/10.1100/tsw.2001.103
Abelho M (2009) Leaf–litter mixtures affect breakdown and macroinvertebrate colonization rates in a stream ecosystem. Int Rev Hydrobiol 94:436–451. https://doi.org/10.1002/iroh.200711019
Abelho M, Graça MAS (1996) Effects of eucalyptus afforestation on leaf litter dynamics and macroinvertebrate community structure of streams in Central Portugal. Hydrobiologia 324:195–204. https://doi.org/10.1007/BF00016391
Albariño RJ, Balseiro EG (2002) Leaf litter breakdown in Patagonian streams: native versus exotic trees and the effect of invertebrate size. Aquat Conserv Mar Freshw Ecosyst 12:181–192. https://doi.org/10.1002/aqc.511
Alvares CA, Stape JL, Sentelhas PC, Gonçalves JLM, Sparovek G (2013) Köppen’s climate classification map for Brazil. Meteorol Z 22:711–728. https://doi.org/10.1127/0941-2948/2013/0507
Bärlocher F, Graça MAS (2002) Exotic riparian vegetation lowers fungal diversity but not leaf decomposition in Portuguese streams. Freshw Biol 47:1123–1135. https://doi.org/10.1046/j.1365-2427.2002.00836.x
Biasi C, Cerezer C, Santos S (2016) Biological colonization and leaf decomposition in a subtropical stream. Ecol Austr 26:189–199
Biasi C, Cogo GB, Hepp LU, Santos S (2019) Shredders prefer soft and fungal-conditioned leaves, regardless of their initial chemical traits. Iheringia Ser Zool 109(2019004):1–7. https://doi.org/10.1590/1678-4766e2019004
Biasi C, Fontana LE, Restello RM, Hepp LU (2020) Effect of invasive Hovenia dulcis on microbial decomposition and diversity of hyphomycetes in Atlantic forest streams. Fungal Ecol 44:100890. https://doi.org/10.1016/j.funeco.2019.100890
Canhoto C, Graça MAS (1996) Decomposition of Eucalyptus globulus leaves and three native leaf species (Alnus glutinosa, Castanea sativa and Quercus faginea) in a Portuguese low order stream. Hydrobiologia 333:79–85. https://doi.org/10.1007/BF00017570
Capellesso ES, Scrovonski KL, Zanin EM, Hepp LU, Bayer C, Sausen TL (2016) Effects of forest structure on litter production, soil chemical composition and litter–soil interactions. Acta Bot Bras 30:329–335. https://doi.org/10.1590/0102-33062016abb0048
Carvalho PER (1994) Circular Técnica: Ecologia, silvicultura e usos da uva-do-japão (Hovenia dulcis Thunberg). Curitiba: EMBRAPA-CNPF, n.20
Casas JJ, Larrañaga A, Menéndez M, Pozo J, Basaguren A, Martínez A, Pérez J, González JM, Casado C, Descals E, Roblas N, Lopéz-González JA, Valenzuela JL (2013) Leaf-litter decomposition of native and introduced tree species of contrasting quality in headwater streams: how does the regional setting matter? Sci Total Environ 458–460:197–208. https://doi.org/10.1016/j.scitotenv.2013.04.004
Castro-Díez P, Alonso A (2017) Effects of non-native riparian plants in riparian and fluvial ecosystems: a review for the Iberian Peninsula. Limnetica 36:525–541. https://doi.org/10.23818/limn.36.19
Chauvet E, Ferreira V, Giller PS, McKie BG, Tiegs SD, Woodward G, Elosegi A, Dobson M, Fleituch T, Graça MAS, Gulis V, Hladyz S, Lacoursière JO, Lecerf A, Pozo J, Preda E, Riipinen M, Rîsnoveanu G, Vadineanu A, Vought LBM, Gessner MO (2016) Litter decomposition as an indicator of stream ecosystem functioning at local-to-continental scales: insights from the European RivFunction Project. Adv Ecol Res 55:99–182. https://doi.org/10.1016/bs.aecr.2016.08.006
Cummins KW (2002) Riparian-stream linkage paradigm. Verh Int Ver Theor Angew Limnol 28:49–58
Cummins KWR, Merritt W, Andrade PCN (2005) The use of invertebrate functional groups to characterize ecosystem attributes in selected streams and rivers in south Brazil. Stud Neotrop Fauna Environ 40:69–89. https://doi.org/10.1080/01650520400025720
Dechoum MS, Castellani TT, Zalba SM, Rejmánek M, Peroni N, Tamashiro JY (2015) Community structure, succession and invasibility in a seasonal deciduous forest in southern Brazil. Biol Invasions 17:1697–1712. https://doi.org/10.1007/s10530-014-0827-6
Dick G, D’ávila M, Schumacher MV (2015) Produção de serapilheira em fragmento de Floresta Estacional Subtropical na região norte do Rio Grande do Sul. Ecol Nut Florest 3:01–08. https://doi.org/10.5902/2316980X16354
Ferreira V, Faustino H, Raposeiro PM, Gonçalves V (2017) Replacement of native forests by conifer plantations affects fungal decomposer community structure but not litter decomposition in Atlantic island streams. For Ecol Manag 389:323–330. https://doi.org/10.1016/j.foreco.2017.01.004
Ferreira V, Boyero L, Calvo C, Correa F, Figueroa R, Gonçalves JF, Goyenola G, Graça MAS, Hepp LU, Kariuki S, López-Rodríguez A, Mazzeo N, M’Erimba C, Monroy S, Peil A, Pozo J, Rezende R, Mello FT (2019) A global assessment of the effects of eucalyptus plantations on stream ecosystem functioning. Ecosystems 22:629–642. https://doi.org/10.1007/s10021-018-0292-7
Gonçalves JF, Callisto M (2013) Organic-matter dynamics in the riparian zone of a tropical headwater stream in Southern Brazil. Aquat Bot 109:8–13. https://doi.org/10.1016/j.aquabot.2013.03.005
Gonçalves JF, Graça MAS, Callisto M (2006) Leaf-litter breakdown in 3 streams in temperate, Mediterranean, and tropical Cerrado climates. J N Am Benthol Soc 25:344–355. https://doi.org/10.1899/0887-3593(2006)25[344:LBISIT]2.0.CO;2
Gonçalves JF, Rezende RS, França JS, Callisto M (2012) Invertebrate colonisation during leaf processing of native, exotic and artificial detritus in a tropical stream. Mar Freshw Res 63:428–439. https://doi.org/10.1071/MF11172
Gonçalves JF, Rezende RS, Gregório RS, Valentin GC (2014) Relationship between dynamics of litterfall and riparian plant species in a tropical stream. Limnologica 44:40–48. https://doi.org/10.1016/j.limno.2013.05.010
Gotelli NJ, Colwell RK (2001) Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol Lett 4:379–391. https://doi.org/10.1046/j.1461-0248.2001.00230.x
Graça MAS (1993) Patterns and processes in detritus-based stream systems. Limnologica 23:107–114
Graça MAS (2001) The role of invertebrates on leaf decomposition in streams: a review. Internat Rev Hydrobiol 86:383–393. https://doi.org/10.1002/1522-2632(200107)86:4/5%3c383::AID-IROH383%3e3.0.CO;2-D
Graça MAS, Ferreira V, Canhoto C, Encalada AC, Guerrero-Bolaño F, Wantzen KM, Boyero L (2015) A conceptual model of litter breakdown in low order streams. Int Rev Hydrobiol 100:1–12. https://doi.org/10.1002/iroh.201401757
Juvenal TL, Matos RLG (2002) O setor florestal no Brasil e a importância do reflorestamento. BNDES Setorial 16:3–30
König R, Hepp LU, Santos S (2014) Colonisation of low- and high- quality detritus by benthic macroinvertebrates during leaf breakdown in a subtropical stream. Limnologica 45:61–68. https://doi.org/10.1016/j.limno.2013.11.001
Larrañaga S, Larrañaga A, Basaguren A, Elosegi A, Pozo J (2014) Effects of exotic eucalypt plantations on organic matter processing in Iberian streams. Int Rev Hydrobiol 99:363–372. https://doi.org/10.1002/iroh.201301665
Leyser G, Zanin EM, Budke JC, Mélo MA, Henke-Oliveira C (2012) Regeneração de espécies arbóreas e relações com componente adulto em uma floresta estacional no vale do rio Uruguai, Brasil. Acta Bot Bras 26:74–83. https://doi.org/10.1590/S0102-33062012000100009
Lisboa LK, da Silva ALL, Siegloch AE, Goncalves Jr JF, Petrucio MM (2015) Temporal dynamics of allochthonous coarse particulate organic matter in a subtropical Atlantic Rainforest Brazilian stream. Mar Freshw Res 66:674–680. https://doi.org/10.1071/MF14068
Marks J (2019) Revisiting the fates of dead leaves that fall into streams. Annu Rev Ecol Evol Syst 50:24.1-24.22. https://doi.org/10.1146/annurev-ecolsys-110218-024755
Martínez A, Larrañaga A, Pérez J, Descals E, Basaguren A, Pozo J (2013a) Effects of pine plantations on structural and functional attributes of forested streams. For Ecol Manag 310:147–155. https://doi.org/10.1016/j.foreco.2013.08.024
Martínez A, Larrañaga A, Pérez J, Basaguren A, Pozo J (2013b) Leaf-litter quality effects on stream ecosystem functioning: a comparison among five species. Fundam Appl Limnol 183:239–248. https://doi.org/10.1127/1863-9135/2013/0514
Medina-Villar S, Alonso A, Aldana BRV, Pérez-Corona E, Castro-Díez P (2015) Decomposition and biological colonization of native and exotic leaf litter in a Central Spain stream. Limnetica 3:293–310. https://doi.org/10.23818/limn.34.23
Mélo MA, Budke JC, Henke-Oliveira C (2013) Relationships between structure of the tree component and environmental variables in a subtropical seasonal forest in the upper Uruguay River valley, Brazil. Acta Bot Bras 27:751–760. https://doi.org/10.1590/S0102-33062013000400015
Menéndez M, Descals E, Riera T, Moya O (2013) Do non-native Platanus hybrida riparian plantations affect leaf litter decomposition in streams? Hydrobiologia 716:5–20. https://doi.org/10.1007/s10750-013-1539-0
Mugnai R, Nessimian JL, Baptista DF (2010) Manual de Identificação de Macroinvertebrados Aquáticos do Rio de Janeiro. Tecnical Books Editora, Rio de Janeiro
Neres-Lima V, Machado-Silva F, Baptista DF, Oliveira RO, Andrade PM, Oliveira AF, Sasada-Sato CY, Silva-Júnior EF, Feijó-Lima R, Angelini R, Camargo PB, Moulton TP (2017) Allochthonous and autochthonous carbon flows in food webs of tropical forest streams. Freshw Biol 62:1012–1023. https://doi.org/10.1111/fwb.12921
Oliveira-Filho A, Budke JC, Jarenkow JA, Eisenlohr PV, Neves DRM (2015) Deving into the variations in tree species composition and richness across South American subtropical Atlantic and Pampean forest. J Plant Ecol 6:242–260. https://doi.org/10.1093/JPE/RTT058
Padilha DL, Loregian AC, Budke JC (2015) Forest fragmentation does not matter to invasions by Hovenia dulcis. Biodivers Conserv 24:2293–2304. https://doi.org/10.1007/s10531-015-0930-8
Pozo J, González E, Díez JR, Molinero J, Elósegui A (1997) Inputs of particulate organic matter to streams with different riparian vegetation. J N Am Benthol Soc 16:602–611. https://doi.org/10.2307/1468147
R Core Team (2017). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. Disponível em: http://www.R-project.org. Acesso em 10 feb. 2017.
Ramírez A, Gutiérrez-Fonseca PE (2014) Functional feeding groups of aquatic insect families in Latin America: a critical analysis and review of existing literature. Rev Biol Trop 62:155–167. https://doi.org/10.15517/rbt.v62i0.15785
Rezende RS, Sales MA, Hurbath F, Roque N, Gonçalves JF, Medeiros AO (2017) Effect of plant richness on the dynamics of coarse particulate organic matter in a Brazilian Savannah stream. Limnologica 63:57–64. https://doi.org/10.1016/j.limno.2017.02.002
Ruschel AR, Guerra MP, Moerschbacher BM, Nodari RO (2005) Valuation and characterization of the timber species in remnants of the Alto Uruguay River ecosystem, southern Brazil. For Ecol Manag 217:103–116. https://doi.org/10.1016/j.foreco.2005.05.054
Schmidt AD, Castellani TT, Dechoum MS (2020) Biotic and abiotic changes in subtropical seasonal deciduous forest associated with invasion by Hovenia dulcis Thunb. (Rhamanaceae). Biol Invasions 22:293–306. https://doi.org/10.1007/s10530-019-02089-4
Schumacher MV, Brun EJ, Illana VB, Dissiuta SI, Agne TL (2008) Biomassa e Nutrientes em um Povoamento de Hovenia dulcis Thunb., Plantado na FEPAGRO Florestas, Santa Maria, RS. Ciência Florestal 18:27–37. https://doi.org/10.5902/19805098519
Seeney A, Pattison Z, Willby NJ, Boon PJ, Bull CD (2019) Stream invertebrate diversity reduces with invasion of river banks by non-native plants. Freshw Biol 64:485–496. https://doi.org/10.1111/fwb.13236
Taylor BR, Andrushchenko IV (2014) Interaction of water temperature and shredders on leaf litter breakdown: a comparison of streams in Canada and Norway. Hydrobiologia 721:77–88. https://doi.org/10.1007/s10750-013-1650-2
Tomanova S, Goitia E, Helešic J (2006) Trophic levels and functional feeding groups of macroinvertebrates in neotropical streams. Hydrobiologia 556:251–264. https://doi.org/10.1007/s10750-005-1255-5
Tonello G, Loureiro RC, Krause P, Silva C, Ongaratto RM, Sepp S, Restello RM, Hepp LU (2014) Colonização de invertebrados durante a decomposição de diferentes detritos vegetais em um riacho subtropical. Rev Bras Biocienc 12:98–105
Tonello G, Naziloski LA, Tonin AM, Restello RM, Hepp LU (2016) Effect of Phylloicus on leaf breakdown in a subtropical stream. Limnetica 35:243–252. https://doi.org/10.23818/limn.35.20
Tonin AM, Restello RM, Hepp LU (2014a) Chemical change of leaves during breakdown affects associated invertebrates in a subtropical stream. Acta Limnol Bras 26:235–244. https://doi.org/10.1590/S2179-975X2014000300003
Tonin AM, Hepp LU, Restello RM, Gonçalves JF (2014b) Understanding of colonization and breakdown of leaves by invertebrates in a tropical stream is enhanced by using biomass as well as count data. Hydrobiologia 740:79–88. https://doi.org/10.1007/s10750-014-1939-9
Tonin AM, Gonçalves JF, Bambi P, Couceiro SRM, Feitoza LAM, Fontana LE, Hamada N, Hepp LU, Lezan-Kowalczuk VG, Leite GFM, Lemes-Silva AL, Lisboa LK, Loureiro RC, Martins RT, Medeiros AO, Morais PB, Moretto Y, Oliveria PCA, Pereira EB, Ferreira LP, Pérez J, Petrucio MM, Reis DF, Rezende RS, Roque N, Santos LEP, Siegloch AE, Tonello G, Boyero L (2017) Plant litter dynamics in the forest-stream interface: precipitation is a major control across tropical biomes. Sci Rep 7:1–14. https://doi.org/10.1038/s41598-017-10576-8
Turchetto F, Fortes FO (2014) Aporte e decomposição de serapilheira em Floresta Estacional Decidual na região do Alto Uruguai, RS. Pesq Florest Bras 34:391–397. https://doi.org/10.4336/2014.pfb.34.80.735
Vannote RL, Minshall GW, Cummins KW, Sedell JR, Cushing CE (1980) The river continuum concept. Can J Fish Aquat Sci 137:130–137. https://doi.org/10.1139/f80-017
Wallace JB, Webster JR (1996) The role of macroinvertebrates in stream ecosystem function. Annu Rev Entomol 41:115–139. https://doi.org/10.1146/annurev.en.41.010196.000555
Webster JR, Benfield EF (1986) Vascular plant breakdown in freshwater ecosystems. Annu Rev Ecol Evol Syst 17:567–594. https://doi.org/10.1146/annurev.es.17.110186.003031
Zhang M, Cheng X, Geng Q, Shi Z, Luo Y, Xu X (2019) Leaf litter traits predominantly control litter decomposition in streams worldwide. Glob Ecol Biogeogr 28:1469–1486. https://doi.org/10.1111/geb.12966
Acknowledgements
This work was carried with Collection License issued by the Chico Mendes Institute for Biodiversity Conservation (ICMBio; Collection License No. 53164-1). The authors to thank the Municipal Park Mata do Rio Uruguai Teixeira Soares for their assistance during the field activities. The authors are grateful to ENGIE Tractebel Energia for providing the rainfall data. LEF thanks the scholarship (CAPES-PROSUP) linked to the URI Erechim Postgraduate Program in Ecology. LUH receives financial support from CNPq (nº 305203/2017-7, 421632/2016-0 and 307212/2020-3).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Luz Boyero.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fontana, L.E., Restello, R.M. & Hepp, L.U. Hovenia dulcis Thunb. (Rhamnaceae) invasion in the riparian zone alters the dynamics and decomposition of organic matter in subtropical streams, but not of associated invertebrate assemblages. Limnology 23, 365–373 (2022). https://doi.org/10.1007/s10201-021-00695-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10201-021-00695-7