Abstract
Lake Malawi is famous for the rapid speciation of cichlid fishes, but the potential diversification of its invertebrate fauna is poorly studied. Using two mitochondrial DNA sequence markers (partial ND1 and Cyt b genes), we investigated the population genetic structure of the only known species of freshwater crab inhabiting Lake Malawi (Potamonautes lirrangensis (Rathbun, 1904)). We detected little overall genetic differentiation among different sampling sites (pairwise ΦST-values = 0.00–0.13). Genetic differentiation between sampling sites was better explained by linear distances than by shoreline distances, suggesting that ‘sweepstake dispersal’ between western and eastern shores occurs. Moreover, several population genetic parameters (Tajima’s D, Fu’s FS, Fay and Wu’s H and mismatch distribution analysis) suggest a recent population expansion, and Bayesian skyline plot analysis confirmed a sudden increase of the effective population size between 70 and 30 ka. Genetic diversity decreased towards the southern, shallower part of the lake, suggesting a more recent colonization of the southern shores. This finding is in line with hypotheses on Lake Malawi’s paleogeography suggesting that the lake largely desiccated during Pleistocene East African megadroughts and re-expanded southwards only recently after ~ 70 ka.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lakes that have persisted for at least 100 ka, i.e. ancient or long-lived lakes (Gorthner 1994; Cohen, 2012; Wilke et al., 2016) tend to harbour biota characterized by high levels of species diversity and endemicity (Brooks, 1950; Gorthner, 1994; Martens et al., 1994). The Great Lakes of the East African Rift Valley (Victoria, Tanganyika, and Malawi) are prime examples of such ancient lakes and are well known for harbouring species flocks of cichlid fishes that most likely evolved sympatrically after the formation of each of these lakes (McKaye et al., 1984; Seehausen, 2000; Lande et al., 2001; Seehausen, 2006). This tremendous diversity of teleost fishes, however, is usually not mirrored by similarly high levels of invertebrate diversity (Michel, 1994; Cumberlidge et al., 1999; Martens & Schön, 1999; Schön et al., 2001; Marijnissen et al., 2006; Genner et al., 2007; Salzburger et al., 2014). While ostracods and gastropods show higher levels of species diversity within the East African Great Lakes compared to the surrounding areas (Martens & Schön, 1999; Schön et al., 2001; Genner et al., 2007), brachyuran crabs do not show a comparable level of elevated intra-lacustrine diversity (Cumberlidge & Meyer, 2011). This is surprising, because African freshwater crabs likely face ample ecological opportunities for adaptive diversification in ancient lakes because they can occupy a range of trophic niches such as shredding (Hill & O’Keeffe, 1992; Dobson et al., 2002; Masese et al., 2014) and predation (West & Cohen, 1994; Weigand et al., 2014). In Lake Tanganyika, primary freshwater crabs of the family Potamonautidae have formed a small endemic species flock comprising nine phylogenetically closely related, but morphologically and ecologically differentiated Platythelphusa species (Cumberlidge et al. 1999; Marijnissen et al., 2004, 2006, 2008, 2009). Nevertheless, their species diversity lags far behind the c. 250 cichlid species in this lake (Turner et al., 2001). Even more extreme is the situation in Lake Malawi, which harbours about 700 cichlid species (Meyer, 1993; Turner et al., 2001), while only one potamonautid crab, the Malawi blue crab has been reported (Daniels & Bayliss, 2012). Comparably low freshwater crab diversity is also found in Lake Victoria (one species, not endemic) and Lake Kivu (four species, two of which are endemic; Cumberlidge & Meyer, 2011).
Comparing species numbers between cichlids and freshwater crabs in the East African Great Lakes supports the view that behavioural components of sympatric speciation, which are thought to be major drivers of accelerated speciation rates in cichlids (Danley & Kocher, 2001), play only a minor role in freshwater crabs. For example, unlike cichlids, East African freshwater crabs do not display sexually dimorphic colour patterns. These colour ornaments evolved through sexual selection and serve (beside other functions) mate attraction (Lande et al., 2001). While ‘sensory drive’—divergent adaptation of sensory and signalling systems in a heterogeneous environment that may cause premating isolation—plays a role in intra-lacustrine cichlid speciation (Seehausen et al., 2008), it has never been proposed to drive speciation in freshwater crabs. Also, small effective population sizes as a consequence of mouth-breeding have been proposed to drive fixation of rare alleles in cichlids (Salzburger et al., 2005), but small population sizes do not apply to the Malawi blue crab which has large populations in Lake Malawi, even if it is a direct developer with comparatively small broods (Yeo et al., 2008).
Nevertheless, the much lower freshwater crab diversity of Lake Malawi compared to Lake Tanganyika is puzzling. The estimated age of the Lake Malawi basin is between 8 and 4 Ma (Delvaux, 1996) which means that Lake Malawi formed roughly at the same time as Lake Tanganyika (8–5 Ma; Lezzar et al., 1996; Cohen et al., 1997). One potential explanation for different evolutionary diversification rates between these two ancient lakes is attributed to the Pleistocene drying of the major lakes of the East African Rift Valley (Van Damme & Gautier, 2013), i.e. Lake Victoria (Johnson et al., 1996), Lake Tanganyika (Cohen et al., 1997) including Lake Malawi (Cohen et al., 2007; Scholz et al., 2011). The respective African megadroughts (Cohen et al., 2007; Lyons et al. 2015; Ivory et al. 2016) have most likely affected the biota of Lake Malawi (maximum depth ca. 700 m; Eccles, 1974) more severely than Lake Tanganyika (ca. 1470 m deep; Felton et al., 2007) (Van Damme & Gautier, 2013). Alternatively, it is possible that if the Malawi blue crab has inhabited Lake Malawi for a long time it might harbour cryptic diversity of morphologically similar but divergently evolving lineages, as has been demonstrated for species of freshwater crabs on the Seychelles (Daniels 2011; Cumberlidge & Daniels, 2014) and in Europe (Jesse et al., 2013). Lake Malawi’s long north–south extension of 570 km (and its relatively narrow east–west extension of only about 70 km) could produce at least some degree of genetic isolation-by-distance along the lake’s north–south axis, and/or (in absence of accidental drift of individuals) genetic differentiation between eastern and western shores. Indeed, freshwater crabs have not been reported from below 200 m depth in Lake Malawi (Cunnington, 1907; Cumberlidge & Meyer, 2011) which is considered anoxic in Lake Malawi (Vollmer et al., 2002).
The Malawi blue crab is currently assigned to the species Potamonautes lirrangensis (Rathbun, 1904), which is not endemic to Lake Malawi, but has a more widespread occurrence, reaching Lake Kivu in the north and the Congo River drainage in the Democratic Republic of the Congo in the west (Reed & Cumberlidge, 2006; Cumberlidge & Meyer, 2011; Daniels & Bayliss, 2012). The name ‘Malawi blue crab’ originates from the aquarium trade and does not indicate that populations of this species within Lake Malawi are morphologically different from populations outside the lake. However, the type locality of P. lirrangensis at Lirranga is at the confluence of Congo and Ubangi Rivers, about 2000 km from Lake Malawi (Rathbun, 1905). Phylogenetic divergence between Lake Malawi and the Upper Congo system was inferred for viviparid freshwater snails at ~4.5 Ma (Schultheiss et al., 2014), and this could well reflect a general biotic pattern. Molecular data indeed indicate considerable genetic divergence across the distribution range of P. lirrangensis (Marijnissen et al., 2006), raising the question of whether Potamonautes orbitospinus (Cunnington, 1907)—whose type locality is Lake Malawi—is actually a valid species. In the following, before more detailed molecular data become available, we will refer to the Malawi blue crab as P. lirrangensis, in line with the latest revisions (Reed & Cumberlidge, 2006; Cumberlidge & Meyer, 2011).
We investigated genetic differentiation of the Malawi blue crab within Lake Malawi based on mitochondrial sequence markers with the following aims: (1) we asked if there is evidence for morphologically cryptic diversity (Pfenninger & Schwenk, 2007; Jesse et al., 2013), (2) we reconstructed the past population demography of the lake’s population(s) to test if the observed patterns confirm hypotheses about the lake’s palaeogeography (Cohen et al., 2007; Scholz et al., 2011; Ivory et al., 2016), and (3) we evaluated genetic similarity/differentiation between populations along the western and eastern lake shores and tested for a potential signature of isolation-by-distance along the shore line.
Materials and methods
Sampling and acquisition of sequence data
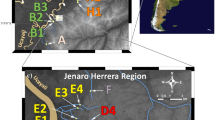
Freshwater crabs were sampled at seven sites along the shores of Lake Malawi during 2012 (N = 60; Fig. 1A) and fixed in 70% ethanol. Crabs were hand caught in shallow water close to the beach—with the exception of the sites Mbamba bay and Chikale at the eastern shores that were sampled in slightly deeper water by local fishermen. We extracted DNA using the CTAB method (Del Sal et al., 1989) and amplified the partial mitochondrial NADH dehydrogenase subunit 1 (ND1, primers 16L11/NDH5, see Jesse et al., 2011; including the partial 16S rRNA gene and the tRNA Leu) and cytochrome b genes (Cytb, primers Cytb-MVZ15/Cytb2; Klaus et al., 2013). PCR conditions were 35 cycles; denaturing at 94°C for 45 s; annealing at 48°C for 1 min; extension at 72°C for 45 s. Sequencing was outsourced to GATC Biotech AG (Konstanz, Germany). Assembly of forward and reverse strands and sequence quality control was done in SEQUENCHER 5.2.3 (GeneCodes Corporation, Ann Arbor, U.S.A.). We submitted all new sequences to the European Nucleotide Archive (ENA; accession numbers LT837525–LT837584 for Cytb; and LT837856–LT837915 for ND1). We aligned the sequences manually, as there were no ambiguous sites, and checked for stop codons to minimize the risk of including pseudogenes. The final total alignment length was 507 bp for ND1 and 291 bp for Cytb.
A Location of the seven sampling sites of Potamonautes lirrangensis in Lake Malawi. Bathymetry is based on Scholz et al. (2011); dashed lines indicate the 500 m depth contour below the present lake level. B Pairwise ΦST-values between sampling sites (significant values indicated by an asterisk), and average number of pairwise nucleotide differences within sampling sites (diagonal values) based on combined mitochondrial Cytb and ND1 genes. Comparisons between and within the three southern sites are highlighted
Data analysis
Unless stated, otherwise, all population genetic analyses were conducted in ARLEQUIN version 3.5.1.2 (Excoffier et al., 2005). We estimated genetic heterogeneity of the total sample (as a proxy for the total lake population) and within each sampling site, namely haplotype diversity (h), which indicates the probability that two randomly drawn haplotypes are different, and nucleotide diversity (π), which indicates the mean number of differences between all pairs of haplotypes in a population, expressed as a percentage value (Nei, 1987).
Pairwise fixation indices (Wright, 1951) were computed based on genetic distances between sampling sites under a Tajima-Nei model (Tajima & Nei, 1984). Significance of fixation indices (ΦST) under the null hypothesis of no differences among populations was tested using a non-parametric permutation approach (Excoffier et al., 1992) with 10,000 permutations of haplotypes among populations. The population genetic structure within the Lake was explored with a series of AMOVAs (Excoffier et al., 1992) to identify the grouping scheme that results in maximal between- and minimal within-group variation. Significance of the results was estimated with 10,000 permutations.
To investigate if genetic similarity/differentiation between population pairs can be explained by linear distances (indicating migration through open waters) and/or shoreline distance, we tested for a correlation between linear as well as shoreline distance matrices (Table 1) and ΦST-values between all sampling sites (Fig. 1B) using a Mantel test with 1000 permutations (Mantel, 1967; Smouse et al., 1986). Geographic distances were measured in Google Earth (Alphabet Inc., Mountain View, U.S.A.).
We asked if the demographic history of P. lirrangensis in Lake Malawi was subject to fluctuation in effective population size. To this end, we calculated a set of neutrality tests, testing for equilibrium between the loss of genetic variation through random drift and the accumulation of molecular diversity through mutation. We calculated Tajima’s D (Tajima, 1989) and Fay and Wu’s H statistic (Fay & Wu, 2000), whereby the latter was computed in DNASP 5.10.01 (Librado & Rozas, 2009). Those indices use the mutation frequency for assessing potential deviations from equilibrium while another index, Fu’s FS (Fu, 1997), is based on haplotype frequencies. The significance of D and FS was estimated by generating random samples under the null hypothesis of selective neutrality and population equilibrium using a coalescent simulation (Hudson, 1990). While D is more sensitive to population expansion, H is largely influenced by purifying selection, thus potentially allowing discrimination between both processes (Fay & Wu, 2000).
Subsequently, we applied a mismatch distribution analysis on the frequency distribution of observed numbers of differences between pairs of haplotypes using a generalized least-squares approach (Schneider & Excoffier, 1999). Populations at equilibrium are expected to show a multimodal distribution, while populations that have recently experienced either a demographic or range expansion are predicted to have a unimodal distribution (Rogers & Harpending, 1992). As an indicator of the smoothness of the distribution, we inferred the raggedness index, rg (Harpending et al., 1993; Harpending, 1994), and tested its significance with a parametric bootstrap approach (10,000 replicates) under the null hypothesis of population expansion.
Moreover, past demographic processes were inferred using a Bayesian skyline plot model (Drummond et al., 2005) as implemented in BEAST 1.8.2 (Drummond et al., 2012) and visualised in TRACER 1.5. This individual-based coalescent approach uses Bayesian MCMC sampling procedures to estimate a posterior distribution of gene genealogies and population parameters. We applied a HKY+G model of sequence evolution and a log-normal relaxed molecular clock model (Drummond et al., 2006) for all gene partitions, and ran the analyses for 507 iterations, sampling every 10,000th iteration, and discarding the first 106 as burn-in. We used an external substitution rate of 2% per Ma for all mitochondrial partitions with a standard deviation of 0.6% per Ma as inferred for mitochondrial genes of the freshwater crab genus Potamon based on the cessation of the Messinian Salinity Crisis, assuming that this event triggered divergence between Potamon fluviatile and Potamon algeriense (see Jesse et al., 2009). We set the number of groups (m) to 10. The resulting posterior distributions were then used to generate credibility intervals that represent phylogenetic and coalescent uncertainty in TRACER 1.6.0 (Fig. 2B). As we used mitochondrial sequence markers, population size estimates reflect the maternal effective population size.
Historical demography of the Malawi blue crab Potamonautes lirrangensis. A Mismatch distribution with identical expected distributions under both spatial and demographic expansion models, with only slightly differing 90% confidence intervals (outer interval boundaries: demographic expansion). B Bayesian Skyline plot indicating past changes in effective population size (median and 95% credibility intervals)
Results
Highest genetic differentiation in the northern lake basin
Overall, we found moderate haplotype diversity but only low levels of nucleotide diversity for the two mitochondrial loci (Table 2), supporting the view that no morphologically cryptic lineages of freshwater crabs exist in Lake Malawi. Irrespective of the grouping scheme of sampling sites, most of the genetic variation was due to diversity within sampling sites (Table 3). However, comparison of different AMOVAs revealed that grouping the three southern sites (Chikale, Nkotakhota bay, Cape McLear) versus the remaining sites maximizes between-group variation (Table 3). The three southern sites were characterized by moderate haplotype (h = 0.51–0.63) and low nucleotide diversity (π = 0.000698–0.001321; Table 2), low mean pairwise nucleotide differences within sites (0.60–1.10; Fig. 1B), and pairwise ΦST-values close to zero (Fig. 1B). In contrast, the northern sites harboured most of the genetic diversity of P. lirrangensis within Lake Malawi (h = 0.79–1.00; π = 0.001606–0.003774; mean pairwise nucleotide differences = 1.30–3.00; pairwise ΦST = 0.00–0.13; Table 2; Fig. 1B). A Mantel test found linear distances between sites to correlate significantly with genetic distances (i.e., pairwise ΦST; r = 0.442, P = 0.02) and to explain 21% of the genetic variance between sites. By contrast, shoreline distances did not correlate with genetic distances (r = 0.071, P = 0.36; < 0.001% variance explained).
Evidence for a recent population expansion
Negative values of Tajima’s D (−2.312; P < 0.001) and Fu’s FS (−24.760; P < 0.001) argue in favour of a recent (demographic or spatial) population expansion, or a scenario of purifying selection during a population bottleneck. The high value of Fay and Wu’s H (0.936), however, suggests that purifying selection most likely plays only a minor role compared to the contribution of a recent population expansion. Mismatch analysis revealed that the observed number of pairwise differences did not differ significantly from the expected distribution for both demographic and spatial expansion models (spatial expansion model: P-value of sum of squared deviations (SSD) = 0.45, rg = 0.062, P-value of rg = 0.44; demographic expansion model: P-value of SSD = 0.30, rg = 0.062, P-value of rg = 0.43; Fig. 2A). The Bayesian Skyline plot yielded particularly small estimates for the maternal effective population size (partly < 0 on a logarithmic scale; Fig. 2B)—most likely reflecting low overall genetic diversity—which can at most be interpreted as an approximation of changes in relative population size. Under this premise and the applied external substitution rate, the Bayesian Skyline plot suggests that population size increased between 70,000 and 30,000 years ago before present (Fig. 2B).
Discussion
We found no evidence for independently evolving—and thus, potentially sympatrically speciating—divergent mitochondrial lineages in the Malawi blue crab P. lirrangensis within Lake Malawi. This contrasts with the extraordinary species richness of the Lake Malawi cichlids (Danley & Kocher, 2001), and the occurrence of several divergent lineages of the gastropod genera Bellamya (Viviparidae; Schultheiss et al., 2011), Lanistes (Ampullariidae; Schultheiss et al., 2009) and Melanoides (Thiaridae; Genner et al., 2007; Schultheiss et al., 2009; Van Bocxlaer et al., 2015). Given the low genetic diversity as observed in our present study, the lake’s population of freshwater crabs most likely originated from a single colonization event, as has also been inferred for gastropods of the genus Lanistes (see Schultheiss et al., 2009); but not for thiarid gastropods (Genner et al., 2007) and haplochromine cichlids (Joyce et al., 2015). At this point, based on the available mitochondrial sequence data, we cannot exclude that there is very recent, ongoing genetic differentiation or incipient speciation in these freshwater crabs. Such a process would possibly go undetected in this study due to incomplete lineage sorting and/or slow evolution of the mitochondrial genes when compared, for example, to faster-evolving nuclear markers such as microsatellite loci or single nucleotide polymorphisms. Nevertheless, we can clearly reject the hypothesis of a completely panmictic population within Lake Malawi—as inferred for some pelagic cichlid species (Shaw et al., 2000)—on the basis of the genetic differentiation between populations from northern and southern sites. The recent population expansion of P. lirrangensis between 70,000 and 30,000 years ago, along with the extremely low genetic diversity at our southern sampling sites, argues in favour of the potential influence of the last East African megadrought. During that period (117–85 ka), Lake Malawi supposedly desiccated to approximately 2% of its original size in a then semiarid environment, and only the northern, deeper parts of the lake remained filled with water (Scholz et al., 2011). After a period with fluctuating lake levels (85–71 ka) and another low-stand at 62 ka (−200 m; Scholz et al., 2011), the lake level rose again (Ivory et al., 2016). The shallow southern parts of Lake Malawi were supposedly flooded last, thus explaining the low genetic diversity of the southern populations. Alternatively, higher levels of gene flow in the southern portion of the lake, along with stronger isolation between populations in the northern parts of the lake (assuming that freshwater crabs do not disperse through the deeper, anoxic waters in the northern parts of the lake), could result in a similar pattern. However, we could show that linear distances are a better predictor of genetic differentiation between sampling sites than shoreline distances. This indicates that gene flow does not strictly follow a stepping-stone model along the coastline (i.e., each population receives migrants mainly from neighbouring populations as, e.g. in the Lake Malawi cichlid Melanochromis auratus, see Albertson et al., 1999; Markert et al., 1999; or in the stone loach of Lake Constance, see Barluenga & Meyer, 2005), but most likely allows for erratic dispersal across the open water throughout the lake. We propose that the results of our present study should be confirmed based on a denser sampling of P. lirrangensis along the lake’s shores.
Our hypothesis that the Malawi blue crab was severely affected by the Pleistocene East African megadrought is congruent with the higher species diversity of Lake Tanganyika’s freshwater crabs (Marijnissen et al., 2004, 2006). The Lake Tanganyika basin is twice as deep as Lake Malawi, and most likely retained several isolated water bodies during times of maximum lake level low-stands (Cohen et al., 1997) that enabled the persistence of ancestral lineages (see Van Damme & Gautier, 2013) and might have triggered allopatric speciation in the isolated water bodies. A similar pattern applies for cichlid lineages, as Lake Malawi, despite its high species number, harbours only cichlids of the tribe Haplochromini, while as many as 14 tribes occur in Lake Tanganyika (Meyer et al., 2015). Also, much smaller Southeast Asian ancient lakes, under more stable palaeoclimatic conditions, harbour a variety of primary freshwater crab species belonging to different trophic guilds (Schubart et al., 2008; Poettinger & Schubart, 2014). Therefore, at least for freshwater crabs, Lake Malawi is apparently not all that ancient, with the time elapsed since the last Pleistocene megadrought being too short for sympatric speciation to occur.
References
Albertson, R. C., J. A. Markert, P. D. Danley & T. D. Kocher, 1999. Phylogeny of a rapidly evolving clade: The cichlid fishes of Lake Malawi, East Africa. Proceedings of the National Academy of Sciences of the U.S.A. 96: 5107–5110.
Barluenga, M. & A. Meyer, 2005. Old fish in a young lake: stone loach (Pisces: Barbatula barbatula) populations in Lake Constance are genetically isolated by distance. Molecular Ecology 14: 1229–1239.
Brooks, J. L., 1950. Speciation in ancient lakes (concluded). The Quarterly Review of Biology 25: 131–176.
Cohen, A. S., 2012. Scientific drilling and biological evolution in ancient lakes: lessons learned and recommendations for the future. Hydrobiologia 682: 3–25.
Cohen, A. S., K.-E. Lezzar, J.-J. Tiercelin & M. Soreghan, 1997. New palaeogeographic and lake-level reconstructions of Lake Tanganyika: implications for tectonic, climatic and biological evolution in a rift lake. Basin Research 9: 107–132.
Cohen, A. S., J. R. Stone, K. R. M. Beuning, L. E. Park, P. N. Reinthal, D. Dettman, C. A. Scholz, T. C. Johnson, J. W. King, M. R. Talbot, E. T. Brown & S. J. Ivory, 2007. Ecological consequences of early Late Pleistocene megadroughts in tropical Africa. Proceedings of the National Academy of Sciences of the U.S.A. 104: 16422–16427.
Cumberlidge, N. & K. S. Meyer, 2011. The freshwater crabs of Lake Kivu (Crustacea: Decapoda: Brachyura: Potamonautidae). Journal of Natural History 45: 1835–1857.
Cumberlidge, N. & S. R. Daniels, 2014. Recognition of two new species of freshwater crabs from the Seychelles based on molecular evidence (Potamoidea: Potamonautidae). Invertebrate Systematics 28: 17–31.
Cumberlidge, N., R. V. Sternberg, I. R. Bills & H. A. Martin, 1999. A revision of the genus Platythelphusa A. Milne-Edwards, 1887 from Lake Tanganyika, East Africa (Decapoda: Potamoidea: Platythelphusidae). Journal of Natural History 33: 1487–1512.
Cunnington, W. A., 1907. Zoological results of the Third Tanganyika Expedition, conducted by Dr. W.A. Cunnington, 1904-1905. Report on the brachyurous Crustacea. Proceedings of the Zoological Society of London 77: 258–276.
Daniels, S. R., 2011. Reconstructing the colonisation and diversification history of the endemic freshwater crab (Seychellum alluaudi) in the granitic and volcanic Seychelles. Molecular Phylogenetics and Evolution 61: 534–542.
Daniels, S. R. & J. Bayliss, 2012. Neglected refugia of biodiversity: mountainous regions in Mozambique and Malawi yield two novel freshwater crab species (Potamonautidae: Potamonautes). Zoological Journal of the Linnean Society 164: 498–509.
Danley, P. D. & T. D. Kocher, 2001. Speciation in rapidly diverging systems: lessons from Lake Malawi. Molecular Ecology 10: 1075–1086.
Del Sal, G., G. Manfioletti & C. Schneider, 1989. The CTAB-DNA precipitation method: a common mini-scale preparation of template DNA from phagemids, phages or plasmids suitable for sequencing. BioTechniques 7: 514–520.
Delvaux, D., 1996. Age of Lake Malawi (Nyasa) and water level fluctuations. In Musée royal de l’Afrique centrale, Tervuren, Dept. Geol. Min. (ed.), Rapport Annuel 1993 & 1994: 99–108.
Dobson, M., A. Magana, J. M. Mathooko & F. K. Ndegwa, 2002. Detritivores in Kenyan highland streams: more evidence for the paucity of shredders in the tropics? Freshwater Biology 47: 909–919.
Drummond, A. J., A. Rambaut, B. Shapiro & O. G. Pybus, 2005. Bayesian coalescent inference of past population dynamics from molecular sequences. Molecular Biology and Evolution 22: 1185–1192.
Drummond, A. J., S. Y. W. Ho, M. J. Phillips & A. Rambaut, 2006. Relaxed phylogenetics and dating with confidence. PLoS Biology 4: e88.
Drummond, A. J., M. A. Suchard, D. Xie & A. Rambaut, 2012. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Molecular Biology and Evolution 29: 1969–1973.
Eccles, D. H., 1974. An outline of the physical limnology of Lake Malawi (Lake Nyasa). Limnology and Oceanography 19: 730–742.
Excoffier, L., G. Laval & S. Schneider, 2005. Arlequin (version 3.0): an integrated software package for population genetics data analysis. Evolutionary Bioinformatics 1: 47. Online.
Excoffier, L., P. E. Smouse & J. M. Quattro, 1992. Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131: 479–491.
Fay, J. C. & C. I. Wu, 2000. Hitchhiking under positive Darwinian selection. Genetics 155: 1405–1413.
Felton, A. A., J. M. Russell, A. S. Cohen, M. E. Baker, J. T. Chesley, K. E. Lezzar, M. M. McGlue, J. S. Pigati, J. Quade, J. C. Stager & J. J. Tiercelin, 2007. Paleolimnological evidence for the onset and termination of glacial aridity from Lake Tanganyika, tropical East Africa. Palaeogeography, Palaeoclimatology, Palaeoecology 252: 405–423.
Fu, Y.-X., 1997. Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics 147: 915–925.
Genner, M. J., J. A. Todd, E. Michel, D. Erpenbeck, A. Jimoh, D. A. Joyce, A. Piechocki & J.-P. Pointier, 2007. Amassing diversity in an ancient lake: evolution of a morphologically diverse parthenogenetic gastropod assemblage in Lake Malawi. Molecular Ecology 16: 517–530.
Gorthner, A., 1994. What is an ancient lake? In Martens, K., B. Goddeeris & G. Coulter (eds), Speciation in Ancient Lakes. Archiv für Hydrobiologie, Beiheft Ergebnisse der Limnologie, 44. Schweizerbart, Stuttgart: 97–100.
Harpending, H. C., 1994. Signature of ancient population growth in a low-resolution mitochondrial DNA mismatch distribution. Human Biology 66: 591–600.
Harpending, H. C., S. T. Sherry, A. R. Rogers & M. Stoneking, 1993. The genetic structure of ancient human populations. Current Anthropology 34: 483–496.
Hill, M. P. & J. H. O’Keeffe, 1992. Some aspects of the ecology of the freshwater crab Potamonautes perlatus Milne Edwards) in the upper reaches of the Buffalo River, eastern Cape Province, South Africa. Southern African Journal of Aquatic Sciences 18: 42–50.
Hudson, R. R., 1990. Gene genealogies and the coalescent process. In Futuyma, D. J. & J. Antonovics (eds), Oxford Surveys in Evolutionary Biology. Oxford University Press, New York: 1–44.
Ivory, S. J., M. W. Blome, J. W. King, M. M. McGlue, J. E. Cole & A. S. Cohen, 2016. Environmental change explains cichlid adaptive radiation at Lake Malawi over the past 1.2 million years. Proceedings of the National Academy of Sciences of the U.S.A. 113: 11895–11900.
Jesse, R., M. Grudinski, S. Klaus, B. Streit & M. Pfenninger, 2011. Evolution of freshwater crab diversity in the Aegean region (Crustacea: Brachyura: Potamidae). Molecular Phylogenetics and Evolution 59: 23–33.
Jesse, R., M. Pfenninger, S. Fratini, M. Scalici, B. Streit & C. D. Schubart, 2009. Disjunct distribution of the Mediterranean freshwater crab Potamon fluviatile—natural expansion or human introduction? Biological Invasions 11: 2209–2221.
Jesse, R., C. D. Schubart & S. Klaus, 2013. Identification of a cryptic lineage within Potamon fluviatile (Herbst) (Crustacea: Brachyura: Potamidae). Invertebrate Systematics 24: 348.
Johnson, T. C., C. A. Scholz, M. R. Talbot, K. Kelts, R. D. Ricketts, G. Ngobi, K. R. M. Beuning, I. Ssemmanda & J. W. McGill, 1996. Late Pleistocene desiccation of Lake Victoria and rapid evolution of cichlid fishes. Science 273: 1091–1093.
Klaus, S., J. C. E. Mendoza, J. H. Liew, M. Plath, R. Meier & D. C. J. Yeo, 2013. Rapid evolution of troglomorphic characters suggests selection rather than neutral mutation as a driver of eye reduction in cave crabs. Biology Letters 9: 20121098.
Lande, R., O. Seehausen & J. J. M. van Alphen, 2001. Mechanisms of rapid sympatric speciation by sex reversal and sexual selection in cichlid fish. Genetica 112(113): 435–443.
Lezzar, K. E., J.-J. Tiercelin, M. D. E. Batist, A. S. Cohen, T. Bandora, P. van Rensbergen, C. Le Turdu, W. Mifundu & J. Klerkx, 1996. New seismic stratigraphy and Late Tertiary history of the North Tanganyika Basin, East African Rift system, deduced from multichannel and high-resolution reflection seismic data and piston core evidence. Basin Research 8: 1–28.
Librado, P. & J. Rozas, 2009. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25: 1451–1452. (Oxford, England).
Lyons, R. P., C. A. Scholz, A. S. Cohen, J. W. King, E. T. Brown, S. J. Ivory, T. C. Johnson, A. L. Deinof, P. N. Reinthalg, M. M. McGlue & M. W. Blome, 2015. Continuous 1.3-million-year record of East African hydroclimate, and implications for patterns of evolution and biodiversity. Proceedings of the National Academy of Sciences 112: 15568–15573.
Mantel, N., 1967. The detection of disease clustering and a generalized regression approach. Cancer Research 27: 209–220.
Marijnissen, S. A. E., F. Schram, N. Cumberlidge & E. Michel, 2004. Two new species of Platythelphusa A. Milne-Edwards, 1887 (Decapoda, Potamoidea, Platythelphusidae) and comments on the taxonomic position of P. denticulata Capart, 1952 from Lake Tanganyika, East Africa. Crustaceana 77: 513–532.
Marijnissen, S. A. E., E. Michel, S. R. Daniels, D. Erpenbeck, S. B. J. Menken & F. Schram, 2006. Molecular evidence for recent divergence of Lake Tanganyika endemic crabs (Decapoda: Platythelphusidae). Molecular Phylogenetics and Evolution 40: 628–634.
Marijnissen, S. A. E., E. Michel, M. Kamermans, K. Olaya-Bosch, M. Kars, D. F. R. Cleary, E. E. van Loon, P. G. Rachello Dolmen & S. B. J. Menken, 2008. Ecological correlates of species differences in the Lake Tanganyika crab radiation. Hydrobiologia 615: 81–94.
Marijnissen, S. A. E., E. Michel, D. F. R. Cleary & P. B. McIntyre, 2009. Ecology and conservation status of endemic freshwater crabs in Lake Tanganyika, Africa. Biodiversity and Conservation 18: 1555–1573.
Markert, J. A., M. E. Arnegard, P. D. Danley & T. D. Kocher, 1999. Biogeography and population genetics of the Lake Malawi cichlid Melanochromis auratus: habitat transience, philopatry and speciation. Molecular Ecology 8: 1013–1026.
Martens, K. & I. Schön, 1999. Crustacean biodiversity in ancient lakes: a review. Crustaceana 72: 899–910.
Martens, K., B. Goddeeris & G. Coulter, 1994. Speciation in ancient lakes—40 years after Brooks. In Martens, K., B. Goddeeris & G. Coulter (eds), Speciation in Ancient Lakes. Archiv für Hydrobiologie, Beiheft Ergebnisse der Limnologie, 44. Schweizerbart, Stuttgart: 75–96.
Masese, F. O., N. Kitaka, J. Kipkemboi, G. M. Gettel, K. Irvine & M. E. McClain, 2014. Macroinvertebrate functional feeding groups in Kenyan highland streams: evidence for a diverse shredder guild. Freshwater Science 33: 435–450.
McKaye, K. R., T. Kocher, P. Reinthal, R. Harrison & I. Kornfield, 1984. Genetic evidence for allopatric and sympatric differentiation among color morphs of a Lake Malawi cichlid fish. Evolution 38: 215–219.
Meyer, A., 1993. Phylogenetic relationships and evolutionary processes in East African cichlid fishes. Trends in Ecology & Evolution 8: 279–284.
Meyer, B. S., M. Matschiner & W. Salzburger, 2015. A tribal level phylogeny of Lake Tanganyika cichlid fishes based on a genomic multi-marker approach. Molecular Phylogenetics and Evolution 83: 56–71.
Michel, E., 1994. Why snails radiate: a review of gastropod evolution in long-lived lakes, both recent and fossil. In Martens, K., B. Goddeeris & G. Coulter (eds), Speciation in Ancient Lakes. Archiv für Hydrobiologie, Beiheft Ergebnisse der Limnologie, 44. Schweizerbart, Stuttgart: 285–317.
Nei, M., 1987. Molecular Evolutionary Genetics. Columbia University Press, New York.
Pfenninger, M. & K. Schwenk, 2007. Cryptic animal species are homogeneously distributed among taxa and biogeographical regions. BMC Evolutionary Biology 7: 121.
Poettinger, T. & C. D. Schubart, 2014. Molecular diversity of freshwater crabs from Sulawesi and the sequential colonization of ancient lakes. Hydrobiologia 739: 73–84.
Rathbun, M. J., 1904. Les crabes d’eau douce (Potamonidae). Archives du Muséum d’histoire naturelle, Paris 4(6): 225–312.
Rathbun, M. J., 1905. Les crabes d’eau douce (Potamonidae). Archives du Muséum d’histoire naturelle, Paris 4(7): 159–321.
Reed, S. K. & N. Cumberlidge, 2006. Taxonomy and biogeography of the freshwater crabs of Tanzania, East Africa. Zootaxa 1262: 1–139.
Rogers, A. R. & H. C. Harpending, 1992. Population growth makes waves in the distribution of pairwise genetic differences. Molecular Biology and Evolution 9: 552–569.
Salzburger, W., T. Mack, E. Verheyen & A. Meyer, 2005. Out of Tanganyika: genesis, explosive speciation, key-innovations and phylogeography of the haplochromine cichlid fishes. BMC Evolutionary Biology 5: 17.
Salzburger, W., B. Van Bocxlaer & A. S. Cohen, 2014. Ecology and evolution of the African Great Lakes and their faunas. Annual Review of Ecology, Evolution, and Systematics 45: 519–545.
Schneider, S. & L. Excoffier, 1999. Estimation of past demographic parameters from the distribution of pairwise differences when the mutation rates vary among sites: application to human mitochondrial DNA. Genetics 152: 1079–1089.
Scholz, C. A., A. S. Cohen, T. C. Johnson, J. King, M. R. Talbot & E. T. Brown, 2011. Scientific drilling in the Great Rift Valley: the 2005 Lake Malawi Scientific Drilling Project—an overview of the past 145,000 years of climate variability in Southern Hemisphere East Africa. Palaeogeography, Palaeoclimatology, Palaeoecology 303: 3–19.
Schön, I., E. Verheyen & K. Martens, 2001. Speciation in ancient lake ostracods: comparative analysis of Baikalian Cytherissa and Tanganyikan Cyprideis. In Williams, W. D. (ed.), Verhandlungen IVL: 27th Congress in Dublin 1998. Verhandlungen IVL. Schweizerbart, Stuttgart: 2674–2677.
Schubart, C. D., T. Santl & P. Koller, 2008. Mitochondrial patterns of intra- and interspecific differentiation among endemic freshwater crabs of ancient lakes in Sulawesi. Contributions to Zoology 77: 83–90.
Schultheiss, R., B. van Bocxlaer, T. Wilke & C. Albrecht, 2009. Old fossils—young species: evolutionary history of an endemic gastropod assemblage in Lake Malawi. Proceedings of the Royal Society B 276: 2837–2846.
Schultheiss, R., T. Wilke, A. Jørgensen & C. Albrecht, 2011. The birth of an endemic species flock: demographic history of the Bellamya group (Gastropoda, Viviparidae) in Lake Malawi. Biological Journal of the Linnean Society 102: 130–143.
Schultheiss, R., B. Van Bocxlaer, F. Riedel, T. von Rintelen & C. Albrecht, 2014. Disjunct distributions of freshwater snails testify to a central role of the Congo system in shaping biogeographical patterns in Africa. BMC Evolutionary Biology 14: 42.
Seehausen, O., 2000. Explosive speciation rates and unusual species richness in haplochromine cichlid fishes: effects of sexual selection. In Rossiter, A. (ed.), Ancient Lakes: Biodiversity, Ecology and Evolution. Advances in Ecological Research, Vol. 31. Elsevier, Amsterdam: 237–274.
Seehausen, O., 2006. African cichlid fish: a model system in adaptive radiation research. Proceedings of the Royal Society B 273: 1987–1998.
Seehausen, O., Y. Terai, I. S. Magalhaes, K. L. Carleton, H. D. J. Mrosso, R. Miyagi, I. van der Sluijs, M. V. Schneider, M. E. Maan, H. Tachida, H. Imai & N. Okada, 2008. Speciation through sensory drive in cichlid fish. Nature 455: 620–626.
Shaw, P. W., G. F. Turner, M. R. Idid, R. L. Robinson & G. R. Carvalho, 2000. Genetic population structure indicates sympatric speciation of Lake Malawi pelagic cichlids. Proceedings of the Royal Society B 267: 2273–2280.
Smouse, P. E., J. C. Long & R. R. Sokal, 1986. Multiple regression and correlation extensions of the Mantel test of matrix correspondence. Systematic Zoology 35: 627.
Tajima, F., 1989. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123: 585–595.
Tajima, F. & M. Nei, 1984. Estimation of evolutionary distance between nucleotide sequences. Molecular Biology and Evolution 1: 269–285.
Turner, G. F., O. Seehausen, M. E. Knight, C. J. Allender & R. L. Robinson, 2001. How many species of cichlid fishes are there in African lakes? Molecular Ecology 10: 793–806.
Van Bocxlaer, B., C. Clewing, J. P. M. Etimosundja, A. Kankonda, O. W. Ndeo & C. Albrecht, 2015. Recurrent camouflaged invasions and dispersal of an Asian freshwater gastropod in tropical Africa. BMC Evolutionary Biology 15: 33.
Van Damme, D. & A. Gautier, 2013. Lacustrine mollusc radiations in the Lake Malawi Basin: experiments in a natural laboratory for evolution. Biogeosciences 10: 5767–5778.
Vollmer, M. K., R. F. Weiss & H. A. Bootsma, 2002. Ventilation of Lake Malawi/Nyasa. In Odada, E. O. & D. O. Olago (eds), The East African Great Lakes: Limnology, Palaeolimnology and Biodiversity. Advances in Global Change Research, Vol. 12. Kluwer Academic Publishers, Dordrecht: 209–233.
Weigand, A. M., The Volkswagen Foundation Lake Malawi Field School 2012 Consortium & M. Plath, 2014. Prey preferences in captivity of the freshwater crab Potamonautes lirrangensis from Lake Malawi with special emphasis on molluscivory. Hydrobiologia 739: 145–153.
West, K. & A. S. Cohen, 1994. Predator–prey coevolution as a model for the unusual morphologies of the crabs and gastropods of Lake Tanganyika. In Martens, K., B. Goddeeris, & G. Coulter (eds), Speciation in Ancient Lakes. Archiv für Hydrobiologie, Beiheft Ergebnisse der Limnologie, 44. Schweizerbart, Stuttgart: 267–283.
Wilke, T., B. Wagner, B. Van Bocxlaer, C. Albrecht, D. Ariztegui, D. Delicado, A. Francke, M. Harzhauser, T. Hauffe, J. Holtvoeth, J. Just, M. J. Leng, Z. Levkov, K. Penkman, L. Sadori, A. Skinner, B. Stelbrink, H. Vogel, F. Wesselingh & J. Just, 2016. Scientific drilling projects in ancient lakes: integrating geological and biological histories. Global and Planetary Change 143: 118–151.
Wright, S., 1951. The genetical structure of populations. Annals of Eugenics 15: 323–354.
Yeo, D. C. J., P. K. L. Ng, N. Cumberlidge, C. Magalhães, S. R. Daniels & M. R. Campos, 2008. Global diversity of crabs (Crustacea: Decapoda: Brachyura) in freshwater. Hydrobiologia 595: 275–286.
Acknowledgements
Field work for this study was conducted in cooperation with and supported by Dr. Dylo Pemba and Dr. Zuze Dulany of Chancellor College Zomba, Malawi. The population at Cape McLear was sampled during a field school supported by the Volkswagen Foundation [Grant No. AZ 86 253]. We thank Orsolya Klára Mák, Kathrin Schleich and Elena Richter who helped acquiring the ND1 data during a practical course at the Universität Frankfurt in 2012. CP was funded by the scholarship program of the German Federal Environmental Foundation (Deutsche Bundesstiftung Umwelt, DBU); JKK is supported by a joint grant of the Ministry of Higher Education, Science and Technology of Kenya (MOHEST) and the German Academic Exchange Service (Deutscher Akademischer Austauschdienst, DAAD) [Grant No. 57139945]; and SK by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) [Grant No. DFG KL2378/2-1]. We thank two anonymous referees for their helpful suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: T. von Rintelen, P. B. Hamilton, R. P. Walter & G. D. Haffner / Speciation in Ancient Lakes – Integrative Approaches to their Biology and Limnology
Rights and permissions
About this article
Cite this article
Kochey, J.K., Daniels, S.R., Plagge, C. et al. Genetic differentiation of the Malawi blue crab reflects Pleistocene desiccation of Lake Malawi (Brachyura, Potamonautidae: Potamonautes lirrangensis (Rathbun, 1904)). Hydrobiologia 843, 1–11 (2019). https://doi.org/10.1007/s10750-017-3292-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-017-3292-2