Abstract
To produce high-, medium- and low-molecular-weight hyaluronic acid (HA) at different temperatures using engineered Bacillus subtilis expressing hyaluronidase (HAase) from leech. By overexpressing the HAase gene hya in the HA-producing strain WmB using temperature-sensitive plasmid pKSV7, the engineered strain WmB-PYh produced HA with different molecular weights (8.61 kDa at 32 °C, 0.615 MDa at 42 °C, and 6.19 MDa at 47 °C). In this study, the molecular weight of HA was regulated by using leech HAase expressed from a temperature-sensitive plasmid. We thus obtained different molecular weight HAs by using a single bacterial strain at different culture temperatures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
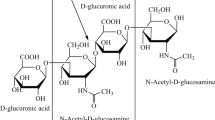
Hyaluronic acid (HA) is a non-sulphated glycosaminoglycan that has been applied in cosmetic, food and pharmaceutical industries (Kogan et al. 2007). N-acetyl-glucosamine (GlcNAc) and glucuronic acid (GlcUA) monomers polymerize via β-1,3- and β-1,4-glycosidic bonds to form HA chains, the length of which determines the molecular weight (MW), and different HAs have different functions (Stern et al. 2006). High-molecular-weight (HMW) HAs (MW > 2 MDa) have good viscoelasticity, strong rigidity, and maintain their properties for a long time. HMW HAs can be used in ophthalmic surgery and are useful for wound healing. Medium-molecular-weight (MMW) HAs (MW 0.1−1 MDa) display good moisture retention and lubricity, and they are suitable for soft tissue filling, deep wrinkle removal, and drug release. Low-molecular-weight (LMW) HAs (MW < 10 kDa) have superior permeability and cell penetration properties, can inhibit tumor proliferation, promote bone formation and angiogenesis, and be used in immunomodulation (Stern et al. 2006; Toole et al. 2008).
In recent years, heterologous HA-producing strains with clear backgrounds have become established as an attractive alternative for HA production due to the high pathogenic risk and paucity of DNA manipulation techniques for Streptococcus species (Blank et al. 2005). Many hosts, including Bacillus subtilis (Widner et al. 2005), Lactococcus lactis (Chien and Lee 2007), Agrobacterium sp. (Mao and Chen 2007), Escherichia coli (Yu and Stephanopoulos 2008), Pichia pastoris (Jeong et al. 2014) and Corynebacterium glutamicum (Cheng et al. 2019), have been engineered extensively for HA production. B. subtilis, an established “Generally Recognized as Safe” strain, is considered ideal for metabolic engineering (Commichau et al. 2015), and has been studied for HA production.
Leech hyaluronidase (LHAase) can specifically hydrolyze HA by acting on the non-reducing end of HA and cleaving the β-1,3-glycosidic bond, thereby decreasing the molecular weight of HA (Linker et al. 1960). In addition, exogenous expression of recombinant LHAase in B. subtilis has no risk of animal cross-infection (Jin et al. 2014). Therefore, LMW HAs for clinical medical treatment can be obtained using recombinant leech HAase. However, industrial processes for producing HAs with different MWs are complex.
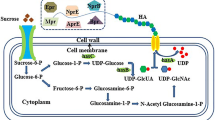
Different MW HAs have been collected by modifying the ribosome binding site (RBS) of hya (encoding LHAase) in different engineered strains (Jin et al. 2016). In the present study, the temperature-sensitive vector pKSV7 was used to overexpress LHAase in B. subtilis. We regulated the expression of LHAase by controlling the temperature. This provides a novel strategy for producing high-, medium- and LMW HAs using a single bacterial strain at different culture temperatures (Fig. 1).
Materials and methods
Media and culture conditions
Luria–Bertani (LB) broth (10 g/L tryptone, 5 g/L yeast extract and 10 g/L NaCl) was used for seed cultivation, with ampicillin (100 mg/L), ergomycin (50 mg/L) and tetracycline (50 mg/L) added as necessary. E. coli and B. subtilis strains were cultured overnight at 37 °C. Fermentation medium (20 g/L sucrose, 3 g/L (NH4)2SO4, 6.5 g/L KH2PO4, 4.5 g/L Na2HPO4, 2 g/L sodium citrate, 3 g/L MgSO4•7H2O, and 0.5 g/L CaCl2•2H2O) and 6 mL/L of a trace metallic elements solution (100 g/L citric acid, 20 g/L FeSO4•7H2O, 5 g/L MnSO4•H2O, 2 g/L CuSO4•5H2O and 2 g/L ZnCl2) was used for HA production. B. subtilis strains were incubated at 32 °C, 42 °C and 47 °C.
Strains and plasmid construction
E. coli DH5α chemically competent cells were prepared as described previously (Russell and Sambrook 2001) and used as hosts for plasmid construction. The hya sequence was downloaded from NCBI and synthesized by Genewiz (Tianjin, China). The signal peptide of the yweA gene was amplified from B. subtilis WB600 and connected to the 5′- end of hya to form a fused yweA–hya fragment. The yweA–hya fragment was subsequently inserted into the temperature-sensitive plasmid pKSV7, resulting in plasmid pKSV7-Yh. Then, the strong promoter P43 was placed in front of yweA–hya and the new fragment P43–yweA–hya was linked to pKSV7 to generate pKSV7-PYh. The constructed plasmids were electrotransformed into B. subtilis WB600 and WmB (Li et al. 2019). The B. subtilis strains and plasmids used in this study are listed in Table 1, and the primers used are listed in Supplementary Table 1.
Hyaluronidase activity analysis
LHAase activity was quantified using the 3,5-dinitrosalicylic acid (DNS) colorimetric spectrophotometric method (Jin et al. 2014). Controls comprising the fermentation supernatant of B. subtilis Wm were prepared and analyzed in an identical manner.
HA quantification
HA fermentation products were collected as described previously (Li et al. 2019), and the HA titer was routinely determined using a modified uronic acid carbazole reaction (Bitter and Muir 1962). As a control, B. subtilis strain WB600 was treated in the same way and the value (background signal) was subtracted.
HA molecular weight measurement
The MW of HA was measured with a multi-angle laser light scattering and size exclusion chromatography system as described previously (Li et al. 2019).
Results and discussion
Expression of hyaluronidase in B. subtilis Wm
Due to the specificity of LHAase for hydrolyzing HA, it can be used to lower the MW of HA (Jin et al. 2016). To accomplish high-level extracellular production of LHAase in B. subtilis, we fused the LHAase gene hya with the signal peptide of yweA in the temperature-sensitive plasmid pKSV7. In theory, hya can be expressed at 32 °C, but there should be no expression of hya at 42 °C due to elimination of plasmid at this temperature (Smith and Youngman 1992). We constructed the engineered strain Wm-Yh expressing LHAase from the spore deletion strain Wm (Li et al. 2019), as confirmed by gel electrophoresis (Supplementary Fig 1). To verify LHAase activity, the resulting Wm-Yh strain was cultivated in flasks and the fermentation supernatant was analyzed by the DNS method (Fig. 2a). The reaction solution comprising Wm-Yh supernatant became reddish brown, while it was still yellow in solution containing Wm supernatant. The results indicated that Wm-Yh could produce active LHAase. The mean hyaluronidase activity of Wm-Yh was 2.04 × 104 U/mL (Fig. 2b). The activity was higher than that for enzyme expressed in Pichia pastoris (1.20 × 104 U/mL) (Jin et al. 2014).
Determination of leech hyaluronidase activity by the DNS method. a Photos of DNS and hyaluronidase color reactions following fermentation by Wm, Wm-Yh and Wm-PYh strains. b Hyaluronidase activity following fermentation by Wm-Yh and Wm-PYh. Data are averages of three independent experiments, and error bars represent standard deviation (SD; *p < 0.05; **p < 0.01; ***p < 0.001; ns, no significance)
Enhancement of hyaluronidase activity in B. subtilis Wm
To further promote the expression of LHAase, the strong promoter P43 was placed in front of the fused yweA-hya fragment in Wm, resulting in strain Wm-PYh. Gel electrophoresis results are shown in Supplementary Fig. 2. The wild-type RBS of hya has been verified to produce the lowest MW HAs (Jin et al. 2016). Compared with Wm-Yh, the product of the DNS reaction was darker for Wm-PYh (Fig. 2a). The mean HAase activity of Wm-PYh was 4.77 × 104 U/mL, significantly higher than that of Wm-Yh (Fig. 2b). These results indicated that the hya of pKSV7-PYh could be expressed at high levels in B. subtilis. Accordingly, plasmid pKSV7-PYh was chosen and engineered to explore its effect on HA MWs.
Production of LMW and MMW HA by B. subtilis WmB-PYh
Recombinant B. subtilis typically produce MMW HAs (Widner et al. 2005; Westbrook et al. 2018; Li et al. 2019). However, HAs with different MWs have different functions, and recombinant LHAase can be expressed to expand the MW range of HAs (Jin et al. 2016). Herein, the temperature-sensitive plasmid pKSV7-PYh was electrotransformed into the HA-producing WmB strain to construct WmB–PYh, and this strain produced LMW (8.61 kDa) and MMW (0.615 MDa) HAs at 32 °C and 42 °C, respectively (Fig. 3a). Additionally, WmB not expressing LHAase produced MMW HAs at both 32 °C (0.392 MDa) and 42 °C (0.678 MDa; Fig. 3a). Compared with WmB, there were no differences in HA MW for WmB-PYh at 42 °C, at which temperature pKSV7-PYh was eliminated. These results suggest that it may be more convenient and practical to produce LMW and MMW HAs by rationally controlling the expression of LHAase than by employing previously reported strategies (Jin et al. 2016). Additionally, the HA titers of WmB (4.01 g/L and 2.66 g/L) and WmB-PYh (4.25 g/L and 2.77 g/L) were not significantly different at 32 °C and 42 °C (Fig. 3b). This indicates that this strategy only affects the MW of HA.
Further increasing the MW of HAs produced by B. subtilis WmB-PYh
Since HA is a growth-associated product (Huang et al. 2006), the MW can be increased by decreasing the HA-producing bacterial cell density using the oxygen vector n-heptane (Westbrook et al. 2018). Moreover, our previous research showed that WmB can produce HMW HAs (6.94 MDa) when the culture temperature is increased to 47 °C (Li et al. 2019). Similarly, WmB-PYh produced HMW HAs (6.19 MDa) at 47 °C in the present work (Fig. 4a). Compared with WmB (1.72 g/L), the HA titer of WmB-PYh (1.88 g/L) was not significantly different at 47 °C (Fig. 4b). These results demonstrate that lower cell density is conducive for extension of HA chains.
Conclusion
In this study, we developed a novel strategy to produce high-, medium- and LMW HAs using WmB-PYh. By adjusting the temperature to control the presence of the pKSV7-PYh plasmid, WmB-PYh produced HAs with different MW (8.61 kDa at 32 °C, 0.615 MDa at 42 °C and 6.19 MDa at 47 °C), and HA production was efficient (4.25 g/L at 32 °C, 2.77 g/L at 42 °C and 1.88 g/L at 47 °C). To our knowledge, this is the first method for producing HAs with different MWs using a single bacterial strain. This strategy could also be employed to produce other valuable polysaccharides with different MWs.
References
Bitter T, Muir HM (1962) A modified uronic acid carbazole reaction. Anal Biochem 4:330–334. https://doi.org/10.1016/0003-2697(62)90095-7
Blank LM, McLaughlin RL, Nielsen LK (2005) Stable production of hyaluronic acid in Streptococcus zooepidemicus chemostats operated at high dilution rate. Biotechnol Bioeng 90:685–693. https://doi.org/10.1002/bit.20466
Cheng F, Yu H, Stephanopoulos G (2019) Engineering Corynebacterium glutamicum for high-titer biosynthesis of hyaluronic acid. Metab Eng 55:276–289. https://doi.org/10.1016/j.ymben.2019.07.003
Chien LJ, Lee CK (2007) Hyaluronic acid production by recombinant Lactococcus lactis. Appl Microbiol Biotechnol 77:339–346. https://doi.org/10.1007/s00253-007-1153-z
Commichau FM, Alzinger A, Sande R et al (2015) Engineering Bacillus subtilis for the conversion of the antimetabolite 4-hydroxy-l-threonine to pyridoxine. Metab Eng 29:196–207. https://doi.org/10.1016/j.ymben.2015.03.007
Huang WC, Chen SJ, Chen TL (2006) The role of dissolved oxygen and function of agitation in hyaluronic acid fermentation. Biochem Eng J 32:239–243. https://doi.org/10.1016/j.bej.2006.10.011
Jeong E, Shim WY, Kim JH (2014) Metabolic engineering of Pichia pastoris for production of hyaluronic acid with high molecular weight. J Biotechnol 185:28–36. https://doi.org/10.1016/j.jbiotec.2014.05.018
Jin P, Kang Z, Zhang N, Du G, Chen J (2014) High-yield novel leech hyaluronidase to expedite the preparation of specific hyaluronan oligomers. Scientific reports 4:4471. https://doi.org/10.1038/srep04471
Jin P, Kang Z, Yuan P, Du G, Chen J (2016) Production of specific-molecular-weight hyaluronan by metabolically engineered Bacillus subtilis 168. Metab Eng 35:21–30. https://doi.org/10.1016/j.ymben.2016.01.008
Kang Z, Ruiyang Z, Congqiang Z, Gregory S, Heng-Phon T (2013) Optimization of amorphadiene synthesis in Bacillus subtilis via transcriptional, translational, and media modulation. Biotechnol Bioeng 110:2556–2561. https://doi.org/10.1002/bit.24900
Kogan G, Soltés L, Stern R, Gemeiner P (2007) Hyaluronic acid: a natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol Lett 29:17–25. https://doi.org/10.1007/s10529-006-9219-z
Li Y, Li G, Zhao X, Shao Y, Wu M, Ma T (2019) Regulation of hyaluronic acid molecular weight and titer by temperature in engineered Bacillus subtilis. 3 Biotech 9(6):225. https://doi.org/10.1007/s13205-019-1749-x
Linker A, Meyer K, Hoffman P (1960) The production of hyaluronate oligosaccharides by leech hyaluronidase and alkali. J Biol Chem 235:924–927
Mao Z, Chen RR (2007) Recombinant synthesis of hyaluronan by Agrobacterium sp. Biotechnol Prog 23:1038–1042. https://doi.org/10.1021/bp070113n
Russell DW, Sambrook (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor, New York
Smith K, Youngman P (1992) Use of a new integrational vector to investigate compartment-specific expression of the Bacillus subtilis spollM gene. Biochimie 74:705–711. https://doi.org/10.1016/0300-9084(92)90143-3
Stern R, Asari AA, Sugahara KN (2006) Hyaluronan fragments: an information-rich system. Eur J Cell Biol 85:699–715. https://doi.org/10.1016/j.ejcb.2006.05.009
Toole BP, Ghatak S, Misra S (2008) Hyaluronan oligosaccharides as a potential anticancer therapeutic. Curr Pharm Biotechnol 9:249–252. https://doi.org/10.2174/138920108785161569
Westbrook AW, Ren X, Moo-Young M, Chou CP (2018) Application of hydrocarbon and perfluorocarbon oxygen vectors to enhance heterologous production of hyaluronic acid in engineered Bacillus subtilis. Biotechnol Bioeng 115:1239–1252. https://doi.org/10.1002/bit.26551
Widner B, Behr R, Von Dollen S et al (2005) Hyaluronic acid production in Bacillus subtilis. Appl Environ Microbiol 71:3747–3752. https://doi.org/10.1128/AEM.71.7.3747-3752.2005
Wu XC, Lee W, Tran L, Wong SL (1991) Engineering a Bacillus subtilis expression-secretion system with a strain deficient in six extracellular proteases. J Bacteriol 173:4952–4958. https://doi.org/10.1128/jb.173.16.4952-4958.1991
Yu H, Stephanopoulos G (2008) Metabolic engineering of Escherichia coli for biosynthesis of hyaluronic acid. Metab Eng 10:24–32. https://doi.org/10.1016/j.ymben.2007.09.001
Acknowledgements
This work was supported by Tianjin Science Technology project, China (Grant No. 18YFZCSY00020, 16JCTPJC50100) and National Natural Science Foundation of China (31900050).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
This work did not include any studies with human participants or animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, Y., Shi, Z., Shao, Y. et al. Temperature-controlled molecular weight of hyaluronic acid produced by engineered Bacillus subtilis. Biotechnol Lett 43, 271–277 (2021). https://doi.org/10.1007/s10529-020-03001-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-020-03001-0