Abstract
Rhithropanopeus harrisii (Gould 1841) has a native distribution from New Brunswick (Canada) to Veracruz (Mexico) and is considered an invasive species in northwestern North American (Oregon and California), South American (Brazil) and European estuaries and rivers. In Europe, it was observed for the first time in 1874, in The Netherlands. We sequenced and analyzed part of the cytochrome oxidase subunit I gene (mitochondrial DNA) of eight populations, three from the east coast of the United States of America (USA) and five from Europe, in order to assess their genetic diversity and to determine a potential founder population. European populations are characterized by a lower number of haplotypes than the whole native region of the eastern USA, suggesting that genetic bottlenecks occurred during the European colonisation. Along the North American East Coast, there is evidence of clearcut genetic heterogeneity, New Jersey being the most similar population in its genetic structure to the postulated Europe-founding population. Also the different European populations are heterogeneous and there is a tendency of higher genetic diversity in the populations founded earlier. R. harrisii is still in the process of expansion in Europe and may have been introduced once or repeatedly by different invasion mechanisms. The pronounced lack of gene flow among populations is of great ecological significance, since it may facilitate rapid adaptation and specialization to local conditions within single estuarine systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exotic species are one important factor in the global diversity crisis, changing community composition and ecosystem functioning. Besides, they often cause significant economic damage (Cohen and Carlton 1998; Stachowicz et al. 1999; Grosholz 2002). For this reason, there is great interest in understanding why and how successful invasions occur (Stachowicz et al. 1999). Some studied examples, mainly for aquatic invertebrates, range from ecological impact assessment (e.g., Marck and d’Antonio 1998; Grosholz 2002) and invasion dynamics (e.g., Neubert et al. 2000) to invasion pathways and genetic characterization (e.g., Müller 2001; ICES 2005). Molecular tools proved to be very useful in this context, since they allow genetic comparisons among any taxa or populations, which is crucial when reconstructing invasion histories (Avise 2004).
Decapod crustaceans are a key group in benthic communities and present characteristics useful for long distance migrations, so it is not surprising that many species have been associated with different invasion processes (Rodríguez and Suárez 2001). Carcinus maenas, outside of Europe (see Yamada et al. 2005), and Eriocheir sinensis, in Europe (see Hänfling et al. 2002), are good examples of the ecological impact that exotic crab species might have on native communities. The latter species is an ecological and economic threat in northern Europe owing to its burrowing activities in the banks of estuaries and predation on commercial fish (Hänfling et al. 2002). But according to Cabral and Costa (1999), E. sinensis in the Tagus Estuary (Portugal) was not a hazard in the 1990s and it was even used as a food source.
Rhithropanopeus harrisii (Gould 1841) is a euryhaline decapod crustacean that presents a larval retention mechanism within estuaries (Cronin 1982; Cronin and Forward 1986). It is easy to collect and, because its larvae show resistance to lower salinities, its ecological and physiological requirements are well studied (e.g., Alvarez et al. 1995; Grosholz and Ruiz 1995; Cripe et al. 2003). The native distribution of this species extends from New Brunswick (Canada) to Veracruz (Mexico). It is considered an invasive species in northwestern North American (Oregon and California), central and southeastern South American (Panama and Brazil), and European estuaries and rivers (Christiansen 1969; Roche and Torchin 2007). In Europe it was observed for the first time in The Netherlands in 1874 (Wolff 1954), and in the following years it was discovered in many other European countries/regions (mentioned chronologically): southeastern France (Adour River); Bulgaria, Romania and Russia (Black Sea area); Germany; Poland; Denmark; north of France (Normandy); Sea of Azov and Caspian Sea (Christiansen 1969); Spain (Cuesta et al. 1991); Portugal (Gonçalves et al. 1995); Italy (Mizzan and Zanella 1996 in Mizzan 1999), and Mediterranean France (Galil et al. 2002). Today it is distributed from the Caspian and Black Sea throughout the Mediterranean and European Atlantic all the way to the Baltic Sea. According to Gonçalves et al. (1995), R. harrisii has a patchy distribution along the coast of the Iberian Peninsula, being present only in the Mondego Estuary (central Portugal) and in the Guadalquivir River (south Atlantic coast of Spain). Recent observations of the presence of this species in the Tagus Estuary have broadened its Iberian distribution. It is also known that this species carries a herpes-like virus (Payen and Bonami 1979) and that this kind of virus can be fatal for juvenile oysters, lobster and shrimp populations (Renault 1998; Shields and Behringer 2004). Therefore, it would be of great importance to evaluate the status of this exotic species, in order to monitor its future spread and to understand its genetic and ecological characteristics. This can be achieved through knowledge of the invasion history and population genetic characterization of R. harrisii along European coasts.
The goals of this study are thus to verify (1) if the European populations of this species can be attributed to only one colonisation event or were introduced several times independently from different areas of origin and (2) if haplotype frequency differentiation exists between European populations, which would imply lack of gene flow and independent evolution after the respective invasions.
Materials and methods
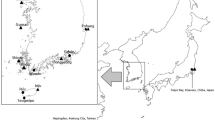
We had access to eight populations; three from the east coast of the United States of America (USA) and five from Europe (see Table 1, Fig. 1). The sequences from one population of the USA, Neuse River (North Carolina), were obtained directly from GenBank, derived from a study on the genetic structure of the invasive populations in California and Oregon as compared to the Neuse River population (Petersen 2006).
Genomic DNA was extracted from the muscle tissue of walking legs using the Puregene kit (Gentra Systems). Parts of the mitochondrial cytochrome-oxidase subunit I (COI) gene, with a length of 650 bp, were amplified through touch down polymerase chain reaction (PCR; 40 cycles: 45 s at 94°C, denaturating; 1 min at 52–48°C, annealing; 1 min at 72°C, extension). Primers used were: COL14 (5′-GCT TGA GCT GGC ATA GTA GG-3′; identical to internal forward primer of Roman and Palumbi 2004) and the new primer COH11R (5′-GCC TTT GGW ATA ATT TCT CA-3′). Sequences were obtained by cycle sequencing, run in an automated sequencer (for details see Reuschel and Schubart 2006), and read and edited with BioEdit (Hall 1999). The sequences are available through GenBank (accession numbers: FJ517398–FJ517532).
The genetic diversity (nucleotide diversity: π and haplotype diversity: h) was assessed through DnaSP 4.0 (Rozas and Rozas 1999). The following additional tests were run with Arlequin 3.11 (Excoffier et al. 2005): AMOVA analysis (Φ statistics), for the analysis of variance; neutrality tests (Tajima’s D and Fu’s Fs), that test whether sampled populations follow the neutrality model (i.e., genetic variability maintained by a balance between mutations and genetic drift; Avise 2004); mismatch distribution analyses using the minimization of the sum of squared deviations (SSD) between the observed mismatch distribution and its expectation, under the model of sudden expansion, discriminating distributions that result from expansion or from stationary populations (Harpending et al. 1993; Schneider and Excoffier 1999); and a raggedness index that quantifies the smoothness of the observed mismatch distribution, showing low values when populations underwent an expansion event, and high values when the populations are stationary or suffered a bottleneck (Harpending et al. 1993; Harpending 1994). The model of DNA substitution that best fit our data (providing a gamma value for the Arlequin analyses) was chosen using the software Modeltest 3.6 (Posada and Crandall 1998).
A statistical parsimony network of haplotypes (Templeton et al. 1992) was constructed using the program TCS (Clement et al. 2000).
Results
Obtained sequences were trimmed to a length of 523 bp in order to make them comparable with the ones from GenBank (Neuse River population). In that set of sequences, a total of 163 sequences were analyzed, revealing 51 different haplotypes with 47 variable sites, of which 30 are parsimony-informative; 55 mutations were detected, of which 7 are non-synonymous. The haplotype and nucleotide diversities within each population revealed that the European populations tend to have lower diversities than the American ones, especially than the population from the Neuse River in North Carolina (Table 2). However, the Neuse River population presents exceptionally high values of genetic diversity and a comparison of the European populations with the New Jersey (NJ) population shows that the European haplotype diversity is not very different from the native one. The Netherlands and Spain present even higher values of both haplotype diversity and nucleotide diversity than NJ (Table 2). Usually, the haplotype diversity and nucleotide diversity follow the same pattern in the studied populations. For Poland this was not quite the case, since this population presents a haplotypic diversity similar to Portugal, but its nucleotide diversity is much smaller (Table 2).
The analyses of variance (Table 3) show that the largest fraction of the variation resides among populations within regions (Europe and USA; >53%). Within populations there is also a great amount of variation, but this value is not significant (P = 0.43). The contrary is observed among regions, where the percentage of variation is small, though significant (P < 0.0001), meaning that populations differ significantly inside each region (Europe and USA), but these regions differ from each other at a small level.
From the neutrality tests we can observe that all populations are close to a mutation-random drift equilibrium (neutral model), since the model was not rejected, except for two populations, Neuse River and Louisiana (Table 2). The first population yields a very large negative value from the Fu’s Fs, which means, because the test is sensitive to population size changes (Excoffier et al. 2005; Fu 1997), that probably the Neuse River population has experienced a demographic expansion. From the pairwise mismatch analyses we obtain the same kind of result, since the Neuse River population does not reject the model of sudden expansion (SSD = 0.002; P = 0.65). Unfortunately, the raggedness index is not significant, but, its low value (mean value of 0.208) should be noted. Taking into account the mismatch distribution graph for the Neuse River population, a unimodal distribution is clear (Fig. 2), so this population has been growing for a long time or had a sudden expansion event in the past (Harpending 1994; Rogers and Harpending 1992). Also the minimum spanning network, produced by TCS, for the Neuse River population (all haplotypes identified with “N”) shows a star-like pattern, which means that this population must have had an expansion event in the past (Slatkin and Hudson 1991; Fig. 3). Some of these results were also obtained by Petersen (2006), with the exception of the neutrality test results and the mismatch distribution graph. The other population that was not in agreement with the neutral model was the Louisiana population. In this case, both tests, Tajima’s D and Fu’s Fs, give significant P values. The later test is not as negatively large as for the Neuse River population, and Tajima’s D gave a significant negative value that might be due to a large insertion/deletion polymorphism or to a bottleneck event (Tajima 1989a; Table 2). Similarly to the Neuse River population, the model of sudden expansion cannot be rejected for the Louisiana population. Once again, the raggedness index is not significant but shows a low value, and through the mismatch distribution graph we can observe a unimodal distribution (Table 2; Fig. 2). TCS analysis also resulted in a star-shape network of the Louisiana haplotypes, even if they are not as numerous as in the Neuse River population (Fig. 3). All of these tests suggest that a population expansion might have occurred, so the fact that the Tajima’s D was significantly negative for Louisiana cannot be attributed to a bottleneck event. As for the other North-American population, New Jersey, we cannot reject any of the neutrality tests, nor the model of sudden expansion. The raggedness index value does not differ significantly from the model, but is also low relative to the mean value, indicating growth for a long time or a burst of expansion (Harpending 1994). As shown by the TCS network, all the haplotypes corresponding to NJ are among other European haplotypes, like B, C, and U (Fig. 3), making it difficult empirically to observe a star-like shape.
Minimum spanning network based on a 523 bp fragment of the COI gene of Rhithropanopeus harrisii with haplotype A as the proposed ancestral haplotype according to the software TCS. Dark grey: USA populations only, light grey: haplotypes shared by European and USA populations, white: European populations only (see Table 1 for abbreviations and details)
For the European populations, it was not possible to reject the neutrality tests and the sudden expansion model. Portugal was the only population that came close to rejecting this model, being at the statistical limit (SSD P = 0.05). The raggedness index for Portugal was highly significant, yielding a value that, relative to the mean, can be considered high (r = 0.675; P < 0.001), so we might infer that it is a stationary or a bottlenecked population that, possibly, did not pass through a sudden population expansion (Harpending 1994). All the other populations did not present significant raggedness indexes.
The TCS network (Fig. 3) shows that the Neuse River (North Carolina) population and the Louisiana animals occupy the opposite extremes of the network and that Louisiana shows four fixed differences with all haplotypes from the American East Coast and Europe. Three frequent haplotypes, A, B, and C, are predominant. These haplotypes are constituted mainly by European individuals, the exception being individual N8 from Neuse River corresponding to haplotype A and several NJ individuals corresponding to haplotypes B and C (Figs. 1, 3). The U haplotype is represented by Spanish and NJ individuals (Table 1; Fig. 1). In Fig. 1 we can also observe that the haplotypes present in Europe exist also in the NJ population as a great majority, and in the North Carolina population as a much smaller fraction. The Netherlands, Portugal, Spain, and Bulgaria have haplotypes of their own that are not represented in the USA populations (Fig. 1; Table 1).
Discussion
The first goal of this study was to find out if, the European populations of R. harrisii can be traced to a single colonisation event or were introduced several times independently from different areas of origin. We show that the NJ population includes individuals with two of the most frequent European haplotypes (B, C). This makes it the most similar population in its genetic structure to the potential founding population of the European colonisation. The fact that the most frequent haplotype (A) from Europe was only found in the USA populations in relatively low frequency (in the Neuse River, North Carolina), can be explained by a sudden spreading of a small founder population in Europe or alternatively by an insufficient sampling of the USA populations. Therefore, we should take these conclusions as preliminary and await a more thorough sampling of native populations. Nevertheless, for the moment, the most likely hypothesis is that the colonisation of European waters was initiated by individuals originating from the northeastern USA, owing to the fact that four distinct haplotypes (A, B, C, and U) are shared among Europe and the USA populations. This demonstrates that at least four females, not necessarily in the same colonization event, with these different haplotypes, were among the European colonists. In order to hypothesize that the colonisation could have occurred during a single event, haplotype A, so far only found in Neuse River (North Carolina) among the USA populations, would have to be present also in the NJ population, or the source population would have to lie between NJ and North Carolina and includes haplotypes A, B, C, and U.
A second goal of this study was to establish whether, differentiation at the level of haplotype frequencies exists between European populations. Our results show that European populations are characterized by a lower number of haplotypes than the assemblage of all native populations in eastern USA. Nevertheless, the Dutch and the Spanish populations showed a similar variability to that of the NJ population and seem to be the most diverse in Europe. This cannot be due to sampling bias, because only 16 sequences from The Netherlands were analyzed. There are two other possibilities: (1) these sampling sites experienced more colonisation events or colonisations by more individuals than, for example, Poland, Portugal, and Bulgaria (which would also explain the similar genetic variability between those European populations and New Jersey); (2) bottlenecks occurred during colonisation from other parts of Europe to Poland, Portugal or Bulgaria. Results for Portugal showed that this population might not have suffered expansion and given the high raggedness index, and due to the fact that it is not a native region, only a bottleneck event can be assumed. Poland and Bulgaria did not reject the model of sudden expansion, but when bottlenecks last for a long time, their model becomes equivalent to a sudden expansion model, with the same pattern, being unimodal but with curves with steep leading faces (Rogers and Harpending 1992), as can be observed for these two populations (Fig. 2). In Poland and Bulgaria this persistent bottleneck might have acted more strongly on the nucleotide differences (their nucleotide diversity are the lowest in Europe), because probably their population size was very small at the time of colonisation (Tajima 1989b). All this information seems to support the second possibility that bottlenecks occured during European colonisations.
The TCS network and Fig. 1 show also that all European populations (except for Poland) include unique haplotypes, found nowhere else. This could imply that new haplotypes might have evolved in the newly colonized areas, or a more exhaustive sampling of the USA populations is needed to detect these haplotypes in the founder population. Since COI in decapod crustaceans has an average mutation rate of about 1.4–2.6% per million year (Knowlton et al. 1993; Knowlton and Weigt 1998; Schubart et al. 1998) and R. harrisii was first observed in Europe in the nineteenth century (Christiansen 1969), the probability of new mutations is very low, and probably most of these haplotypes also occur in low frequencies in the founder population.
All populations of R. harrisii showed a pronounced lack of gene flow (greater fraction of the variability was among populations in each region), and this might be mostly due to the fact that this species shows a larval retention mechanism, retaining the larvae within the vicinity of the parental population (Cronin 1982; Cronin and Forward 1986). This is of great ecological significance, since it may facilitate rapid adaptation and specialization to local conditions within single estuarine systems, which could have contributed to the success of this species outside its native range. For example, in the Portuguese population, which seems to be a stable one, this adaptation could be verified by the authors, since in a period of 10 years this population has moved around 6 km upward in Mondego River, disappearing completely from the previous sampling site.
The invasion pathway for R. harrisii to the European coast is assumed to be through ballast water or fouling communities on the hulls of vessels (Christiansen 1969). Ballast water might play an important role for current invasion episodes, but not for the first ones of R. harrisii, since it was a mechanism that was not used at the time of the first invasions (Carlton 1985 in Rodríguez and Suárez 2001). Therefore, the first invasions were most likely as a byproduct of live animal shipment for aquaculture like oysters or through fouling communities on ship hulls. Oyster trading seems a viable theory, since it started as early as the seventeenth century between New England and Europe and it was also the invasion vector into California waters (Rodríguez and Suárez 2001; Kirby 2004). The oyster trade to Europe was carried out throughout several northern and southern regions of the USA (Kirby 2004), so it is possible that the founder individuals arrived through several colonisation events, supporting the first hypothesis mentioned previously, that the Dutch and Spanish populations of R. harrisii might have had more founders than other European populations. Transport as fouling material on ship hulls can also be considered a possible invasion mechanism for the first invasions. For example, in German waters about one half of introduced species arrived through the hulls of sea trade ships (Nehring 2005). But since the 1970s, ship hulls and ballast water seem to have similar impacts on invasion processes (Nehring 2001).
An additional new insight from this study is the evidence that the Louisiana population is genetically distinct from the European as well as from the eastern USA populations. This means that this population did not contribute to the European colonisations, and it also constitutes evidence for genetic differentiation along the North American coast. Eventually this difference could be minimized from south to north, if some larvae of R. harrisii could be taken by the Gulf Stream current and also from north to south if southward nearshore currents, from Georgia to Cape Canaveral, would transport them. Although more sampling of populations from the northeastern USA would be needed to confirm this dispersal, we can already say that probably these population differences would continue, owing to the larval retention mechanism of this species. Genetic distinctness of Gulf of Mexico populations is well studied in many animal groups along the North American East Coast, most of which have a hybrid/frontier zone in Florida (e.g., Briggs 1974; Reeb and Avise 1990; Young et al. 2002), and this is also known for other crustaceans (e.g., Schubart et al. 2000; Mathews et al. 2002; Young et al. 2002). What leads to this differentiation might depend on several factors, for example, for the Crassostrea virginica oyster it depends on the species ecology and their life history, but also on historical and contemporary environmental events (Reeb and Avise 1990).
According to Lee (2002), invasive species present very intriguing characteristics, not only in their dispersal methods, but also in their evolution after the invasion. R. harrisii is still a species in expansion and its ecological success might be due to the occupation of vacant niches (Nehring 2000). It is also ominous that R. harrisii is a transport vector for a herpes-like virus (Payen and Bonami 1979) that provokes death in oyster, shrimp, lobster, and crab communities (Arzul et al. 2002; Shields and Behringer 2004). But, for now it remains unknown if R. harrisii is a real hazard for European estuarine communities. This is why invasive species are an current and major subject of investigation.
References
Alvarez F, Hines AH, Reaka-Kudla ML (1995) The effects of parasitism by the barnacle Loxothylacus panopaei (Gissler) (Cirripedia: Rhizocephala) on growth and survival of the host crab Rhithropanopeus harrisii (Gould) (Brachyura: Xanthidae). J Exp Mar Biol Ecol 192:221–232. doi:10.1016/0022-0981(95)00068-3
Arzul I, Renault T, Thébault A, Gérard A (2002) Detection of oyster herpesvirus DNA and proteins in asymptomatic Crassostrea gigas adults. Virus Res 84:151–160. doi:10.1016/S0168-1702(02)00007-2
Avise JC (2004) Molecular markers, natural history and evolution. Sinauer Associates, Suderland
Briggs JC (1974) Marine zoogeography. McGraw-Hill, New York
Cabral HN, Costa MJ (1999) On the occurrence of the Chinese mitten crab, Eriocheir sinensis, in Portugal (Decapoda, Brachyura). Crustac 72:55–58
Carlton JT (1985) Transoceanic and interoceanic dispersal of coastal marine organisms: the biology of ballast water. Oceanogr Mar Biol Annu Rev 23:313–371
Christiansen ME (1969) Crustacea Decapoda Brachyura. Marine invertebrates of Scandinavia, vol 2. Universitetsforlaget, Oslo
Clement M, Posada D, Crandall KA (2000) TCS: a computer program to estimate gene genealogies. Mol Ecol 9:1657–1659. doi:10.1046/j.1365-294x.2000.01020.x
Cohen AN, Carlton JT (1998) Accelerating invasion rate in a highly invaded estuary. Science 279:555–558. doi:10.1126/science.279.5350.555
Cripe GM, McKenney CL Jr, Hoglund MD, Harris PS (2003) Effects of fenoxycarb exposure on complete larval development of the xanthid crab, Rhithropanopeus harrisii. Environ Pollut 125:295–299. doi:10.1016/S0269-7491(02)00414-1
Cronin TW (1982) Estuarine retention of larvae of the crab Rhithropanopeus harrisii. Estuar Coast Mar Sci 15:207–220
Cronin TW, Forward RB Jr (1986) Vertical migration cycles of crab larvae and their role in larval dispersal. Bull Mar Sci 39:192–201
Cuesta JA, García-Raso JE, González JI (1991) Primera cita de Rhithropanopeus harrisii (Gould, 1841) (Crustacea, Decapoda, Brachyura, Xanthidae) en la Península Ibérica. Boll Inst Esp Oceanogr 7:149–153
Excoffier L, Laval G, Schneider S (2005) Arlequin ver 3.0—an integrated software package for population genetics data analysis. Computational and Molecular Genetics Lab, Institute of Zoology, University of Berne, Berne
Fu Y-X (1997) Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics 147:915–925
Galil B, Froglia C, Noel P (2002) CIESM atlas of exotic species in the Mediterranean 2. Crustaceans: decapods and stomatopods. CIESM Publishers, Monaco
Gonçalves F, Ribeiro R, Soares AMVM (1995) Rhithropanopeus harrisii (Gould), an American crab in the estuary of the Mondego River, Portugal. J Crustac Biol 15:756–762. doi:10.2307/1548824
Grosholz E (2002) Ecological and evolutionary consequences of coastal invasions. Trends Ecol Evol 17:22–27. doi:10.1016/S0169-5347(01)02358-8
Grosholz ED, Ruiz GM (1995) Does spatial heterogeneity and genetic variation in populations of the xanthid crab Rhithropanopeus harrisii (Gould) influence the prevalence of an introduced parasitic castrator? J Exp Mar Biol Ecol 187:129–145. doi:10.1016/0022-0981(94)00175-D
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hänfling B, Carvalho GR, Brandl R (2002) mt-DNA sequences and possible invasion pathways of the Chinese mitten crab. Mar Ecol Prog Ser 238:307–310. doi:10.3354/meps238307
Harpending HC (1994) Signature of ancient population growth in a low-resolution mitochondrial DNA mismatch distribution. Hum Biol 66:591–600
Harpending HC, Sherry ST, Rogers AR, Stoneking M (1993) The genetic structure of ancient human populations. Curr Anthropol 34:483–496. doi:10.1086/204195
ICES (2005) Vector pathways and the spread of exotic species in the sea, ICES cooperative research report, No. 271
Kirby MX (2004) Fishing down the coast: historical expansion and collapse of oyster fisheries along continental margins. Proc Natl Acad Sci USA 101:13096–13099. doi:10.1073/pnas.0405150101
Knowlton N, Weigt LA (1998) New dates and new rates for divergence across the isthmus of panama. Proc R Soc Lond B Biol Sci 265:2257–2263. doi:10.1098/rspb.1998.0568
Knowlton N, Weigt LA, Solorzano LA, Mills DK, Bermingham E (1993) Divergence in proteins, mitochondrial DNA, and reproductive compatibility across the isthmus of panama. Science 260:1629–1632. doi:10.1126/science.8503007
Lee CE (2002) Evolutionary genetics of invasive species. Trends Ecol Evol 17:386–391. doi:10.1016/S0169-5347(02)02554-5
Marck MC, D’Antonio CM (1998) Impacts of biological invasions on disturbance regimes. Trends Ecol Evol 13:195–198. doi:10.1016/S0169-5347(97)01286-X
Mathews LM, Schubart CD, Neigel JE, Felder DL (2002) Genetic, ecological, and behavioural divergence between two sibling snapping shrimp species (Crustacea: Decapoda: Alpheus). Mol Ecol 11:1427–1437. doi:10.1046/j.1365-294X.2002.01539.x
Mizzan L (1999) Le specie alloctone del macrozoobenthos della laguna di Venezia: il punto della situazione. Boll Mus Civ Storio Nat Venezia 49:145–177
Mizzan L, Zanella L (1996) First record of Rhithropanopeus harrisii (Gould, 1841) (Crustacea, Decapoda, Xanthidae) in the Italian water. Boll Mus Civ Storio Nat Venezia 46:109–120
Müller J (2001) Invasion history and genetic population structure of riverine macroinvertebrates. Zool Jena 104:346–355. doi:10.1078/0944-2006-00040
Nehring S (2000) Zur Bestandssituation von Rhithropanopeus harrisii (Gould, 1841) in deutschen Gewässern: die sukzessive Ausbreitung eines amerikanischen Neozoons (Crustacea: Decapoda: Panopeidae). Senckenberg marit 30:115–122
Nehring S (2001) After the TBT era: alternative anti-fouling paints and their ecological risks. Senckenberg marit 31:341–351
Nehring S (2005) International shipping—a risk for aquatic biodiversity in Germany. In: Nentwig W, Bacher S, Cock M, Dietz HJ, Gigon A, Wittenberg R (eds) Biological invasions—from ecology to control. NEOBIOTA 6, Prague, pp 125–143
Neubert MG, Kot M, Lewis MA (2000) Invasion speeds in fluctuating environments. Proc R Soc Lond B Biol Sci 267:1603–1610. doi:10.1098/rspb.2000.1185
Payen GG, Bonami JR (1979) Mise en evidence de particles d’allure virale associees aux noyaux des cellules mesodermiques de la zone germinative testiculaire du crabe Rhithropanopeus harrisii (Gould) (Brachyoure, Xanthide). Rev Trav Inst Peches Mar 43:361–365
Petersen C (2006) Range expansion in the northeast Pacific by an estuary mud crab—a molecular study. Biol Invasions 8:565–576. doi:10.1007/s10530-005-0160-1
Posada D, Crandall KA (1998) MODELTEST: testing the model of DNA substitution. Bioinformatics 14:817–818. doi:10.1093/bioinformatics/14.9.817
Reeb CA, Avise JC (1990) A genetic discontinuity in a constinuously distributed species: mitochondrial DNA in the American oyster, Crassostrea virginica. Genetics 124:397–406
Renault T (1998) Infections herpétiques chez les invertébrés: détection de virus de type herpès chez les mollusques bivalves marins. Virology 2:401–403
Reuschel S, Schubart CD (2006) Phylogeny and geographic differentiation of Atlanto-Mediterranean species of the genus Xantho (Crustacea: Brachyura: Xanthidae) based on genetic and morphometric analyses. Mar Biol (Berl) 148:853–866. doi:10.1007/s00227-005-0095-1
Roche D, Torchin M (2007) Established population of the North American Harris mud crab, Rhithropanopeus harrisii (Gould 1841)(Crustacea: Brachyura: Xanthidae) in the Panama Canal. Aquat Invasions 2:155–161
Rodríguez G, Suárez H (2001) Anthropogenic dispersal of decapod crustaceans in aquatic environments. Interciencia 26:282–289
Rogers AR, Harpending H (1992) Population growth makes waves in the distribution of pairwise genetic differences. Mol Biol Evol 9:552–569
Roman J, Palumbi SR (2004) A global invader at home: population structure of the green crab, Carcinus maenas, in Europe. Mol Ecol 13:2891–2898. doi:10.1111/j.1365-294X.2004.02255.x
Rozas J, Rozas R (1999) DnaSP version 3: an integrated program for molecular population genetics and molecular evolution analysis. Bioinformatics 15:174–175. doi:10.1093/bioinformatics/15.2.174
Schneider S, Excoffier L (1999) Estimation of past demographic parameters from the distribution of pairwise differences when the mutation rates vary among sites: application to human mitochondrial DNA. Genetics 152:1079–1089
Schubart C, Diesel R, Hedges SB (1998) Rapid evolution to terrestrial life in Jamaican crabs. Nature 393:363–365. doi:10.1038/30724
Schubart CD, Neigel JE, Felder DL (2000) Use of the mitochondrial 16S rRNA gene for phylogenetic and population studies of Crustacea. Crustac Issues 12:817–830
Shields JD, Behringer DC Jr (2004) A new pathogenic virus in the Caribbean spiny lobster Palinurus argus from the Florida keys. Dis Aquat Organ 59:109–118. doi:10.3354/dao059109
Slatkin M, Hudson RR (1991) Pairwise comparisons of mithochondrial DNA sequences in stable and exponentially growing populations. Genetics 129:555–562
Stachowicz JJ, Whitlatch RB, Osman RW (1999) Species diversity and invasion resistance in a marine ecosystem. Science 286:1577–1579. doi:10.1126/science.286.5444.1577
Tajima F (1989a) Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123:585–595
Tajima F (1989b) The effect of change in population size on DNA polymorphism. Genetics 123:597–601
Templeton AR, Crandall KA, Sing CF (1992) A cladistic analysis of phenotypic associations with haplotypes inferred from restriction endonucleases mapping and DNA sequence data. III. Cladogram estimation. Genetics 132:619–633
Wolff T (1954) Occurence of two East American species of crabs in European waters. Nature 174(4421):188–189. doi:10.1038/174188a0
Yamada SB, Dumbauld BR, Kalin A, Hunt CE, Figlar-Barnes R, Randall A (2005) Growth and persistence of a recent invader Carcinus maenas in estuaries of the northeastern Pacific. Biol Invasions 7:309–321. doi:10.1007/s10530-004-0877-2
Young AM, Torres C, Mack JE, Cunningham CW (2002) Morphological and genetic evidence for vicariance and refugium in Atlantic and Gulf of Mexico populations of the hermit crab Pagurus longicarpus. Mar Biol (Berl) 140:1059–1066. doi:10.1007/s00227-002-0780-2
Acknowledgments
We would like to thank F. Martinho, J. P. Domingues, N. R. Duarte, C. Fidalgo, R. Ribeiro and F. Gonçalves for helping with the sampling in the Mondego estuary (Portugal); C. d’Udekem d’Acoz, J. A. Cuesta, R. Forward, P. Jivoff, L. Mizzan, M. Normant, S. Algarín Vélez and S. Uzumov for their help with sampling European and North American populations; C. Petersen for making available additional sequence information; R. Jesse, T. Santl, S. Klaus L. Heine and P. Koller for advice and support; S. Reuschel, I. Silva and A. I. Catarino for statistical discussions; N. T. Goulart and M. Bruneaux for figures enhancements; J. Heinze and the entire group from Biologie 1, Universität Regensburg for making this study possible in every way and to the Deutscher Akademischer Austausch Dienst (DAAD) for the financial support. The authors would like also to acknowledge the comments of two anonymous reviewers that greatly improved this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Projecto-Garcia, J., Cabral, H. & Schubart, C.D. High regional differentiation in a North American crab species throughout its native range and invaded European waters: a phylogeographic analysis. Biol Invasions 12, 253–263 (2010). https://doi.org/10.1007/s10530-009-9447-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-009-9447-y