Abstract
Objectives
This study focuses on dehalogenation of halogenated organic substrate (3-Chloropropiophenone) using both free and hydrogel entrapped microalgae Chlorella emersonii (211.8b) as biocatalyst. We aimed at successful immobilization of C. emersonii (211.8b) cells and to assess their biotransformation efficiency.
Results
Aquasorb (entrapping material in this study) was found to be highly biocompatible with the cellular growth and viability of C. emersonii. A promising number of entrapped cells was achieved in terms of colony-forming units (CFUs = 2.1 × 104) per hydrogel bead with a comparable growth pattern to that of free cells. It was determined that there is no activity of hydrogenase that could transform 1-phenyl-2-propenone into 1-phenyl-1-propanone because after 12 h the ratio between two products (0.36 ± 0.02) remained constant throughout. Furthermore, it was found that the entrapped cells have higher biotransformation of 3-chloropropiophenone to 1-phenyl-1-propanone as compared to free cells at every interval of time. 1-phenyl-2-propenone was excluded from the whole-cell biotransformation as it was also found in the control group (due to spontaneous generation).
Conclusion
Hence, enhanced synthesis of 1-phenyl-1-propanone by entrapped Chlorella (211.8b) can be ascribed to either an enzymatic activity (dehalogenase) or thanks to the antioxidants from 211-8b, especially when they are in immobilized form. The aquasorb based immobilization of microalgae is highly recommended as an effective tool for exploiting microalgal potentials of biocatalysis specifically when free cells activities are seized due to stress.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biotransformation is the chemical reaction performed by microorganisms and catalyzed by the enzymes within the microbial cells. This method harnesses cell cultures, chemical recycling, and the naturally occurring microbial catabolic diversity to degrade, transform and produce a wide range of compounds including oil, polychlorinated biphenyls, polyaromatic hydrocarbons, pharmaceutical substances, halogenated organic substrates, and metals (Raza et al. 2021a; Wang et al. 2020). To achieve efficient biotransformation, immobilized or entrapped microbial cells, isolated enzymes, and immobilized enzymes may be applied (Zur et al. 2020). Microalgal whole-cell biotransformation has been widely used to study freshwater alga, Chlorella vulgaris, with different natural (estradiol, estriol, estrone, and hydroxyestrone) and synthetic (estradiol valerate and ethinylestradiol) estrogens for its biotransformation or bioconcentration capabilities. Another study reported the potential use of freshwater microalgae as bioreactors. They studied the effects of some Rhodophyta and Chlorophyta on progesterone and found that Chlorella emersonii C211-8h induced hydroxylation of substrate yielding 2OH-hydroxyprogesterone as the main bioproduct (Della Greca et al. 1996). Another study proved that non-natural prochiral ketones could be reduced by microalgal photo-biocatalysis with high efficiency (Yang et al. 2012).
Halogenated organic compounds include vinyl chloride, carbon tetrachloride, methylene chloride, polychlorinated biphenyls, 3-chloropropiophenone, and many more. Some of these compounds are important precursors or intermediates in the synthesis of pharmaceuticals, agrochemicals, flavors, and functional materials (Janeczko and Kostrzewa-Susłow 2014). One such halogenated organic compound is 3-chloropropiophenone which upon hydrogenation, may be transformed into 3-chloro-1-phenylpropanol which is essential for preparing antidepressants like (S)-fluoxetine, nisoxetine, and (R)-tomoxetine (Xu et al. 2015). Upon dehalogenation, it synthesizes propiophenone which can be used as a flavoring or aroma ingredient. This dehalogenation-based biotransformation of halogenated organic substrates is often carried out using a whole-cell biocatalytic system. The higher efficiency of whole-cell biocatalysis allows minimizing the need for expensive external cofactors. Furthermore, the indigenous enzymes can be effectively utilized because of the protective nature of the cellular envelope in the stabilization of the enzymes under severe conditions. Besides, advances in synthetic biology together with the rapid development of genetic engineering tools, have brought about a revival of whole-cell biocatalysis (Lin and Tao 2017). The whole-cell biocatalytic system might be used either as free cells or immobilized (entrapped) ones under optimized conditions.
The use of photosynthetic microalgae as biocatalysts have received considerable attention in recent years. Microalgal products have been used in cosmetics, aquaculture, food, and pharmaceutical industries. However, the whole-cell biocatalyst stability and activity of microalgae during biotransformation might be affected by several parameters such as shaking speed, temperature, and most importantly the substrate concentration, which can be enhanced by techniques of whole-cell immobilization or entrapment. The advantages in entrapped or immobilized whole-cells as biocatalysts include higher reaction rates, increased cell densities, capable of continuous biotransformation, longer and stable cellular activity, and reusability of biocatalysts (Arabi et al. 2010). Most of the immobilization techniques with microorganisms, in general, can be applied to microalgae, with the only limitation of light transmission if living cells are intended to be immobilized. The gel entrapment is the most widely used for algal immobilization. This can be performed by the use of synthetic polymers (acrylamide, photo cross-linkable resins, polyurethanes), proteins (gelatine, collagen, or egg white), or natural polysaccharides (agars, carrageenans, or alginates) (Raza et al. 2021b; Kaparapu 2017). In this study, we have attempted a novel technique for Chlorella whole-cell entrapment using Aquasorb as entrapment material.
Aquasorb is a water retainer that, when absorbs water, becomes hydrogel. Upon incorporation into soil or water, it is capable of absorbing and retaining up to 500 times more water and nutrients than its dry weight (Ghisleri et al. 2017). Aquasorb consists of a set of polymeric chains that are regularly linked to each other by cross-linking agents such as potassium acrylate and acrylamide in parallel to each other, forming a network. When water comes into contact with chains of the polymers, it rapidly migrates into the interior of Aquasorb to form a hydrogel. Conversely, the hydrogel is also able to release up to 95% of the absorbed water into the exterior when the environment is deficient in water (Śpitalniak et al. 2019). Due to the efficient water absorption-release cycle of Aquasorb, we have utilized it for whole-cell entrapment of C. emersonii (211.8b) for the first time and its biocompatibility was assessed. We have also studied and compared the biotransformation (dehalogenation) efficiency of both free and entrapped C. emersonii (211.8b) cells for halogenated organic substrate (3-chloropropiophenone).
Materials and methods
Immobilization of C. emersonii (211.8b) cells in hydrogel (Aquasorb)
Chlorella emersonii strain 211.8b was kindly donated by Professor Przemyslaw Malec of Jagiellonian University, Poland. It was grown in BG-11 medium in 250 mL flask containing 100 mL culture medium, under continuous shaking (100 rpm) and illumination of white fluorescent light (25 µmol m−2 s−1) using Philips 40-Watt 4 ft. ALTO Supreme Linear T12 Fluorescent Light Bulb, Cool White (4100 K), without supply of external CO2 at 30 ± 1 °C for 7 days (Jang et al. 2018). The sequence of events from Aquasorb to the hydrogel and then to cellular growth inside the hydrogel is depicting in Fig. 1. When becoming hydrogel, Aquasorb beads can swell up to 500 times of its initial dry volume withdrawing water from the solution in which it is placed (the absorption capacity may vary depending upon the composition of solution absorbed). Aquasorb beads with an average weight of 5 ± 0.5 mg were used as “expandable containers” for Chlorella 211.8b cell cultures (Fig. 1i). In more details, a seed Chlorella 211.8b culture was grown till OD750 = 0.5, and the Aquasorb beads were added to absorb medium containing cells for about 30 min (Fig. 1ii). The Aquasorb hydrogels were then removed from the culture and washed gently several times with sterilized distilled water to remove the cells attached to the surface of hydrogels. To assess the biocompatibility, these hydrogels were further added to a fresh medium, and the entrapped cells were allowed to grow under the same conditions inside the hydrogel. Green color patches can be observed in the fully swollen hydrogel representing successful immobilization (entrapment) of the cells (Fig. 1iii). The hydrogels along with entrapped cells were added to fresh BG-11 medium to assess the biological and metabolic activities of Chlorella 211.8b cells inside the hydrogel. In Fig. 1, the hydrogel carrying entrapped cells is highlighted by a red circle (Fig. 1iv). Another most important aspect is that the water absorption-release cycle of Aquasorb enables the slow release of cells into the medium after saturation inside the hydrogel (Fig. 1v). After growth of the cells to an OD750 value of 1, the substrate was added to the culture to investigate the biotransformation capability of both free and hydrogel entrapped 211.8b cells (Fig. 1vi).
Events of the hydrogel (Aquasorb) entrapping C. emersonii strain 211.8b cells. The arrows indicate the sequence of events from i–vi. Aquasorb beads in its original form (i), Aquasorb beads in cell suspension medium (ii), Aquasorb hydrogel with green patches of entrapped cells (iii), the addition of hydrogel along with entrapped cells into fresh medium (iv), growth of cells within hydrogel and their release into the medium (v) and hydrogel entrapped cells fully populated and ready for substrate addition (vi)
Quantification of cell density per hydrogel bead and assessment of growth
The hydrogel beads with entrapped Chlorella 211.8b cells (at day 1, 2, 4, and 6) were homogenized in distilled water using a tissue grinder, and the material was sonicated (Ultrasonic homogenizer Scientz-IID, Ningbo Scientz Biotechnology Co., Ltd. Fujian, China) at 200 W and 20 kHz for 1 min on ice. After mechanical treatment, ten-fold dilution of the sample was performed and the last three suspensions of the dilution series were spread onto BG-11 plates and incubated at 30 ± 1 °C under 24 h fluorescent light (25 µmol m−2 s−1). After one week the number of colony-forming units (CFUs) were counted on the plates where each plate represented the number of cells entrapped in a single hydrogel bead. A similar procedure was followed for the assessment of growth of entrapped cells at different intervals of times (at days 1, 2, 4, 6, 8, 10, and 12) (Isaka et al. 2016). To avoid uneven light scattering in sample with hydrogel, the sample containing hydrogel was homogenized to minimize possible scattering phenomena and then compared the OD of the homogenized sample with the liquid phase (containing cells) coming from the culture media with hydrogel, which resulted in a similar absorbance pattern. Hence, the liquid phase from hydrogel containing samples were taken out for OD measurement which was representative of the hydrogel entrapped cells. The recyclability and biocompatibility of the Aquasorb with the entrapped cells were also monitored for several generations upon transferring the hydrogel beads with entrapped cells into fresh BG-11 medium.
Spectrofluorometric analysis of C. emersonii (211.8b) cells
To see the impact of 5 mM substrate on the viability of free cells as well as hydrogel entrapped Chlorella 211.8b, fluorescence emission spectra of both free and entrapped C. emersonii (211.8b) were recorded over the 620–720 nm range with a Jasco FP-550 spectrofluorometer equipped with a Peltier thermostat. The fluorescence was recorded at different intervals of time after incubating the cells (20 mL culture) with 16 mg (5 mM) of the substrate (3-CPP). A quartz cuvette of 1 mL volume was used. The excitation wavelength used was 435 nm, which corresponds to the maximum absorption of chlorophyll a in fluorescence (Christoffers and Ernst 1983).
Biotransformation of 3-chloropropiophenone (3-CPP) with Chlorella 211.8b
An equivalent volume of culture (150 mL each) for both free and hydrogel entrapped cells of C. emersonii (211.8b) were prepared in 500 mL flasks having an OD750 value of 1. To find out the suitable concentration of the substrate which can be tolerated by both free and entrapped cells, different concentrations of 3-CPP (0, 2, 5, and 10 mM) were added directly to the cultures. Hence, the optimum concentration of the substrate was obtained which can withstand by both the free and entrapped cells (Supplementary Fig. S1). To determine the biotransformation capacities of both free and entrapped microalgae for halogenated organic substrate, the optimal concentration (5 mM) of 3-CPP (Supplementary Fig. S1) was added to both the cultures. To check the stability of the substrate in the medium, the treated samples were also compared with the medium supplemented with the substrate (5 mM) only as abiotic control (without cells). After the addition of substrate, 30 mL of sample was extracted in a separating funnel by liquid–liquid extraction via ethyl acetate at five time points (6 h, 12 h, 24 h, 48 h, and 72 h). The extracts were dehydrated by adding anhydrous Na2SO4 and then dried using a rotary evaporator (IKA® RV 10 digital). The dried extracts were then re-dissolved in 2 mL of methanol and were analyzed by gas chromatography-mass spectrometry (GC–MS).
Analysis via GC–MS
The samples were analyzed by Agilent 7890B-5977A with a triple-axis detector (TAD) and HP-5MS column (30 m × 250 μm × 0.25 μm) with a split ratio of 50:1. Both the injector and the detector temperatures were kept at 250 °C. The column temperature varied from 80 °C (1 min) to 250 °C (0.5 min) at the increasing rate of 30 °C min−1. Helium was used as the carrier gas at a flow rate of 1 mL min−1. After analyzing the control (3-CPP), 10 min of retention time was fixed for both substrate and product. All reported results were the averages of experimental triplicates. For this analysis, the average error was less than 1.0%. The substrate to products conversion ratio in terms of peak areas was calculated using the formula reported earlier (Eq. 1) (Xu et al. 2016).
where “Product” is the cumulative of P1 and P2.
Antioxidant activity measurements of microalgae culture
The antioxidant activity of microalgae 211-8b culture in all conditions tested was measured by the method of Brand-Williams et al. (1995). Briefly, spectra of a 67 µM 2,2-diphenyl-1-picrylhydrazyl (DPPH•) (Sigma-Aldrich) in methanol as well as in the presence of 12 µM of ascorbic acid were recorded at 25 °C by a Jasco V-650 UV/Vis spectrophotometer. In the case of spectra of DPPH• with microalgae, 60 µL of 211-8b of microalgae culture were mixed with 1 mL of DPPH• in methanolic solution (final concentration 67 µM). A steady-state microalgae culture with initial OD750 of 1 Abs was divided into fractions (5 mL) and let to grow under autotrophic conditions (white fluorescent light (25 µmol m−2 s−1) and 30 ± 1 °C) in the absence or presence of hydrogel (obtained adding dry Aquasorb beads with an average weight of 5 ± 0.5 mg mL−1). These microalgae culture was then treated with 5 mM 3-CPP as described in Sect. Biotransformation of 3-chloropropiophenone (3-CPP) with Chlorella 211.8b. In all cases, spectra were recorded with 1 cm path length, blank subtracted and baseline corrected.
Results and discussion
Aquasorb hydrogel-based entrapment of C. emersonii (211.8b) cells
Hydrogel (Aquasorb) is water retaining copolymer of acrylamide and potassium acrylate. When introduced into the soil or aqueous medium, it can absorb a large amount of water and nutrients, and gradually release the retained water and nutrients. It thus enables the living material (especially plants) to maintain the supply of water and nutrients in a prolonged way for their growth and development. Considering the “absorption-release cycle” of water by Aquasorb hydrogel, we have exploited its application in the immobilization of C. emersonii (211.8b) cells. Using Aquasorb, we cannot only provide water and necessary nutrients for Chlorella cellular growth but also protect the cells from direct exposure to toxic or stressed environmental conditions.
To date, this Aquasorb mediated cell-entrapment was the first-ever reported technique for microalgal immobilization. Earlier reports regarding bacterial gel entrapping using different approaches are available in which they have used a gel carrier consists of PEG (a pre-polymer) and tetramethylene diamine (a promoter). They dissolved both the PEG and tetramethylene diamine in water and mixed the culture with the solution before polymerization was induced upon the addition of an initiator (potassium persulfate) (Isaka et al. 2016). These results suggest that the Aquasorb upon absorbing medium with cells are favorable for the normal Chlorella 211.8b cell growth and metabolism. They are biocompatible materials for immobilization of cyanobacterial and microalgal cells, which are thus suitable to be used in biotransformation or biocatalysis. Several studies based on immobilization and entrapment with increasing cell density of microalgae have recently been reported using silica hydrogel as well as poly-acrylic polymers, but the techniques used were more complicated, time-consuming, and costly (Homburg et al. 2019).
Growth curve of both free and entrapped Chlorella (211.8b)
Biocompatibility of Aquasorb hydrogel with Chlorella (211.8b) was studied upon evaluating the cellular growth by spectrophotometric measurement using the absorbance of OD750. Similar growth patterns of both free and entrapped cells were observed initially for four days, following a slower but comparatively lengthy exponential phase of entrapped cells till the 8th day of culture (Fig. 2). Comparatively higher OD750 was observed after the 9th day in hydrogel entrapped cells. It suggested that the initial slower lag and exponential phases were possibly due to the acclimation of cells to the new environment. Highest absorbance was noted on 12th day (OD750 = 1.33 ± 0.05) for hydrogel entrapped cells in comparison to free cells (OD750 = 1.12 ± 0.03). These results proved that the Aquasorb hydrogel was biocompatible to support the normal and healthier growth of cells (Fig. 2).
Cell density (CFUs) per hydrogel bead
After the hydrogel-based entrapment was successfully performed, the number of cells in terms of CFUs entrapped per hydrogel was estimated. Their growth inside hydrogel was also monitored by the serial dilution method and CFU assay. Each assay was performed three times (using three equivalent size hydrogel beads) (Fig. 3). The mean value obtained right after the entrapment was 1.25 × 104 CFUs/hydrogel bead. An initial decline in cell density (in terms of CFUs) was observed and noted as 1.21 × 104 CFUs/hydrogel bead, which can be ascribed to acclimation of the cells to the new environment. Later on, exponential growth was observed on day 4th and 6th, where the number of colonies obtained was 1.42 × 104 and 2.1 × 104 CFUs/hydrogel bead, respectively. This increase in CFUs per hydrogel bead suggests that the Aquasorb is a biocompatible material for immobilization/entrapment of Chlorella 211.8b cells. The photographed Petri plate shown in Fig. 3, portrays the number of colonies per hydrogel after rescuing and serial dilution. Similar approaches by other researchers can be seen for quantification of bacteria entrapped in the gel as well as estimation of the viability of Chlorella after oxidative stress (Isaka et al. 2016).
Cell density in terms of CFUs of Chlorella 211.8b per hydrogel bead. A merged photograph of the Petri plate depicts how the number of CFUs was measured. The size of each circle corresponds to the number of cells entrapped in the gel. Each point indicates the average of experimental triplicates in which the errors are represented by error bars
Effect of the substrate (3-chloropropiophenone) concentrations on 211.8b cells
As a general rule, substrate concentration affects the activity and stability of biocatalyst (in this case Chlorella 211.8b cells), as well as the rate of biotransformation. Therefore, to find out the suitable concentration of the substrate which can be tolerated by both free and entrapped cells, different concentrations of 3-CPP (0, 2, 5, and 10 mM) were applied. The optimum concentration of the substrate without adversely affecting the activity of both free and entrapped cells was found to be 5 mM (Supplementary Fig. S1). In addition, the Chlorella cells were incubated with 16 mg (~ 5 mM) concentrations of 3-chloropropiophenone for different intervals of time (0, 4, 8, 24, and 52 h), to see its impact on the viability of free cells as well as hydrogel entrapped Chlorella 211.8b (Fig. 4). The viability of C. emersonii in the presence of 3-CPP was monitored by analyzing the variation of fluorescence emitted by the chlorophyll of microalgae, based on the fact that cell death causes the disappearance of chlorophyll (Schulze et al. 2011). The culture of free Chlorella 211.8b shows fluorescence spectra that rapidly decrease as a function of time (approx. > 90% decrease after 52 h) that indicate destruction of chlorophyll and death of maximum of free cells. The fluorescence spectra underwent a marked intensity decrease (> 90% after 52 h) and a redshift of the λmax of emission from 681 to 668 nm after 52 h (Fig. 4). On the contrary, in the presence of 5 mM of 3-CPP, the hydrogel entrapped cells show viability in terms of chlorophyll fluorescence even after 52 h. These spectral variations indicate a disappearance of the chlorophyll likely due to its aggregation after cell death. No growth was observed when the free cells were re-cultured after treating with 3-CPP while on the other hand, the hydrogel entrapped cells maintained their viability when re-cultured after treating with 3-CPP. A similar toxicity test was recently reported by Gatidou et al., for Chlorella sorokiniana incubated with different concentrations of benzotriazoles (Gatidou et al. 2019). The mechanism on how the entrapped C. emersonii cells are protected by the hydrogel is not yet fully understood. Since the hydrogel is approximately 95% water with a heterogeneous, three-dimensional structure that includes loose fibers, flow channels, and micropores, the gelatinous matrix with bulk liquid phase in the hydrogel is thought to provide a safeguard and enable less exposure of the entrapped cells to the halogenated substrate (or its products). A reduction of the local concentration of the substrates in the surrounding of the cells might be the effect of physical adsorption of the halogenated substrate on the hydrogel.
Effect of the substrate (3-chloropropiophenone) concentrations on the viability of Chlorella 211.8b cells. 3-CPP (5 mM) was supplemented to both free and hydrogel entrapped cells of Chlorella 211.8b and then their fluorescence spectra were recorded over 620–720 nm after 0, 4, 8, and 52 h. Solid lines represent the hydrogel entrapment while dashed lines indicate free cells
Determination of biotransformation reaction through GC–MS analysis
The biotransformation (dehalogenation) of 3-chloropropiophenone (3-CPP), catalyzed by both free and immobilized C. emersonii (211.8b) cells was carried out on a small scale (150 mL). The biotransformation was monitored by GC–MS analysis after the biocatalyzed product was extracted from the reaction mixture with ethyl acetate at different intervals of time. As a general rule, the addition of a suitable substrate concentration enhances the biocatalytic capabilities of microorganisms without impacting their physicochemical activities (Xu et al. 2015). Therefore, it was of great importance to evaluate the biocatalytic dehalogenation capability of both free and immobilized C. emersonii (211.8b) cells, using 5 mM concentration of 3-CPP during this study.
GC–MS analysis of 3-chloropropiophenone after 6 h of treatment with 211.8b
The GC spectra obtained after 6 h of treatment are shown in Supplementary Fig. S2. The chromatogram acquired from the abiotic control (substrate only, without cells) displayed the standard peak of 3-CPP at retention time (RT) of 5.125 min and another smaller peak around 3.911 min ascribed to 1-phenyl-2-propenone (Supplementary Fig. S2a). This suggests that the 3-CPP was not stable in the reaction medium and its chemical (spontaneous) dehalogenation produced 1-phenyl-2-propenone. At the same time, an increase in the initial reaction rate and the product (1-phenyl-2-propenone) was observed when the 3-CPP was treated with free cells (Supplementary Fig. S2b). As compared to spontaneous dehalogenation of 3-CPP in abiotic control, a higher substrate conversion can be seen to the same product at RT 3.907 min upon treatment with free cells. This high rate of reaction and quantity of the product can be ascribed to a biocatalyst or antioxidants from the Chlorella 211.8b cells (Table 1).
Supplementary Fig. S2c shows the gas chromatogram obtained after 6 h of treatment of 3-CPP with hydrogel entrapped Chlorella 211.8b illustrated a minute peak for the product (RT = 4.049 min). This slower initial biotransformation by entrapped cells can be attributed to the slower absorption of the substrate to the hydrogel and also less number of exposed cells to the substrate. Supplementary Fig. S2d shows the extracted ions chromatogram (EIC) of the 3-CPP treated with hydrogel entrapped cells for 6 h, with no prominent peak for the biotransformed products (either 1-phenyl-2-propenone, Mol. wt. 132 or 1-phenyl-1-propanone, Mol. wt. 134). In the EIC chromatogram, the ions of a particular molecular mass (134 in this case) were taken into account, it thus extracted specific information (in terms of EIC) mathematically, from the crude data of total ions chromatogram (TIC).
GC–MS analysis of 3-CPP after 12 h of treatment with 211.8b
Efficient biotransformation of 3-CPP was observed in both treatments (free and entrapped cells of Chlorella 211.8b) after 12 h of incubation (Supplementary Fig. S3). Similar peaks for both 3-CPP and its spontaneous dehalogenated product 1-phenyl-2-propenone were observed even after 12 h in the abiotic control at retention times 5.129 min and 3.907 min, respectively (Supplementary Fig. S3a). But its intensity is relatively lower as compared to the corresponding peaks noted in both the treatments. As depicted in Supplementary Fig. S3b, the gas chromatogram of 3-CPP treated with free cells for 12 h displayed a sharp peak at RT = 3.911 min, corresponds to the biotransformed product by Chlorella 211.8b cells. It is reported by several researchers that entrapment (immobilization) of cells exhibited a greater impact on the rate of initial reaction, product yield, and biotransformation (Quezada et al. 2009). Not surprisingly, similar results were also observed in the current study after treating the substrate (3-CPP) with hydrogel entrapped 211.8b cells for 12 h. Supplementary Fig. S3c displays comparatively slower initial biotransformation of 3-CPP with entrapped Chlorella 211.8b. The peak intensity of the product (1-phenyl-2-propenone) at RT = 3.911 min in Supplementary Fig. S3c was lower than that observed in Supplementary Fig. S3b at the same retention time, suggesting a faster initial biotransformation activity in free cells as compared to hydrogel entrapped Chlorella 211.8b. EIC of the 3-CPP treated with hydrogel entrapped cells for 12 h is shown in Supplementary Fig. S3d. EIC chromatogram, corresponding to TIC chromatogram in Supplementary Fig. S3c, clearly shows that it consists of the two major compounds [1-phenyl-2-propenone (RT = 3.911 min) and 1-phenyl-1-propanone (RT = 3.959 min)]. The latter (1-phenyl-1-propanone) was obtained only after treatment with 211.8b cells and can be attributed to a cellular activity from the microalgae 211.8b.
GC–MS analysis of 3-CPP after 24 h of treatment with 211.8b
As illustrated in Fig. 5, the rate of biotransformation increased significantly as the time proceeded to 24 h. The rate of spontaneous conversion of 3-CPP to product (1-phenyl-2-propenone) became slower and reached its maximum value of the substrate to product ratio (0.21), which can be observed in Fig. 5a as well as in Table 1. No further increase in spontaneous conversion of 3-CPP was observed over time. Figure 5b shows the gas chromatogram of the substrate treated with free cells. Approximately half (2.51 ± 0.2 mM) of the substrate has been transformed into the products 1-phenyl-2-propenone and 1-phenyl-1-propanone (RT = 3.907 min) after 24 h. It has been observed that the substrate to product ratio for the aforementioned treatment reached its maximum (0.50), with no further change in the ratio. We postulate that it could be because the integrity of the free cell membrane decreased severely as the exposure time exceeds. Contrary to free cells, the biotransformation capability of hydrogel entrapped cells was increased after 24 h and continued to transform 3-CPP to 1-phenyl-2-propenone and 1-phenyl-1-propanone as the exposure time was further extended. As shown in Fig. 5c, the peak intensity of the product (RT = 3.911 min) was higher than the peak for 3-CPP (RT = 5.129 min), suggesting a better biotransformation capability of the entrapped Chlorella 211.8b cells. After 24 h, a total of 3.18 ± 0.07 mM out of 5 mM substrate has been biotransformed into the products 1-phenyl-2-propenone and 1-phenyl-1-propanone (RT = 3.911 min) by the hydrogel entrapped cells. In the latter case, the substrate to product ratio was also higher (0.64) than the preceding free cell treatment (Table 1). Although immobilized cells exhibited a lower initial biotransformation rate than the free cells, enhanced biotransformation by hydrogel entrapped Chlorella 211.8b cells was observed after 24 h (Table 1). Furthermore, the stability of immobilized cells was much better than the free cells (Xu et al. 2015). The corresponding EIC (Fig. 5d) of the TIC (Fig. 5c) indicated the highest conversion of the substrate to product where 1-phenyl-1-propanone at RT = 3.954 min was found to be the major component of the biotransformed product by the entrapped cells.
GC–MS analysis of 3-chloropropiophenone (3-CPP) after 24 h of treatment. a Control showing peaks of 3-CPP and its spontaneous conversion to 1-phenyl-2-propenone, b biotransformation of the substrate with free cells of Chlorella 211.8b. c Biotransformation of 3-CPP with hydrogel entrapped Chlorella 211.8b after 24 h, d EIC of 3-CPP treated with hydrogel entrapped cells displaying the major peak of 1-phenyl-1-propanone (RT 3.954 min) which was the major product of biotransformation
Assessment of biotransformation efficiency of free and entrapped Chlorella 211.8b cells
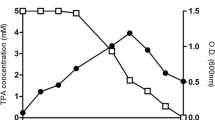
The biotransformation efficiency of both free and hydrogel entrapped Chlorella strain 211.8b was evaluated in terms of the ratio of peak areas. These ratios were obtained as a result of the GC–MS analysis of 3-CPP treated with 211.8b cells at different intervals of time (Fig. 6). As displayed in Supplementary Figs. S2, S3, and Fig. 5, the position of a peak on the x‐axis of a gas chromatogram is a measure of retention time (RT) and a representative of the structure of a compound. Similarly, the area under the peak is a function of the concentration of that compound in the sample. Therefore, calculation of the ratio of peak areas (substrate to product) provides necessary information about the rate of biotransformation and the estimated concentration of the product as well. Figure 6 articulates that all the treatments including both the abiotic controls and free cells reached their maximum values (substrate to product ratios) after 24 h of exposure, as no or minor changes in the ratio were observed. On contrary, even after a slower initial rate, an increasing tendency in biotransformation efficiency of the hydrogel entrapped Chlorella 211.8b cells were noted even after 48 h of exposure. This suggests that Aquasorb was able to provide a biocompatible medium for microalgal cell entrapment, enhanced biotransformation and biocatalytic capabilities of the biocatalysts (Chlorella 211.8b cells in this case). Similar results were also reported by several other researchers that immobilization has promoted the stability of the microbial cells along with augmentation of their biocatalytic capabilities (Xu et al. 2015).
Comparison of the ratio of peak areas (substrate to product) obtained after GC–MS analysis of different treatments of 3-chloropropiophenone (3-CPP) at different intervals. This trend indicates the rate of biotransformation of the substrate to product and compares the efficiency of free and hydrogel entrapped C. emersonii (211.8b). The error bars representing the SD among experimental triplicates
Antioxidant activity of microalgae culture
To shed the light on the higher conversion of 3-CPP to 1-phenyl-1-propanone when biotransformation was carried out with hydrogel entrapped cells, the antiradical activity of microalgae cultures using the free radical DPPH• was analyzed. The rationale of this experiment is based on the assumption that the elimination of chlorine from 3-CPP could generate a carbocation or a radical intermediate, which in the presence of an H− or H• donor present in microalgae culture can evolve to 1-phenyl-1-propanone is known to have antioxidant activity. DPPH• has an absorption band at 515 nm, which disappears upon reduction by an antiradical compound such as ascorbic acid (Supplementary Fig. S4). The absorption band of 67 μM DPPH• decreases from 0.370–0.284 Abs in the presence of 12 μM of ascorbic acid. Analogously, when treating 1 mL of 67 μM DPPH• with 60 μL of microalgae culture, a decrease in the intensity at 515 nm compared to the spectra of 67 μM DPPH• only was observed (Fig. 7). This result confirms the presence of antiradical compounds in the microalgae culture. Interestingly, 211-8b microalgae culture grown for 24 h in the presence of hydrogel showed a band at 515 nm significantly less intense (0.207 Abs) than that observed for 211-8b grown in free conditions (0.284 Abs), suggesting a higher antioxidant activity for 211-8b grown with hydrogel. A further increase of antioxidant activity appears to occur for hydrogel entrapped 211-8b cells treated with 5 mM 3-CPP for 2 h [the absorbance at 515 nm decreased to 0.080 Abs, (Fig. 7)]. A similar trend was observed when the 211-8b microalgae cultures were treated with 5 mM 3-CPP for 4.5 h (data not shown).
Absorption spectra of 67 μM 2,2-diphenyl-1-picrylhydrazyl in methanol in the absence (a) and the presence of 211-8b grown in free conditions (b) or in the presence of hydrogel (d). Spectra c and e refer to 211-8b grown as for b and d, respectively, but in the presence of 5 mM 3-CPP. In all cases, spectra with microalgae were obtained by adding 60 μL of 211-8b in 0.9 mL of 67 μM 2,2-diphenyl-1-picrylhydrazyl methanolic solution
The antioxidant activity data showed, indicating a direct correlation between the formation of 1-phenyl-1-propanone and the antioxidant activity in microalgae culture. Both the highest conversion ratio to 1-phenyl-1-propanone was obtained with 211-8b grown in the presence of hydrogel, which also seems to favor antioxidant formation compared to that grown in free cell modality. These results suggest that the formation of the alkane product might depend on the chemical reduction of a carbocation or a radical intermediate by antioxidants produced by the 211-8b, especially when in an immobilized form.
Composition of the dehalogenated biotransformed product
Two distinct peaks were observed in all the EICs corresponding to two closely related products, namely 1-phenyl-2-propenone (RT = 3.911 min) and 1-phenyl-1-propanone (RT = 3.954 min). The former was the result of spontaneous conversion observed in the abiotic control, while the latter was from biotransformation of 3-CPP with entrapped Chlorella 211.8b, suggesting the activity of either a dehalogenase or antioxidants from C. emersonii 211.8b. The possibility of a hydrogenase to transform 1-phenyl-2-propenone into 1-phenyl-1-propanone was excluded because the ratio between 1-phenyl-1-propanone/1-phenyl-2-propenone (0.36 ± 0.02) remains unchanged after 12 h (Fig. 8). Furthermore, the biotransformed product consists of two products (1-phenyl-2-propenone and 1-phenyl-1-propanone) where conversion to 1-phenyl-1-propanone was found higher in case of entrapped cells as compared to free cells treatment. The ratio of P1/P2 that is 1-phenyl-1-propanone/1-phenyl-2-propenone was obtained by determining the peak area of the product by considering the whole range of fragments obtained by MS analysis (TIC) modality for the total product (Fig. 8), and in EIC modality for the peak area of 1-phenyl-1-propanone only (Fig. 5).
Composition of the biotransformed product. The ratio of peak areas of the two products (1-phenyl-1-propanone and 1-phenyl-2-propenone) indicating that the hydrogel entrapped cells have higher conversion of the substrate to 1-phenyl-1-propanone as compared to free cells. The ratio of P1/P2 that is 1-phenyl-1-propanone/1-phenyl-1-propenone is obtained by determining the peak area of the product by considering the whole range of fragments obtained by MS analysis (TIC) modality
Conclusions
A halogenated organic substrate (3-Chloropropiophenone) was biotransformed using both free and hydrogel entrapped microalgae C. emersonii (211.8b) as biocatalyst. Aquasorb (used as entrapping material) was found to be highly biocompatible with no adverse effects on cellular growth and viability. Besides, significantly higher biocatalysis of 3-CPP was observed in treatment with entrapped cells. No activity of hydrogenase was found that could transform 1-phenyl-2-propenone into 1-phenyl-1-propanone because of the ratio between 1-phenyl-1-propanone/1-phenyl-2-propenone remained constant after 12 h. Of the total dehalogenated product, 1-phenyl-1-propanone was found to be the main product obtained only after cellular treatment. The product 1-phenyl-2-propenone was excluded to be the outcome of biotransformation as it was also found in the control (abiotic) group (produced due to spontaneous generation). Hence, the synthesis of 1-phenyl-1-propanone can be either ascribed to enzymatic activity (probably a dehalogenase) from Chlorella strain 211.8b or a chemical reduction of a carbocation or a radical intermediate by antioxidants produced by the 211-8b cells, especially when in immobilized form. Consequently, the Aquasorb based immobilization of microalgae is highly recommended as an effective tool for exploiting microalgal potentials of biocatalysis specifically when free cell activities are inhibited due to stress.
References
Arabi H, Yazdi MT, Faramarzi M (2010) Influence of whole microalgal cell immobilization and organic solvent on the bioconversion of androst-4-en-3, 17-dione to testosterone by Nostoc muscorum. J Mol Catal B-Enzym 62:213–217. https://doi.org/10.1016/j.molcatb.2009.10.006
Brand-Williams W, Cuvelier M-E, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol 28:25–30. https://doi.org/10.1016/S0023-6438(95)80008-5
Christoffers D, Ernst D (1983) The in-vivo fluorescence of chlorella fusca as a biological test for the inhibition of photosynthesis. Toxicol Environ Chem 7:61–71. https://doi.org/10.1080/02772248309357017
Della Greca M, Fiorentino A, Pinto G, Pollio A, Previtera L (1996) Biotransformation of progesterone by the green alga Chlorella emersonii C211–8H. Phytochemistry 41:1527–1529. https://doi.org/10.1016/0031-9422(95)00786-5
Gatidou G, Anastopoulou P, Aloupi M, Stasinakis AS (2019) Growth inhibition and fate of benzotriazoles in Chlorella sorokiniana cultures. Sci Total Environ 663:580–586. https://doi.org/10.1016/j.scitotenv.2019.01.384
Ghisleri F, Fu P, Secundo F (2017) Water-retaining polymers in organic solvent increase lipase activity for biodiesel synthesis. Insights Enzyme Res 1:1–6. https://doi.org/10.21767/2573-4466.100008
Homburg SV, Venkanna D, Kraushaar K, Kruse O, Kroke E, Patel AV (2019) Entrapment and growth of Chlamydomonas reinhardtii in biocompatible silica hydrogels. Colloid Surf B 173:233–241. https://doi.org/10.1016/j.colsurfb.2018.09.075
Isaka K, Udagawa M, Kimura Y, Sei K, Ike M (2016) Biological wastewater treatment of 1, 4-dioxane using polyethylene glycol gel carriers entrapping Afipia sp. D1. J Biosci Bioeng 121:203–208. https://doi.org/10.1016/j.jbiosc.2015.06.006
Janeczko T, Kostrzewa-Susłow E (2014) Enantioselective reduction of propiophenone formed from 3-chloropropiophenone and stereoinversion of the resulting alcohols in selected yeast cultures. Tetrahedron 25:1264–1269. https://doi.org/10.1016/j.tetasy.2014.08.006
Jang H, Namgoong JW, Sung M-G, Chang Y, Kim JP (2018) Synthesis and characterization of fluorescent dyes and their applications for the enhancement of growth rate of Chlorella vulgaris. Dyes Pigments 158:142–150. https://doi.org/10.1016/j.dyepig.2018.05.003
Kaparapu J (2017) Micro algal immobilization techniques. J Algal Biomass Utln 8:64–70
Lin B, Tao Y (2017) Whole-cell biocatalysts by design. Microb Cell Fact 16:106. https://doi.org/10.1186/s12934-017-0724-7
Quezada M, Carballeira J, Sinisterra J (2009) Monascus kaoliang CBS 302.78 immobilized in polyurethane foam using iso-propanol as co-substrate: optimized immobilization conditions of a fungus as biocatalyst for the reduction of ketones. Bioresource Technol 100:2018–2025. https://doi.org/10.1016/j.biortech.2008.07.068
Raza S, Wen H, Peng Y, Zhang J, Li X, Liu C (2021a) Fabrication of SiO2 modified biobased hydrolyzed hollow polymer particles and their applications as a removal of methyl orange dye and bisphenol-A. Eur Polym J 144:110199. https://doi.org/10.1016/j.eurpolymj.2020.110199
Raza S, Zhang J, Raza M, Li X, Wen H, Liu C (2021b) Biomass furfural-derived green polymer microspheres: synthesis and applications for the removal of environmental pollutants from wastewater. Micropor Mesopor Mat 318:110966. https://doi.org/10.1016/j.micromeso.2021.110966
Schulze K, López DA, Tillich UM, Frohme M (2011) A simple viability analysis for unicellular cyanobacteria using a new autofluorescence assay, automated microscopy, and image J. BMC Biotechnol 11:1–8. https://doi.org/10.1186/1472-6750-11-118
Śpitalniak M, Lejcuś K, Dąbrowska J, Garlikowski D, Bogacz A (2019) The influence of a water absorbing geocomposite on soil water retention and soil matric potential. Water 11:1731. https://doi.org/10.3390/w11081731
Wang J, Ma Q, Zhang Z, Li S, Diko CS, Dai C, Zhang H, Qu Y (2020) Bacteria mediated fenton-like reaction drives the biotransformation of carbon nanomaterials. Sci Total Environ 746:141020. https://doi.org/10.1016/j.scitotenv.2020.141020
Xu P, Xu Y, Li X-F, Zhao B-Y, Zong M-H, Lou W-Y (2015) Enhancing asymmetric reduction of 3-chloropropiophenone with immobilized Acetobacter sp. CCTCC M209061 cells by using deep eutectic solvents as cosolvents. ACS Sustain Chem Eng 3:718–724. https://doi.org/10.1021/acssuschemeng.5b00025
Xu P, Du P-X, Zong M-H, Li N, Lou W-Y (2016) Combination of deep eutectic solvent and ionic liquid to improve biocatalytic reduction of 2-octanone with Acetobacter pasteurianus GIM1.158 cell. Sci Rep 6:26158. https://doi.org/10.1038/srep26158
Yang Z-H, Luo L, Chang X, Zhou W, Chen G-H, Zhao Y, Wang Y-J (2012) Production of chiral alcohols from prochiral ketones by microalgal photo-biocatalytic asymmetric reduction reaction. J Ind Microbiol Biot 39:835–841. https://doi.org/10.1007/s10295-012-1088-y
Zur J, Piński A, Michalska J, Hupert-Kocurek K, Nowak A, Wojcieszyńska D, Guzik U (2020) A whole-cell immobilization system on bacterial cellulose for the paracetamol-degrading Pseudomonas moorei KB4 strain. Int Biodeterior Biodegrad 149:104919. https://doi.org/10.1016/j.ibiod.2020.104919
Funding
All authors are thankful for the financial support by the Research Start-Up Funds from Hainan University in China (KYQD_ZR2017212).
Author information
Authors and Affiliations
Contributions
SK performed the experiments and drafted the manuscript, PF had the idea for the article and administered the work, ADF performed spectrofluorometric study, IM performed the antioxidant studies, FS administered the work and critically revised the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no financial or personal interests that can inappropriately influence our work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Khan, S., Fu, P., Di Fonzo, A. et al. Enhanced whole-cell biotransformation of 3-chloropropiophenone into 1-phenyl-1-propanone by hydrogel entrapped Chlorella emersonii (211.8b). Biotechnol Lett 43, 2259–2272 (2021). https://doi.org/10.1007/s10529-021-03194-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-021-03194-y