Abstract
Terephthalic acid (TPA) is an endocrine disruptor widely used as a plasticizer and as a monomer in the manufacturing of PET bottles. However, because of various harmful effects on humans and the environment, it is now recognized as a priority pollutant whose environmental level needs to be controlled. In the present work, the TPA biodegradation efficacy of the bacterium Rhodococcus erythropolis (MTCC 3951) was studied in mineral salt media with TPA as the sole carbon and energy source. R. erythropolis was observed to degrade 5 mM and 120 mM TPA within 10 h and 84 h of incubation, respectively. The degradation efficiency was further optimized by varying the culture conditions, and the following optimum conditions were obtained: inoculum size- 5% (v/v), temperature- 30 °C, agitation speed- 200 rpm, and pH- 8.0. The bacterium was found to use an ortho-cleavage pathway for TPA degradation determined based on enzymatic and GC–MS studies. Moreover, during the degradation of TPA, the bacterium was observed to produce polyhydroxyalkanoate (PHA)—a biopolymer. Biodegradation of 120 mM TPA resulted in an accumulation of PHA. The PHA granules were visualized using fluorescence and transmission electron microscopy and were later characterized using FTIR spectroscopy. Furthermore, the robustness of the bacterium was demonstrated by its ability to degrade TPA in real industrial wastewater. Overall, R. erythropolis (MTCC 3951) hold the potential for controlling TPA pollution in the environment and vis-à-vis the production of PHA biopolymer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plasticizers are chemical additives that improve plastic strength, durability, flexibility, and fire resistance properties (Bereketoglu and Pradhan 2022). Phthalates, bisphenol, terephthalic acid, and polybrominated biphenyl are commonly utilized plasticizers and cover the largest share of the total plasticizers market (Kumar et al. 2023). India ranks third in global plastic production, trailing only China and the United States, with 368 million tonnes produced in 2020 excluding polyethylene terephthalate and polyacrylic fibres (https://www.plastindia.org/plastic-industry-status-report). In the same line, plasticizer consumption has also increased dramatically owing to the demand for plastics with desirable properties. Most of these properties could be achieved using plasticizers (Boll et al. 2020).
As the majority of plasticizers are usually not chemically bonded with the parent plastics, there is a high tendency for them to leach into the environment (Qadeer et al. 2022). These leach-out contaminants have been detected in many environmental systems, viz. aquatic, biotic, and terrestrial systems (Bridson et al. 2021; Tetu et al. 2019). Similar to plastics, these plasticizers are also recalcitrant compounds and difficult to degrade. Aside from recalcitrance, plasticizers are also regarded as hazardous compounds owing to their potential endocrine disruption and carcinogenic, mutagenic, and bioaccumulation properties (Kumar et al. 2023; Qadeer et al. 2022). Hence, these contaminants also need to be remediated from the environmental systems and other pollutants.
Phthalates are extensively used plasticizers, accounting for 85% of the total plasticizer market (Qadeer et al. 2022). Among phthalates, terephthalic acid (TPA)- a paraform of phthalate is among the top 50 abundantly produced chemicals worldwide and is used extensively as alternate plasticizers (Amiri et al. 2022; Ball et al. 2012; Das et al. 2021). Along with industrial wastewater originating from purified TPA manufacturing industry (petrochemical plant), polyethylene terephthalate (PET), -based plastics have also been identified as the major source of TPA. In PET synthesis, TPA is used as one of the monomers in combination with ethylene glycol (EG); however, wastewater originating from purified TPA manufacturing industries is identified as the major source of TPA in the environment. Apart from PET synthesis, TPA also finds applications in dyes, pesticides, textile fibres, and films used in photographic, audiovisual, and packaging fields (Garg and Prasad 2017; Zhang et al. 2013a). In this reference, the global annual production of TPA increased from 47 million metric tons in 2012 to 84 million metric tons in 2022. It is expected to rise to 105 million metric tons in 2029 with a surge in demand (https://www.statista.com/statistics/1245249/purified-terephthalic-acid-marketvolume-worldwide/).
During the production of 1-ton TPA, about 3–4 m3 wastewater having a COD level of 5–20 g/L is generated (Garg and Prasad 2017; Karthik et al. 2008), which needs to be treated to lower the COD and TPA level before being discharged into the open environment. Nevertheless, the toxicity of TPA was observed to increase with the rise in concentration, especially at concentrations > 1000 mg/L (Garg and Prasad 2017). Toxicity of TPA includes alterations in functions of vital organs, viz. urinary bladder, liver, and testis, through damaging these organs and encouraging adipocyte dysfunction (Molonia et al. 2022). Moreover, it also has been identified as one of the mutagens and carcinogenic chemicals (Aksu et al. 2021). Considering its wide prevalence, recalcitrance nature, and toxicity, USEPA has listed TPA as one of the priority pollutants whose level needs to be controlled (Garg and Prasad 2017).
At present, major physicochemical processes, viz. adsorption, advanced oxidation, electro-coagulation, and coagulation using metal salts, are used to treat wastewater originating from TPA manufacturing plants. However, secondary pollution and high cost are some of the limitations of these processes (Shahedi et al. 2020). On the other hand, biological treatment using microorganisms and activated sludge emerged as a sustainable option for treating TPA-containing wastewater (Rylott and Bruce 2020). Various microorganisms, for instance, Rhodocoocus biphenylivoran (Suwanawat et al. 2019) and Arthrobacter sp. 0574 (Zhang et al. 2013b) have been used for the biodegradation of TPA. However, a higher concentration of TPA significantly beyond 10,000 mg/L is considered highly toxic to microbes; thus, treatment of wastewater containing such a high level of TPA usually needs to be improved. Rhodocoocus erythropolis MTCC 3951 used in the present study was able to degrade up to 20,000 mg/L (120 mM) of TPA efficiently as the sole carbon source. Indeed, to the best of our knowledge, this is the first study demonstrating the TPA biodegradation efficiency of R. erythropolis MTCC 3951.
In the present study, the degradation potential of R. erythropolis MTCC 3951 towards TPA was studied in mineral salt media with TPA as the sole carbon source. The effect of various culture conditions and high concentrations of TPA on biodegradation was determined. The pathway for biodegradation was elucidated using enzymatic assay and through the identification of key intermediate metabolites using Gas Chromatography-Mass Spectrometry (GC–MS). Finally, the application of the bacterium was demonstrated through i) TPA remediation from industrial wastewater and ii) concomitant production of polyhydroxyalkanoate (PHA)- a bioplastic along with TPA degradation.
Material and methods
Materials
Terephthalic acid (TPA) with a purity greater than 99% was procured from Sigma Aldrich (USA). Media components were procured from Himedia Laboratories, India. Organic solvents used were of HPLC grade. All other chemicals belongs to analytical grade.
Microorganism
Bacterium Rhodococcus erythropolis MTCC 3951 was procured from Microbial Type Culture Collection and Gene Bank (MTCC), Chandigarh, India. The bacterium was maintained on a nutrient agar slant (stored at 4 °C) and glycerol stock (stored at -80 °C) with periodic subculturing.
Media and culture conditions
Mother culture of the bacterium was prepared by transferring loopful culture from the slant to 20 ml nutrient broth (NB) containing in (g/L) peptone 5.0, NaCl 0.5, and yeast extract 5.0, pH 7.4. The culture was grown at 30 °C and 120 rpm until the OD600 reached 0.8.
Two percent of overnight grown mother culture was withdrawn from the mother culture and centrifuged at 10,000 × g for 10 min. The cell pellet thus obtained was washed with saline solution (0.85% w/v NaCl) and finally suspended in saline solution. The resultant cell suspension was inoculated to 100 mL mineral salt medium (MSM) of pH 7.0 containing g/L 3: (NH4)2SO4; 1: KH2PO4; 0.2: MgSO4·7H2O; 0.02: FeSO4·7H2O; 0.2: yeast extract; 5 mM TPA (Suwanawat et al. 2019). TPA was added to MSM from the previously prepared 120 mM stock solution. The stock solution of TPA was prepared by adding TPA (10 g) in 500 mL water followed by heating the solution at 95 °C, during heating NaOH pellets (6 g) were added to the solution till complete dissolution of TPA (Wang et al. 2005; Wen et al. 2006). Inoculated MSM was incubated at 30 °C with a shaking speed of 200 rpm for 18 h. Uninoculated MSM containing 5 mM TPA as a control set was also treated as above. During incubation, aliquots of samples (2 mL) were repeatedly withdrawn from both flasks to estimate the growth and residual TPA concentrations.
Effect of varying culture conditions on TPA degradation
To characterize the TPA degradation efficiency of R. erythropolis MTCC 3951, the effect of inoculum size (2, 5, and 10% v/v), temperature (25, 30, 37, and 45 °C), pH (6.0, 6.5, 7.0, 7.5, 8.0, 8.5, 9.0, 9.5, and 10.0) and shaking speed (50, 100, 150, 200, and 250 rpm) were separately monitored on TPA biodegradation. A TPA concentration of 5 mM was used for this study, and samples were withdrawn at periodic intervals for up to 12/24 h to estimate the remaining TPA in the culture medium.
Effect of varying TPA concentrations on the degradation of TPA
To determine the effect of varying concentrations of TPA, MSM containing 5, 10, 20, 60, and 120 mM TPA was individually inoculated with 5% (v/v) culture of the bacterium as described above. The flasks were incubated at 30 °C and 120 rpm for 84 h; afterwards, the residual TPA content was estimated using HPLC (Described below).
Identification of the TPA biodegradation pathway
Enzymatic assay
To identify the pathway followed by R. erythropolis for TPA degradation, protocatechuate 3,4-dioxygenase (P34O) and protocatechuate 4,5-dioxygenase (P45O) activities were estimated in the culture sample. The bacterium was grown in MSM containing 5 mM TPA for 10 h under optimum conditions (pH 8.0, 30 °C temperature, and 200 rpm agitation speed). Afterwards, the culture media was centrifuged at 10000 × g for 15 min under 4 °C, and the pellet thus obtained was washed with saline and finally suspended in lysis buffer [25 mM Tris HCl (pH 8.0), 400 mM NaCl, 2% (v/v) glycerol]. The cells were then subsequently lysed using an ultrasonic homogenizer (Biologics, Inc. Model 30000) on the pulse for 15 s "On" and 20 s "Off" for 30 min under cold conditions. The lysed cells thus obtained were centrifuged at 13000 × g for 40 min at 4 °C, and the supernatant was used for estimating the enzyme activities according to the method of Xu et al. (2021). Briefly, 500 μL of enzyme extract was added to 50 mM (1300 μL) phosphate buffer (pH 7.2), followed by the addition of 10 mM (200 μL) protocatechuic acid (PCA) substrate (Xu et al. 2021). The reaction mixture was incubated at 35 °C for 10 min, and the absorbance was separately recorded at 290 nm and 410 nm for estimating P34O and P45O activities, respectively. The same reaction without substrate (PCA) was also carried out as a control reaction. One unit of enzyme activity was defined as the amount of protocatechuic acid (µmole) consumed by P34O per minute under assay conditions (Xu et al. 2021).
Identification of intermediate metabolites using GC–MS
As explained above, cells were grown in the presence of 5 mM TPA for 10 h. During growth, samples were withdrawn periodically at an interval of 2 h, followed by centrifugation of the sample at 6000 × g for 10 min. The resultant supernatant was mixed with an equal volume of ethyl acetate and was kept at 200 rpm and 30 °C for 48 h. The aqueous phase was extracted thrice, and the pooled ethyl acetate fraction/phase was concentrated using a rotary evaporator. The concentrated fraction was then dissolved in 2 mL of HPLC-grade methanol, followed by filtration through a 0.2 mm nylon membrane filter (Axiva, filter syringe). The filtrate thus obtained was injected into GC–MS (Agilent 8890, USA) for the identification of metabolites of TPA degradation (Tang et al. 2016). The separation was performed using a 95% dimethylpolysiloxane capillary column (15 m × 0.25 mm × 0.25 µm). The detector interface temperature was 280 °C. The injection temperature was maintained at 250 °C. The initial oven temperature was kept at 80 °C for 2 min hold time. It was raised to 280 °C with an increase of 10 °C/min. The final temperature of 280 °C was hold for 10 min. The Helium (1 mL/min) was used as a carrier gas. The compounds were identified using a standard inbuilt NIST (National Institute of Standards and Technology) library.
Quantification of TPA
TPA concentration was estimated using High- Performance Liquid Chromatography (HPLC) (Agilent Technologies HPLC Series 1100, USA) equipped with a C18 column (14.6 cm × 5.5 cm × 5.5 cm) with UV-Diode Array detector (Furukawa et al. 2018). The mobile phase used was 70% (v/v) MilliQ, 20% (v/v) acetonitrile, and 1% (v/v) formic acid. The oven temperature was maintained at 40 °C, and the flow rate was set at 0.5 mL/min. The samples were filtered through a 0.22 µm filter before loading and were analyzed for TPA at 254 nm. The method was standardized using known concentrations of TPA before analysis of unknown TPA levels (Figure S1).
TPA removal from industrial wastewater
The industrial wastewater samples were collected from a Common Effluent Treatment Plant (CETP), Okhla, New Delhi. The samples had a pH value of 7.6. The TPA was externally added to these samples to make the final concentration of 5 mM. The performance of R. erythropolis MTCC 3951 was evaluated for degradation of TPA in the industrial wastewater samples. For this purpose, the mother culture of the R. erythropolis was prepared by transferring loopful culture from the agar slant to 20 mL nutrient broth (NB) containing in (g/L): peptone 5.0, NaCl 0.5, yeast extract 5.0 and pH 7.4. The culture was grown at 30 °C and 120 rpm until the OD600 reached 0.8. After that, 5% of this mother culture was inoculated into a 50 mL sample. The mixture was incubated at 30 °C with constant shaking at 200 rpm for 84 h. The samples were withdrawn periodically at an interval of 12 h. The residual TPA was estimated by HPLC.
Polyhydroxyalkanoate (PHA) production
For polyhydroxyalkanoate (PHA) production, R. erythropolis (5% v/v) was inoculated to MSM amended with 120 mM TPA and treated as described earlier. After complete degradation of TPA (84 h), the cells were harvested using centrifugation (8000 × g for 10 min). The cell pellet was washed with saline solution and was fixed overnight using 2.5% (v/v) glutaraldehyde solution (EM grade). After that, the cells were again washed and suspended in phosphate buffer (pH 7.2), followed by processing for Transmission Electron Microscopy (TEM) at All India Institute of Medical Sciences (AIIMS), New Delhi, India. The TEM micrographs were recorded using TECNAI 200 kV TEM (Fei, Electron Optics) to visualize PHA granules inside the cells. PHA production was also detected through Nile red assay as per the method described by Liu et al. (2021).
Extraction of PHA
The NaOCl-chloroform extraction method was used for the extraction of PHA (Martínez-Herrera et al. 2020). After degradation of 120 mM TPA, cells were collected by centrifugation at 10000 × g for 15 min at 4 °C. Cells were treated with sodium hypochlorite (NaOCl) (5% v/v) and kept for 2 h at 30 °C for digestion. The digested product (cell mass) was collected using centrifugation (10000 × g for 15 min) and was washed with distilled water. After that, it was treated with a mixture of methanol, diethyl ether, and acetone (1:1:1) for 10 min, followed by centrifugation (10000 × g for 15 min). The pellet containing PHA was treated with chloroform at 100 °C for 1 min. The extracted product was collected in a Petri plate and dried at 30 °C. The dried residue was weighed and characterized using Fourier Transform Infrared Spectroscopy (FTIR).
PHA characterization
Characteristics functional group was analyzed through FTIR spectroscopy (Nicolet IS-50, Thermo Fischer Scientific). The PHA residue and KBr were mixed and pressed to form a KBr pellet. The pellet was scanned in the range of 400–4000 cm−1 with 128 added scans at the resolution of 4 cm−1.
Results and discussion
Growth and TPA degradation
R. erythropolis MTCC 3951 was obtained from the Microbial Type Culture Collection Facility (MTCC), Institute of Microbial Technology (IMTECH), Chandigarh, India (Ghosh and Khare 2017) and is known to degrade a variety of aromatic hydrocarbons viz. 7- ketocholesterol, mono, di- and tri-chlorophenols, cresols, catechol, toluene, and benzoic acid. In the present study, the bacterium was used to degrade TPA; the culture grew robustly in MSM containing TPA as a carbon source. The bacterium utilized TPA and its metabolites for growth with concomitant degradation of TPA. Bacterium growth was observed to be exponential from 0 to 12 h; afterwards, the declining phase was obtained (14–18 h). Maximum TPA degradation rate (0.01 mM/h-0.35 mM/h) was recorded during this exponential growth phase (6–12 h) (Fig. 1). Moreover, during the initial 6 h of incubation, there was no significant change in the initial 5 mM TPA content; however, degradation of TPA increased, and complete removal of TPA was observed within 18 h of growth. The uninoculated control setup showed no significant removal of TPA during the course of incubation. Thus, removal of TPA was observed to be growth-dependent, and the bacterium utilizes TPA and its metabolites for growth.
Growth and terephthalic acid (TPA) degradation profile of Rhodococcus erythropolis MTCC 3951. The bacterium (2%,v/v) was grown in mineral media (pH 7.0) with5mM TPA as sole carbon source. The culture was incubated at 30 °C and 20 rpm for 18 h and during incubation aliquots of sample was withdrawn for recording growth (OD600 nm) and residual TPA concentration
Moreover, the declining phase observed (decrease in OD600 nm) during the growth of the bacterium (Fig. 1) is due to the presence of dead cells and subsequent lysis of the dead cells (Liu et al. 2015). To visualize the live and dead cells in the samples, Confocal Laser Scanning Microscopy (CLSM) (FLUOVIEW FV1200 inverted motorized model Olympus IX83) was performed using the dual staining method (Fatima et al. 2023), and images were obtained. From Figure S2, it is figured out the number of dead cells exceeds the live cells in the 16 h sample, proving the assumption, i.e. that dead cells are present.
The cell hydrophobicity of the R. erythropolis MTCC 3951 was also determined to establish the interaction between the hydrophobic TPA and the bacterial surface, as this interaction is a prerequisite for the degradation of TPA. Cell hydrophobicity improves the ability to interact with partial or insoluble pollutants, facilitating their bioremediation (Ivshina et al. 2022). The hydrophobicity of the bacterial surface was elucidated using the Microbial Adhesion to Hydrocarbon Method (MATH) (Aono and Kobayashi 1997). A cell with more than 80% MATH value is considered highly hydrophobic (Aono and Kobayashi 1997). R. erythropolis MTCC 3951 cells have MATH value of 85.50 ± 2 to 67.54 ± 2% during TPA degradation as determined using MATH assay (Figure S3). From the results, it is inferred that initially, cells were more hydrophobic (85% MATH value), which aids in attachment with TPA and helps in TPA degradation. Cell hydrophobicity decreases with degradation of TPA (67% MATH value after degradation).
Optimization of culture conditions
To optimize the degradation efficiency, the effect of inoculum size, temperature, pH, and agitation speed were individually monitored using one—variable at a- time approach. In the case of inoculum size, faster TPA removal was detected with 5% (v/v), while lower (2%) and higher (10%) sizes resulted in lower degradation rates (Fig. 2a). In the case of lower inoculum size, several active cells might have resulted in a lower degradation rate. In contrast, higher inoculum size usually depletes oxygen and nutrient levels, thus affecting the degradation rate (Bhattacharya et al. 2018). Thus, in the present study, 5% (v/v) inoculum size was determined to be the optimum w.r.t to TPA degradation.
Effect of varying culture conditions on terephthalic acid (TPA) degradation by Rhodococcus erythropolis MTCC 3951 (a) Inoculum size (b) Initial pH (c) Temperature (d) Agitation speed. The bacterium was grown in MSM containing 5 mM TPA as sole carbon source and culture conditions were optimized by varying one parameter at a time approach. Samples were periodically withdrawn for estimation residual TPA levels
Regarding the effect of pH, maximum degradation was detected with an initial pH of 8.0 (Fig. 2b). Lower (6.0–7.0) and higher (9.0–10) pH are considered extreme or tolerable to the bacterium as lower growth rates were observed. The optimum temperature and agitation speed were observed at 30 °C and 200 rpm, respectively (Fig. 2c and d). Lower (25 °C) and higher (45 °C) temperatures showed lower degradation rates owing to decreased metabolic rate and enzyme activity at these respective temperatures (Jin and Kirk 2018). Agitation helps in the proper availability and mixing of nutrients and substrates and the enhanced oxygen supply. Hence, slow degradation was detected in the present study at lower agitation speeds (50–150 rpm).
Degradation of varying TPA concentrations under optimum conditions
After determining the optimum culture conditions, the effect of varying concentrations of TPA was studied. Under optimum conditions, the bacterium was observed to completely degrade the initial 5 mM TPA within 12 h of incubation. On the other hand, the same trend was observed at 18 h under unoptimized conditions. With further increase in concentrations of TPA (10–120 mM), the degradation rate was observed to increase, and 10, 20, 40, and 60 mM initial TPA levels were completely removed at 24, 36, 48, and 60 h of incubation, respectively (Fig. 3). At the same time, a higher concentration of 120 mM TPA was completely degraded at 84 h of incubation. This increase in degradation rate with an increase in the level of TPA was owing to the high mass transfer of substrate for the growth of the bacterium. Table 1 compares the TPA degradation performance of R. erythropolis MTCC 3951 with other reported bacterial species. The table shows that the R. erythropolis MTCC 3951 showed an enhanced removal rate of higher TPA compared to other bacteria.
Effect of varying terephthalic acid concentrations on degradation of terephthalic acid. R. erythropolis MTCC 3951 (5%, v/v inoculum) was grown in MSM (pH 8.0) containing varying concentrations of TPA, at 30 °C and 200 rpm (optimum conditions). Samples were taken out during incubation for estimating remaining TPA concentrations
Identification of the TPA biodegradation pathway
Once the TPA degradation was established, the degradation pathway (Figure S4) was elucidated by estimating the activity of key enzymes involved in degradation and also through the identification of intermediate metabolites using GC–MS. According to Vamsee-Krishna and Phale (2008), protocatechuate 3,4-dioxygenase (P34O) and protocatechuate 4,5-dioxygenase (P45O) are two rate-determining enzymes in the entire biodegradation pathway of TPA. The activity of these enzymes decides the ring-cleavage pattern of protocatechuic acid (PCA). P34O results in cleavage at 1 and 2 positions, resulting in the generation of cis, cis-muconic acid; the compound is further metabolized through a cascade of enzymes and results in the formation of succinic acid and acetyl-CoA, which then finally enters the Kreb’s cycle for further metabolism (Vamsee-Krishna and Phale 2008). On the other hand, cleavage at the 3 and 4 positions of protocatechuate results in the formation of 2-hydroxymuconic semialdehyde, which also enters the Kreb’s cycle, through pyruvate and oxaloacetate metabolites after a sequence of transformation through a series of enzymes (Vamsee-Krishna and Phale 2008).
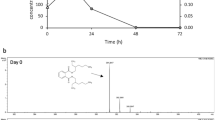
In the present study, R. erythropolis MTCC 3951 showed P34O activity (64 nmol/ min/mL), while no detectable activity of P45O was observed. This indicates that the bacterium followed the ortho-pathway for the metabolism of TPA. For R. erythropolis MTCC 3951, time course changes in P34O activity during TPA degradation showed a steady increase in enzyme activity with simultaneous degradation of TPA (Fig. 4a). The ortho-pathway used by the bacterium for TPA degradation is further corroborated with the identification of TPA intermediate metabolites viz derivative of succinic acid i.e., succinic acid, 2-chloro-6-flourophenyl tetrahydrofurfuryl ester (Fig. 4b) through GC–MS. The succinic acid metabolite is identified as a downstream metabolite of the ortho-degradation pathway. Patrauchan et al. (2005) observed the expression of the P34O gene from Rhodococcus sp. strain RHA1 in the presence of benzoate and phthalate, indicating ortho-cleavage of the compounds. On the other hand, Kim et al. (2002) used Rhodococcus sp. strain DK17 and detected the presence of both ortho- and meta-ring cleavage pathways based on substrate specificity.
(a) Time course changes in protocatechuate 3,4-dioxygenase (P340) activity during degradation of terephthalate (TPA) (b) Identification of intermediate metabolite during TPA degradation using GC–MS. R. erythropolis MTCC 3951 (5%,v/v) was grown in MSM (pH 8.0) containing 5 mM TPA. The culture was incubated at 30 °C and 200 rpm for 10 h. During incubation culture sample was withdrawn repeatedly for estimating the P340 activity. For identification of intermediate metabolites, samples were processed as detailed in methodology section and analyzed using GC–MS
TPA removal from industrial wastewater
The degradation performance of the bacterium R. erythropolis MTCC 3951 was also evaluated in the real industrial wastewater collected from the common effluent treatment plant. The bacterium was observed to completely remove an initial 5 mM TPA from the wastewater within 84 h of treatment (Fig. 5). The TPA degradation efficiency of the bacterium in real heterogeneous and complex wastewater further strengthens the potential of the bacterium towards its application in TPA bioremediation. Uninoculated wastewater showed no prominent removal of TPA during incubation, and this also supports the applicability of the bacterium towards bioremediation.
Removal of terephthalic acid from industrial wastewater using Rhodococcus erythropolis MTCC 3951. The bacterium (5%,v/v) was extraneously added to industrial wastewater (pH 7.67) and incubated at 30 °C and 200 rpm for 84 h. Control wastewater without inoculation with R. erythropolis MTCC 3951 was also treated as above. During treatment, samples were withdrawn and assessed for residual TPA levels
PHA production and characterization
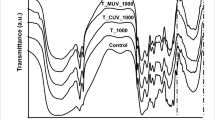
The bacterium R. erythropolis MTCC 3951 was observed to accumulate intracellular polyhydroxyalkanoate (PHA) during the degradation of TPA. The accumulation of PHA was demonstrated through TEM micrographs. The bacterium showed the development of endocytotic vesicles containing PHA as detected in cells grown for 84 h in the presence of 120 mM TPA (Fig. 6a). Fluorescent Nile red staining of cells also showed the accumulation of PHA (Fig. 6b). Control cells grown in media without TPA were stained using Nile red, and accumulation of PHA was not detected in control cells. PHA granules were further visualized using CLSM (Figure S5). PHA production adds another dimension to the study as it is identified as an important alternative to conventional plastics. So, in this study, the bacterium not only degrades the TPA (a metabolite of PET degradation) but also results in the simultaneous production of bioplastics-PHA. FTIR spectra of the produced PHA (Fig. 6c) show bands at 1620 cm−1 corresponding to aliphatic ester carbonyl C = O, while the band at 2920 cm−1, corresponds to CH of CH3 and CH2 groups related to aliphatic compounds like PHA (Rao et al. 2019). A strong band confirms the presence of an OH group at 3420 cm−1. Bands at 1520 cm−1 and 1050 cm−1 are due to CH3 and vibration of C-O and C–C bonds (Anjali et al. 2014), respectively. The OH group is associated with the reported band between 3200 and 3500 cm−1, and the CH vibrational characteristics of PHAs are associated with the band at 2928 cm−1 (Vega-Castro et al. 2016).
Polyhydroxyalkanoate (PHA) production by Rhodococcus erythropolis MTCC 3951 during terephthalic acid (TPA) degradation (a) TEM micrographs showing accumulation of PHA granules (b) Nile red staining of cells showing PHA containing colonies whereas control cells grown in media without TPA (c) FTIR spectra of extracted PHA. The bacterium (5%, v/v) was grown in presence of 120 mM TPA in mineral salt medium, under optimum conditions (pH 8.0, Temperature 30 °C, agitation speed 200 rpm). After 84 h of growth, samples were withdrawn and processed for TEM, Nile red staining, and FTIR spectroscopy as described in methodology section
Conclusions
R. erythropolis MTCC 3951 was observed to efficiently biodegrade up to 120 mM TPA within 84 h of incubation under optimum conditions. P34O activity and intermediates metabolite viz. succinic acid, 2-chloro-6-flourophenyl tetrahydro furfuryl ester confirmed the ortho biodegradation pathway for TPA. The bacterium was also effective in remediating TPA from industrial wastewater. Finally, the production of polyhydroxyalkanoate biopolymer during TPA biodegradation further adds value to the study. Overall, the bacterium could be used for abatement of TPA pollution and vis-à-vis production of bioplastic-polyhydroxyalkanoate.
Data availability
NA.
References
Aksu D, Vural C, Karabey B, Ozdemir G (2021) Biodegradation of terephthalic acid by isolated active sludge microorganisms and monitoring of bacteria in a continuous stirred tank reactor. Braz Arch Biol Technol 64. https://doi.org/10.1590/1678-4324-2021200002
Amiri H, Martinez SS, Shiri MA, Soori MM (2022) Advanced oxidation processes for phthalate esters removal in aqueous solution: A systematic review. Rev Environ Health. https://doi.org/10.1515/reveh-2021-0147
Anjali M, Sukumar C, Kanakalakshmi A, Shanthi K (2014) Enhancement of growth and production of polyhydroxyalkanoates by Bacillus subtilis from agro-industrial waste as carbon substrates. Compos Interfaces 21:111–119
Aono R, Kobayashi H (1997) Cell surface properties of organic solvent-tolerant mutants of Escherichia coli K-12. Appl Environ Microbiol 63:3637–3642
Ball GL, McLellan CJ, Bhat VS (2012) Toxicological review and oral risk assessment of terephthalic acid (TPA) and its esters: A category approach. Crit Rev Toxicol 42:28–67
Bereketoglu C, Pradhan A (2022) Plasticizers: Negative impacts on the thyroid hormone system. Environ Sci Pollut Res 29:38912–38927
Bhattacharya A, Gupta A, Kaur A, Malik D (2018) Remediation of phenol using microorganisms: sustainable way to tackle the chemical pollution menace. Curr Org Chem 22:370–385
Boll M, Geiger R, Junghare M, Schink B (2020) Microbial degradation of phthalates: Biochemistry and environmental implications. Environ Microbiol Rep 12:3–15
Bridson JH, Gaugler EC, Smith DA, Northcott GL, Gaw S (2021) Leaching and extraction of additives from plastic pollution to inform environmental risk: A multidisciplinary review of analytical approaches. J Hazard Mater 414:125571
Das MT, Kumar SS, Ghosh P, Shah G, Malyan SK, Bajar S, Thakur IS, Singh L (2021) Remediation strategies for mitigation of phthalate pollution: Challenges and future perspectives. J Hazard Mater 409:124496
Fatima H, Bhattacharya A, Khare SK (2023) Efficient remediation of meropenem using Bacillus tropicus EMB20 β-lactamase immobilized on magnetic nanoparticles. J Environ Manag 329:117054
Furukawa M, Kawakami N, Oda K, Miyamoto K (2018) Acceleration of enzymatic degradation of poly(ethylene terephthalate) by surface coating with anionic surfactants. Chemsuschem 11:4018–4025
Garg KK, Prasad B (2017) Treatment of toxic pollutants of purified terephthalic acid waste water: A review. Environ Technol Innov 8:191–217
Ghosh S, Khare SK (2017) Biodegradation of 7-Ketocholesterol by Rhodococcus erythropolis MTCC 3951: Process optimization and enzymatic insights. Chem Phys Lipids 207:253–259
Ivshina IB, Krivoruchko AV, Kuyukina MS, Peshkur TA, Cunningham CJ (2022) Adhesion of Rhodococcus bacteria to solid hydrocarbons and enhanced biodegradation of these compounds. Sci Rep 12:21559
Jin Q, Kirk MF (2018) pH as a primary control in environmental microbiology: 1. thermodynamic perspective. Front Environ Sci 6:21
Karthik M, Dafale N, Pathe P, Nandy T (2008) Biodegradability enhancement of purified terephthalic acid wastewater by coagulation–flocculation process as pretreatment. J Hazard Mater 154:721–730
Kim D, Kim YS, Kim SK, Kim SW, Zylstra GJ, Kim YM, Kim E (2002) Monocyclic aromatic hydrocarbon degradation by Rhodococcus sp. strain DK17. Appl Environ Microbioly 68:3270–3278
Kumar A, Chavan PV, Bhatkhande DS, Sadavarte VS, Santosh Mada SSNM, Pande SM (2023) Migration of energetic plasticizer in advanced energetic composite propellant grains. Propellants Explos Pyrotech 48:e202200185
Liu K, Zhao E, Ilyas G, Lalazar G, Lin Y, Haseeb M, Czaja MJ (2015) Impaired macrophage autophagy increases the immune response in obese mice by promoting proinflammatory macrophage polarization. Autophagy 11:271–284
Liu P, Zhang T, Zheng Y, Li Q, Su T, Qi Q (2021) Potential one-step strategy for PET degradation and PHB biosynthesis through co-cultivation of two engineered microorganisms. Eng Microbiol 1:100003
Martínez-Herrera RE, Alemán-Huerta ME, Almaguer-Cantú V, Rosas-Flores W, Martínez-Gómez VJ, Quintero-Zapata I, Rivera G, Rutiaga-Quiñones OM (2020) Efficient recovery of thermostable polyhydroxybutyrate (PHB) by a rapid and solvent-free extraction protocol assisted by ultrasound. Int J Biol Macromol 164:771–782
Molonia MS, Muscarà C, Speciale A, Salamone FL, Toscano G, Saija A, Cimino F (2022) The p-Phthalates terephthalic acid and dimethyl terephthalate used in the manufacture of PET induce in vitro adipocytes dysfunction by altering adipogenesis and thermogenesis mechanisms. Molecules 27:7645
Patrauchan MA, Florizone C, Dosanjh M, Mohn WW, Davies J, Eltis LD (2005) Catabolism of benzoate and phthalate in Rhodococcus sp. strain RHA1: redundancies and convergence. J Bacteriol 187:4050–4063
Qadeer A, Kirsten KL, Ajmal Z, Jiang X, Zhao X (2022) Alternative plasticizers as emerging global environmental and health threat: another regrettable substitution? Environ Sci Technol 56:1482–1488
Rao A, Haque S, El-Enshasy HA, Singh V, Mishra BN (2019) RSM-GA based optimization of bacterial PHA production and in silico modulation of citrate synthase for enhancing PHA production. Biomolecules 9:872
Rylott EL, Bruce NC (2020) How synthetic biology can help bioremediation. Curr Opin Chem Biol 58:86–95
Shahedi A, Darban AK, Taghipour F, Jamshidi-Zanjani A (2020) A review on industrial wastewater treatment via electrocoagulation processes. Curr Opin Electrochem 22:154–169
Suwanawat N, Parakulsuksatid P, Nitayapat N, Sanpamongkolchai W (2019) Biodegradation of terephthalic acid by Rhodococcus biphenylivorans isolated from soil. Int J Environ Sci 10:30–33
Tang WJ, Zhang LS, Fang Y, Zhou Y, Ye BC (2016) Biodegradation of phthalate esters by newly isolated Rhizobium sp. LMB-1 and its biochemical pathway of di- n -butyl phthalate. J Appl Microbiol 121:177–186
Tetu SG, Sarker I, Schrameyer V, Pickford R, Elbourne LDH, Moore LR, Paulsen IT (2019) Plastic leachates impair growth and oxygen production in Prochlorococcus, the ocean’s most abundant photosynthetic bacteria. Commun Biol 2:184
Vamsee Krishna C, Phale PS (2008) Bacterial degradation of phthalate isomers and their esters. Indian J Microbiol 48:19–34
Vega-Castro O, Contreras Calderon J, León E, Segura A, Arias M, Pérez L, Sobral PJA (2016) Characterization of a polyhydroxyalkanoate obtained from pineapple peel waste using Ralsthonia eutropha. J Biotechnol 231:232–238
Wang Q, Xu H, Li X (2005) Solubilities of terephthalic acid in dimethyl sulfoxide + water and in N, N-dimethylformamide + water from (301.4 to 373.7) K. J Chem Eng Data 50:719–721
Wang ZJ, Teng LH, Zhang J, Huang XL, Zhang JF (2011) Study on optimal biodegradation of terephthalic acid by an isolated Pseudomonas sp. Afr J Biotechnol 10:3143–3148
Wen YZ, Tong SP, Zheng KF, Wang LL, Lv JZ, Lin J (2006) Removal of terephthalic acid in alkalized wastewater by ferric chloride. J Hazard Mater 138:169–172
Xu M, Liu D, Sun P, Li Y, Wu M, Liu W, Maser E, Xiong G, Guo L (2021) Degradation of 2,4,6-trinitrotoluene (TNT): Involvement of protocatechuate 3,4-dioxygenase (P34O) in Buttiauxella sp. S19–1. Toxics 9:231
Zhang WL, Zhang JF, Li Z, Gong JX (2011) The isolation, identification and fermentation of Bacillus for degradation of terephthalic acid. Adv Mat Res 183:942–946
Zhang W, Zhang YJ, Chen D, Zhang R, Yu XY, Gao YW, Liu WQ (2013a) Quantitative analysis of overlapping x-ray fluorescence spectra for Ni, Cu, Zn in Soil by orthogonal signal correction and partial least squares algorithm. In Adv Mat Res 705:70–74
Zhang YM, Sun YQ, Wang ZJ, Zhang J (2013b) Degradation of terephthalic acid by a newly isolated strain of Arthrobacter sp. 0574. S Afr J Sci 109:4
Acknowledgements
The first author (ACM) would like to acknowledge Council of Scientific and Industrial Research (CSIR), Government of India for providing financial support in the form of a fellowship (File no. 09/086(1350)/2018-EMR-I). The authors are thankful to the Sophisticated Analytical Instrumentation Facility (SAIF) of All India Institute of Medical Sciences (AIIMS) New Delhi for TEM studies.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study's conception and design. ACM: Conceptualization, Investigation, Carried out experiments, Data analysis, Writing—original draft. AB: Conceptualization, Investigation, and Evaluation of the draft manuscript. SKK: Validation, Review, Editing and Supervision.
Corresponding author
Ethics declarations
Ethical approval
NA.
Consent to participate
NA.
Consent to publish
All authors mutually agree to submit the MS in the Journal of Environmental Science and Pollution Research.
Competing of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Responsible Editor: Gerald Thouand
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Maurya, A.C., Bhattacharya, A. & Khare, S.K. Biodegradation of terephthalic acid using Rhodococcus erythropolis MTCC 3951: Insights into the degradation process, applications in wastewater treatment and polyhydroxyalkanoate production. Environ Sci Pollut Res (2023). https://doi.org/10.1007/s11356-023-30054-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11356-023-30054-1