Abstract
Objectives
Major lignocellulosic inhibitory compounds found in sugarcane-based industrial hydrolysate samples were tested in laboratory and industrial yeast strains, as well as in lactic acid bacteria, in order to verify their effects on important physiological parameters.
Results
Saccharomyces cereviaise SA-1, an industrial strain, stood out as compared to the remaining strains for virtually all inhibitors investigated. This strain presented the highest growth rate and the lowest lag-phase in the presence of acetic acid, levulinic acid, p-coumaric acid, ferulic acid, and HMF, when compared to the other strains. In sugarcane-based hydrolysate fermentations, both SA-1 and CEN.PK113-7D presented similar fermentation performances. Industrial isolates of contaminating lactic acid bacteria were evaluated in the presence of an inhibitory cocktail, containing a mixture of 76.6 mM acetic acid, 1.3 mM HMF, 7.1 mM furfural, and 1.9 mM p-coumaric acid. Whilst all yeast strains were unable to grow under such conditions, bacteria had an average inhibition of roughly 50% on their growth rates.
Conclusions
Overall, industrial strain SA-1 might be a promising microbial chassis for second generation ethanol production and for future metabolic and evolutionary engineering strategies, and for strain robustness understanding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The production of ethanol from sugarcane is highly relevant in the upcoming transition from fossil to sustainable transportation fuels. During industrial production of ethanol from sugar fermentation, yeasts face a variety of stress conditions, which includes high concentrations of ethanol and salts (Auesukaree et al. 2009; Benjaphokee et al. 2012; Della-Bianca and Gombert 2013; Lam et al. 2014), high temperatures (Auesukaree et al. 2009; Benjaphokee et al. 2012; Della-Bianca and Gombert 2013; Caspeta and Nielsen 2015), and constant contamination by lactic acid bacteria (Basso et al. 2014; Reis et al. 2018). In addition to these stress factors, sugarcane-based fermentation differs significantly from other processes, such as in corn-based, because in the former yeast cells are subjected to a continuous recycling process that lasts for the whole harvest season (comprising around 250 days a year). This is an important characteristic that highlights the occurrence of a heterogeneous yeast population dynamics in which industrial strains become selected by adapting not only to the stress factors mentioned above, but also outcompeting wild yeasts and bacterial contaminants. Such bacterial strains do display robust growth profiles at acidic pH values normally found during the fermentation step (Della-Bianca et al. 2013).
Additional obstacles arise in second-generation (2G) ethanol production processes, in which lignocellulosic residues are the feedstock for fermentation. In addition to the problems of pentose fermentation, physical-chemical pretreatment steps generate various microbial inhibitors that severely affect yeast physiology and therefore compromise the fermentation process as a whole (Palmqvist and Hahn-Hägerdal 2000; Klinke et al. 2004; Skerker et al. 2013; Caspeta et al. 2015). Lignocellulosic-derived inhibitors are formed during the pretreatment of biomass and depend mainly on the type of biomass used and on the process conditions (Klinke et al. 2004; van Maris et al. 2006). Many studies have been performed on the formation of by-products during the pretreatment of sugarcane bagasse. Knowledge on the formation of by-products from lignocellulosic material is very beneficial when the decomposed lignocellulose is used in a fermentation process. These by-products can result in problems downstream, since they can inhibit growth and impair fermentative performance of microorganisms (van der Pol et al. 2014).
In the present study, we have investigated the composition of industrial lignocellulosic hydrolysates, and we have evaluated the severity of the main lignocellulosic inhibitory compounds on cell growth rate and on elongation of the lag phase in four strains of Saccharomyces cerevisiae, as well as on lactic acid bacteria, isolated from industrial sugarcane ethanol processes. Yeast strains evaluated included the laboratory strain S. cerevisiae CEN.PK113-7D and its diploid version CEN.PK112, and two industrial strains from the fuel ethanol industry, namely S. cerevisiae SA-1 and JAY270, a derivative strain from PE-2. The effects of varying concentrations of representative inhibitory compounds, such as weak acids, furan derivatives and phenolics, were evaluated on physiological parameters (Adeboye et al. 2014).
In general, this study revealed the outstanding performance of one of the industrial strains, namely SA-1, in comparison to the other strains evaluated. The majority of the inhibitors tested had a smaller impact on the growth rate and on the lag phase of this strain when compared to the remaining strains. This observation corroborates the hypothesis that industrial yeast strains from the first-generation (1G) ethanol industry, selected to withstand stress conditions inherent to this process, are interesting candidates for increased robustness in 2G ethanol production.
Material and methods
Yeast and lactic acid bacteria strains and maintenance
Two reference laboratory strains for physiological studies and functional genomics were used, being one haploid CEN.PK 113-7D (van Dijken et al. 2000) and one diploid CEN.PK 112 (van den Broek et al. 2015) was obtained from the Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands. We also used two industrial strains widely employed in the Brazilian sugarcane ethanol industry, namely SA-1 and JAY270, a derivative of PE-2 (Basso et al. 2008; Della-Bianca et al. 2013). The genotypic characteristics of the strains used in this work are summarized in Table 1. The bacterial strains used in this work were 4 lactic acid bacteria obtained from fermented molasses in industrial ethanol plants located in Brazil (Zimotec, Luiz de Queiroz College of Agriculture, Piracicaba, Brazil). Lactic acid bacteria were grown in plates containing solid MRS broth, incubated at 37 °C for 48 h.
Stock cultures were prepared by growing cells in shake flasks containing 100 mL MRS (for lactic acid bacteria) or YP medium (10 g yeast extract L−1, 20 g bacteriological peptone L−1) with 20 g L−1 initial glucose (for yeasts). After overnight growth at 30 °C and 200 rpm, 20% (final concentration, v/v) glycerol was added and 1 mL aliquots were stored at − 80 °C. Stock cultures were used to prepare pre-cultures for shake flask cultivations.
Lignocellulosic hydrolysates
The lignocellulosic hydrolysates samples for inhibitory compound determinations were kindly provided by Novozymes Latin America Ltda. (Araucária, Brazil) and by the Brazilian Bioethanol Science and Technology Laboratory (CTBE, CNPEM, Campinas, Brazil). Two sugarcane bagasse samples were pre-treated by steam explosion (STEX) with dilute phosphoric acid (9.5 mg H3PO4 g dry solids−1) at 180 °C for 5 min. The pretreated material, both liquor (samples 1 and 2) and solid cellulose-lignin (samples 3 and 4) fractions were then digested with the Cellic® CTec3 enzyme mixture at 50 °C for 72 h. Sample from CTBE (sample 5) was obtained according to Zetty-Arenas et al. (2019). In brief, the sugarcane bagasse was hydrothermally pretreated in a 350-L Hastelloy C-276 reactor (POPE Scientific Inc., Saukville, USA) under 160 °C, 60 min, and solid-to-liquid ratio of 1:10. The pretreated liquor was then collected and filtered (Nutsche filter, POPE Scientific, USA) and subsequently transferred to the acid-post-hydrolysis step in a 2-L stainless steel reactor (PARR Instrument Company, Moline, USA) using H2SO4 aqueous solution (0.4 wt %) at 130 °C and 200 rpm for 30 min.
Cultivation medium
Yeast cells were cultured in YPD (10 g yeast extract L−1, 20 g bacteriological peptone L−1, and containing 20 g glucose L−1) whereas lactic acid bacteria was cultured in MRS (containing 20 g glucose L−1). Yeast cells were also cultured in a defined medium, containing glucose (10 g L−1) as the only carbon and energy source. The composition of the defined medium used during shake flask cultivations was the one described by Luttik et al. (2000). The medium contained (in g L−1): NH2CONH2 (urea), 2.3; KH2PO4, 3.0; K2SO4, 6.6; MgSO4·7H2O, 0.5; and trace elements consisting of (mg L−1) EDTA, 15, ZnSO4·7H2O, 4.5, MnCl2·2H2O, 0.84; CoCl2.6H2O, 0.3; CuSO4·5H2O, 0.3; Na2MoO4·2H2O, 0.4; CaCl2·2H2O, 4.5, FeSO4·7H2O, 3.0, H3BO3, 1.0, KI, 0.1. A solution containing vitamins was filter-sterilized and added to the medium to a final concentration of (mg L−1) d-biotin, 0.05; calcium pantothenate, 1.0; nicotinic acid, 1.0; myo-inositol, 25; thiamine·HCl, 1.0; pyridoxine.HCl, 1.0, and p-aminobenzoic acid, 0.20. The medium had its pH adjusted to 6.0 with addition of KOH.
Yeast and lactic acid bacteria cultivations
The effect of various inhibitors on the growth rate ×and on the lag phase of yeast strains was performed in 50 mL-falcon tubes, using 25 mL of the defined media described above, containing glucose as the only carbon and energy source. This defined media, containing a single inhibitory compound, was prepared using the concentrations reported in Table 2. For the physiological characterization of yeast strains in the presence or in the absence of p-coumaric acid (5 mM), cultivations were performed in 500-mL shake flasks, containing 100 mL of the defined media with glucose as the only carbon and energy source. Precultures for these experiments were grown overnight in an orbital shaker at 30 °C and 150 rpm in 250-mL shake flasks containing 50 mL of the defined medium plus glucose (without the addition of any inhibitory compound). All cultivations were inoculated to an initial OD600 of 0.3 and incubated in an orbital shaker at 30 °C and 150 rpm.
The growth kinetics of yeast and lactic acid bacteria strains were performed in the presence or in the absence of a combination of inhibitory compounds (denoted here as an “inhibitory cocktail”) containing a mixture of 76.6 mM acetic acid, 1.3 mM HMF, 7.1 mM furfural, and 1.9 mM p-coumaric acid. Precultures for these cultivations were grown overnight in an orbital shaker at 30 °C and 150 rpm in 250-mL shake flasks containing 50 mL of complex medium (YPD for yeasts and MRS for lactic acid bacteria) without the addition of any inhibitory compound. Cultivations were inoculated to an initial OD600 of 0.3 and performed in 250-mL shake flasks containing 50 mL of complex medium (YPD for yeasts and MRS for lactic acid bacteria) with and without the addition of the “inhibitory cocktail”. Flasks were incubated in an orbital shaker at 30 °C and 150 rpm.
Hydrolysate fermentations were performed with hydrolysate sample 1 and sample 2 (Table 2), both supplemented with 10 g yeast extract L− 1 and 20 g bacteriological peptone L− 1. In addition to that, sample 1 was also supplemented with glucose (~ 110 g L− 1) to match sample 2. In order to evaluate the fermentability of the hydrolysates, sample 1 was used for fermentations in two conditions: without pH adjustment (pH 3.9) and with pH adjustment (pH 4.9), using KOH. An additional set of fermentation experiments were performed with sample 2 in two conditions as well: without dilution and with dilution with demineralized water, by adding 0.75 volumes of hydrolysate and 0.25 volumes of water. Fermentations using strains CEN.PK113-7D and SA_1 were performed in 50 mL-falcon tubes, containing 25 mL of the of these treated hydrolysate samples. For that, precultures were previously grown overnight in an orbital shaker at 30 °C and 150 rpm in 250 mL shake flasks containing 50 mL of YPD. Fermentations were inoculated to an initial OD600 of 1.0 with each strain separately and incubated in an orbital shaker at 30 °C and 250 rpm. Samples were collected for glucose, glycerol and ethanol quantifications.
Determination of biomass concentration
During cultivations, biomass concentration was indirectly assessed using optical density measurements performed on culture samples, using a spectrophotometer, at 600 nm (OD600). During shake flask cultivations, cell concentration was indirectly determined by spectrophotometry and the data were converted into cell concentration in terms of grams dry cell weight per volume. For this purpose, in each cultivation the final sample had both its OD600 and its dry cell weight determined. Dry weight was determined gravimetrically, in duplicate, by filtering a 10 mL culture sample through a 0.45 μm (for yeasts) or 0.22 μm (for lactic acid bacteria) filter membrane. The filter was subsequently washed with distilled water, dried in a microwave oven and weighed (Olsson and Nielsen 1997).
Analytical determinations
Culture supernatants and hydrolysate samples for analytical determinations were obtained by centrifugation and used for determining the concentrations of sugars (glucose), organic acids, furan derivatives, ethanol, and glycerol. The compounds were separated by HPLC using an HPX-87H ion-exchange column (Bio-Rad) at 60 °C, with 5 mM H2SO4 as the mobile phase, at a flow rate of 0.6 L min−1. Glucose, ethanol, acetate, glycerol, succinate, and lactate were detected using refractive index detector.
The determination and quantification of phenolic compounds in hydrolysate samples were based on the modified methodology proposed for Kammerer et al. (2004), samples were analysed via high performance liquid chromatography with Supelco Inc. C18 column waters Spherisorb ODS-25 5 µm, 250 mm × 4.6 mm, the mobile phase used were compound for 2% (v/v) acetic acid in water (eluent A) and 0.5% acetic acid in water and acetonitrile (50:50, v/v; eluent B) using a gradient program: 10–15% B (10 min), 15% B isocratic (3 min), 15 to 25% B (7 min), 25 to 55% B (30 min), 55 to 100% B (1 min), 100% B isocratic (5 min), 100 to 10% B (0.1 min). The total run time was 60 min, with 1.0 mL/min flow rate and oven temperature of 30 °C. The injection volume for all samples was 10 µL. Monitoring was performed with a Shimadzu UV detector at wavelengths of 280 nm and 320 nm simultaneously. The concentrations of the compounds were calculated from calibration curves obtained from standard solutions. All analytical quantifications were performed as single injections.
MALDI-TOF calibration, sample preparation, and MS analysis
Identification of the lactic acid bacteria was performed according to the protocol described by Avanzi et al. (2017) (Avanzi et al. 2017). MS calibration was performed using protein calibration standard I. Equipment calibration and spectra acquisition had the same method established following Bruker Daltonics standards. Each spectrum had 240 reads from 50 laser shots from different positions (automated mode) in positive linear mode. Single colonies were used for mass spectra analysis in a MALDI Biotyper Ultraflextreme (Bruker Daltonics, Bremen, Germany). A previous step of protein extraction was necessary in order to increase the spectra quality. This protein extraction was performed by adding 50 µL of 70% formic acid directly in the bacterium colony, followed by addition of 30 µL acetonitrile and sonication. The cell extract was transferred to the MALDI Biotyper polished steel target plates followed by addition of 1 µL of α-cyano-4-hydroxycinnamic acid in saturated solution with 50% acetonitrile and 2.5% trifluoroacetic acid. The Biotyper 3.0 software compared the obtained spectra with a reference database spectra from Bacteria, Archaea, and Eukarya domains (total of 5625 species) and expressed the resulting similarity value as a log score. A score higher than 2.0 indicated identification of species, a score higher than 1.7 indicated genus identification, whereas any score under 1.7 meant no significant similarity of the spectrum with any database entry.
Calculation of physiological parameters, graph generation and statistical analysis
The maximum specific growth rate was obtained by plotting the natural logarithm of OD600 values against time and then calculating the slope of the straight line corresponding to the exponential growth phase (by linear regression). The lag phase was estimated visually, when the slope of the natural logarithm data presented a positive value. Yield coefficients (conversion yield) were calculated as the absolute value of the slope of a straight line: the biomass yield (Ysx) from a plot of cell concentration data against substrate concentration data (for this purpose the OD600 values were first converted into grams dry cell weight per volume) and the product (ethanol or glycerol) yield (Yse or Ysg) from a plot of product concentration data against substrate concentration data, as reported by Della-Bianca and Gombert (2013).
Graph generation and statistical analysis were performed using GraphPad Prism 7.00 (GraphPad Software, Inc.). Results are expressed as mean ± standard deviation or as mean ± deviation of the mean.
Results and discussion
Lignocellulosic inhibitors compounds present in industrial hydrolysates
Initially, we aimed to identify and to quantify major lignocellulosic inhibitory compounds in various sugarcane bagasse hydrolysates obtained by two different sources. For that, we used high-performance liquid chromatography in reverse phase and normal phase, depending on the class of the compound analysed. Thus, the most abundant organic acid found in the samples was acetic acid, as reportedly previously (Palmqvist and Hahn-Hägerdal 2000). Similarly, the main phenolic compound was p-coumaric acid, whereas furan derivatives found were HMF and furfural (Table 2). These results are in agreement with a detailed study published on sugarcane bagasse hydrolysates (van der Pol et al. 2014).
Effect of main lignocellulosic inhibitory compounds on industrial and laboratorial S. cerevisiae strains
Expanding the global production of lignocellulosic ethanol requires microorganisms with increasing resistance to pre-treatment process inhibitors (Almeida et al. 2007; Adeboye et al. 2014). Understanding how these molecules impact on the performance of S. cerevisiae is of paramount importance to implement strategies to increase its robustness toward lignocellulosic inhibitory compounds, such as evolutionary engineering. In this sense, we deemed to investigate in a systematic fashion the physiological effects of major organic acids (acetic and levulinic acid), phenolic compounds (p-coumaric and ferulic acid), and furan derivatives (HMF and furfural) found in sugarcane-based hydrolysates on S. cerevisiae strains that are normally found in Brazilian ethanol plants (Basso et al. 2008). For that purpose, four strains, including two laboratory (CEN.PK113-7D and CEN.PK112, the haploid and the diploid versions, respectively) and two industrial (SA-1 and JAY270, a derivative of PE-2) strains, were evaluated in the presence of these inhibitors. The impact of these compounds on quantitative physiological parameters, such as maximum specific growth rate and duration of the lag phase, were assessed in defined medium supplemented with individual compounds. Concentration thresholds were based on previous studies performed by our group as well as on literature data (van der Pol et al. 2014; Adeboye et al. 2014).
Furans derivatives
During the pre-treatment process, the thermal oxidation of pentoses and hexoses lead to the formation of 2-furaldehyde (furfural) and 5-hydroxymethyl furfural (HMF), respectively (Palmqvist and Hahn-Hägerdal 2000). In cultures containing 20 mM furfural, CEN.PK113-7D and CEN.PK112 presented the largest extension of the lag phase in relation to the remaining industrial yeasts. In this condition, SA-1 exhibited the lowest lag phase elongation (Table 3). In addition, none of the strains could grow at 40 mM furfural.
Despite the increase of the lag phase in the laboratory strains, their growth rates were higher when compared with the two industrial strains (Fig. 1). This observation seems to be related to the difference in how these yeasts metabolize furfural. The laboratory strains, on one hand, only grew after complete exhaustion of furfural from the growth medium. The industrial strains, on the other hand, resumed growth before exhaustion of furfural (data not shown). According to Almeida et al. (2007), the growth observed in the presence of furan derivatives is due to the possibility of S. cerevisiae to convert furfural and HMF to less toxic alcohols. The reduction of furfural is preferentially dependent on NADH, and the reduction of HMF has been mainly associated with the consumption of NADPH (Wahlbom and Hahn-Hägerdal 2002). These reductions provide NADP + sufficient for NADPH regeneration, maintaining a redox balance during the detoxification of these compounds (Liu et al. 2009). Overall, growth rates were reduced for all strains when compared to the control condition, according to previous investigations around furfural (Azhar et al. 1981; Boyer et al. 1992; Navarro 1994).
Growth rate of laboratory (CEN.PK113-7D and CEN.PK112) and industrial (SA-1 and JAY270) yeast strains cultured in the presence of major lignocellulosic inhibitory compounds encountered in sugarcane bagasse hydrolysate. Results are given as average values from duplicate experiments and error bars represent the deviation of the mean
Strain CEN.PK113-7D did not grow at both concentrations of HMF tested (16 and 32 mM). The diploid strain, on the other hand, was able to grow at 16 mM, albeit with 1/3 of the growth rate without HMF and presenting a slight elongation of the lag phase (Table 3). Industrial strains grew on the two concentrations tested, and lag phase was only slightly delayed. Remarkably, only the industrial strains grew at the highest concentration tested (32 mM).
Organic acids
Organic acids released from the pre-treatment of lignocellulosic materials are known as weak acids, due to their moderate values of pka, being 4.25 for acetic acid, 3.75 for formic acid (Adeboye et al. 2014), and 4.66 for levulinc acid (Soni et al. 1982), for example. Upon entering the cell, via the plasma membrane, weak acids dissociate and lead to an increase in cell intracellular pH (Pampulha and Loureiro-Dias 1990). To restore intracellular pH, cells export protons at the expense of ATP (Larsson et al. 1999). This mechanism is responsible for the reduced growth rate often observed in cells exposed to weak acids (Larsson et al. 1999; Caspeta et al. 2015). In the present investigation, none of the strains could grow at 200 mM acetic acid or 200 mM levulinic acid. 50 mM acetic acid in non-buffered medium exhibited an elevated toxic effect on cell growth, and SA-1 was the only strain able to grow under these conditions. In cultures containing 25 mM of levulinc acid, the inhibitory effect was very intensive for all strains, except for SA-1 (Fig. 1).
Cunha et al. (2015) reported that concentrations of 1.9 to 3.0 g L−1of weak acid (like acetic acid) in laboratory (CCUG53310) and industrial (PE-2) yeasts produce a large inhibitory effect, two mechanisms are proposed to explain this fact according to (Russell 1992). The first theory proposes that accumulation of the dissociated form of acid in the cell (due to the low extracellular pH) leads to intracellular acidification and consequent toxic effects at various levels of cellular metabolism. The second, known as decoupling theory, states that the decrease in cytoplasm pH resulting from weak acid flux activates ATP-dependent proton pumps to neutralize pH, leading to ATP depletion.
Phenolic compounds
Analyzing the influence of the phenolic compounds in the growth rate of the strains, we observed that, in general, the industrial strains showed a better performance in comparison to the laboratory strains. Strain SA-1 was again superior as compared to the other strains, presenting no elongation in the lag phase and its maximum specific growth rate was virtually unaffected by the presence of the inhibitory compounds, except for 7 mM p-coumaric acid, that caused a reduction of 30% as compared to the control condition (Fig. 1, Table 3). In accordance with previous results reported by Adeboye et al. (2014), the presence of these type of phenolic compounds did not lead to elongation of the lag phase, but only reduction in the growth rates, at a dose-dependent manner.
During the experiments performed with ferulic acid, strain CEN.PK113-7D was the most affected. This effect can be observed by an elongation of the lag phase at 1.3 mM ferulic acid. Surprinsinly, an increase in the concentration of ferulic acid from 0.4 to 1.3 mM did not cause a further decrease in the growth rate. These data demonstrate the different inhibition profiles among the phenolic compounds used, also verified by Adeboye et al. (2014). The inhibitory effect of phenolic compounds is a function of the combination of the occurrence of functional side groups (such as methoxy groups) and the presence of unsaturated bonds in the structure of the compound (Adeboye et al. 2014). Ferulic acid and p-coumaric acid are both caracterizated to have 2 carbon atoms sharing a double bond and linking the carboxylic group to the aromatic ring. They only differ in the presence of a methoxy group present in the aromatic ring, in the case of ferulic acid.
Effect of p-coumaric acid on yeast physiology
We also aimed to investigate the effects of p-coumaric acid, the major phenolic compound found in sugarcane lignocellulosic materials, on the physiology of industrial yeast strains. For that, strains of S. cerevisiae, including one laboratory (CEN.PK113-7D) and two industrial strains (JAY270 and SA-1), were grown in the presence of this compound, in defined medium. The presence of 5 mM p-coumaric acid resulted in a lower growth rate of strain CEN.PK 113-7D (Fig. 2) as compared to the industrial strains. Under these conditions, biomass yields were higher in JAY270 and SA-1 when compared to the lab strain, whereas ethanol yield was lower in the industrial strains as compared to the lab one (Fig. 2). Interestingly, the growth of the laboratory strain and the industrial JAY270 strain were virtually abolished at 7 mM p-coumaric acid, whereas SA-1 was still able to grow under this condition, with a maximum specific growth rate of 0.20 h−1 (data not shown). Adeboye et al. (2014) reported that p-coumaric acid presented a dose-dependent inhibition to growth rate on industrial strain Ethanol Red, cultured in synthetic media with glucose as the carbon and energy source. Moreover, p-coumaric acid completely arrested yeast growth at 9.7 mM.
Effect of p-coumaric acid on the physiology of laboratory and industrial yeast strains. Growth rates and yield coefficients in defined medium containing 5 mM of p-coumaric acid for strains CEN.PK113-7D (black bar), JAY270 (white bar), and SA-1 (grey bar). Legend: *p < 0.05 vs. CEN.PK113-7D (unpaired Student’s t test). Ysx, biomass yield; Yse, ethanol yield; Ysg, glycerol yield. Results are given as average values from triplicate experiments and error bars represent the standard deviation
Differential effects of lignocellulosic inhibitors between yeast and lactic acid bacteria
In industrial processes to produce ethanol from sugarcane, yeasts are not the only players. Lactic acid bacteria are present in virtually all industrial plants (Costa et al. 2008; Basso et al. 2008; Della-Bianca et al. 2013), and will certainly be present in second-generation processes (Collograi et al. 2019). Therefore, 4 industrial lactic acid bacteria isolates from first generation process were evaluated in the presence of selected inhibitors. The lactic acid bacteria strains were firstly identified using MALDI Biotyper as being L. paracasei, except for LAB1 which was not identified by this method (Table 4). They were also characterized, in terms of conversion yields, in MRS medium.
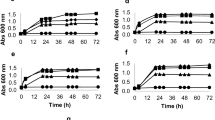
The growth kinetics of lactic acid bacteria LAB1, LAB2, LAB3 and LAB4, in the presence of an inhibitory cocktail containing a mixture of 76.6 mM acetic acid, 1.3 mM HMF, 7.1 mM furfural, and 1.9 mM p-coumaric acid, was analysed. The formulation of this cocktail was based on the compounds identified in hydrolysate samples and literature data (van der Pol et al. 2014). Whilst the two yeast strains, including the industrial one, were unable to grow under such conditions (Fig. 3e, f), bacteria had an average inhibition of roughly 50% on their growth rates (Fig. 3a–d). Analysing the fraction of inhibition in relation to the control (expressed as the relation between growth rate with inhibitors/ growth rate without inhibitors) for each isolate we could find 0.56, 0.50, 0.21 and 0.47 for the isolates LAB1, LAB2 LAB3 and LAB4, respectively. L. paracasei is part of the L. casei group, that consists of obligatory homofermentative and facultative heterofermentative bacteria (De Angelis and Gobbetti 2011).
Effect of inhibitory compounds on the growth kinetics of industrial lactic acid bacteria and the yeasts S. cerevisiae CEN.PK-1137D and SA-1. Cultivations were performed using MRS, in the case of LAB1-4 (a–d, respectively), or YPD, in the case of S. cerevisiae CEN.PK113-7D (e) and S. cerevisiae SA-1 (f), supplemented with (closed symbols) or without (open symbols) a cocktail of lignocellulosic inhibitors containing (in g L−1): 4.6 acetate, 0.17 HMF, 0.68 furfural, and 0.31 p-coumaric acid). Results are given as average values from duplicate experiments and error bars represent the deviation of the mean
The interest in the study of lactic acid bacteria in industrially relevant conditions for the production of biofuels is due to the fact that such strains are tolerant to the various stresses present in these processes, such as high concentrations of ethanol and salts, and extremes of pH and temperature (Bosma et al. 2017). In this way, it is pertinent to evaluate their performance (in terms of growth) in the presence of lignocellulosic inhibitors.
The inhibitory effects of sugarcane-based lignocellulosic hydrolysates on S. cerevisiae strains
In order to test the fermentability of the hydrolysate samples investigated in this study, two of the samples (samples 1 and 2, Table 2), containing high concentrations of HMF, furfural, acetic acid, and p-coumaric acid, were tested. For this purpose, strain CEN.PK113-7D and strain SA-1 were inoculated with a starting OD600 of 1.0 in the hydrolysates, that were previously supplemented with yeast extract (10 g L−1) and peptone (20 g L−1).
Sample 1 was also supplemented with glucose (~ 110 g L−1) in order to have a similar concentration as the other hydrolysate (sample 2). In fermentations performed with this sample, glucose was fully consumed within 80 h by both strains, and ethanol reached around 50 g L−1. Surprisingly, CEN.PK113-7D consumed glucose faster than the industrial strain (Fig. 4a), but ethanol yield was virtually identical for both strains (0.43 ± 0.01 g g−1 for CEN.PK113-7D, and 0.43 ± 0.00 g g− 1 for SA-1). When pH was adjusted from 3.9 to 4.9 with KOH addition, glucose was consumed within 20 h, and no difference in terms of glucose consumption and ethanol production was observed between the strains (Fig. 4b).
Fermentation of sugarcane-based lignocellulosic hydrolysates by S. cerevisiae CEN.PK113-7D and SA-1. a Hydrolysate (sample 1) supplemented with glucose without pH adjustment (pH 3.9); b hydrolysate (sample 1) supplemented with glucose with pH adjustment (pH 4.9); c hydrolysate (sample 2) without pH adjustment (pH 4.8); d hydrolysate (sample 2) diluted with water (hydrolysate/water, 3:1, by vol.). Glucose (closed symbols) and ethanol (open symbols) concentrations in fermentations performed by CEN.PK113-7D (circles) and SA-1 (squares) strains. Results are given as average values from duplicate experiments and error bars represent the deviation of the mean
In fermentations performed with the other hydrolysate (sample 2), glucose consumption and ethanol production were not observed by both strains (Fig. 4c). Glucose was only consumed in this medium when the hydrolysate was diluted with water, by adding 0.75 volumes of hydrolysate and 0.25 volumes of water. In this condition, ethanol titres were slightly higher in fermentations performed with SA-1 than with CEN.PK113-7D (Fig. 4d). It is worth mentioning that glycerol titres were lower in fermentations performed with the industrial strain (2.13 ± 0.08 g L−1) when compared to the laboratorial strain (2.56 ± 0.27 g L−1), which could be an indicative of a reduced stress response in the former strain (van Dijken et al. 2000; Basso et al. 2008).
Conclusions
The present investigation identified, and quantified major lignocellulosic inhibitory compounds present in industrial samples of sugarcane bagasse hydrolysates. Their effects on important physiological parameters were evaluated in laboratory and industrial S. cerevisiae yeast strains, that could serve as candidate microbial platforms in the 2G ethanol industry. Therefore, we conclude that robust 1G yeast strains might be ideal candidates for lignocellulosic processes. However, lactic acid bacteria will certainly be a source of concern in 2G processes due to their potential resistance toward these lignocellulosic inhibitory compounds as compared to yeast strains.
References
Adeboye PT, Bettiga M, Olsson L (2014) The chemical nature of phenolic compounds determines their toxicity and induces distinct physiological responses in Saccharomyces cerevisiae in lignocellulose hydrolysates. AMB Express 4:1–10. https://doi.org/10.1186/s13568-014-0046-7
Almeida JR, Modig T, Petersson A et al (2007) Increased tolerance and conversion of inhibitors in lignocellulosic hydrolysates by Saccharomyces cerevisiae J Chem Technol Biotechnol 82:340–349. https://doi.org/10.1002/jctb.1676
Argueso JL, Carazzolle MF, Mieczkowski PA et al (2009) Genome structure of a Saccharomyces cerevisiae strain widely used in bioethanol production. Genome Res 19:2258–2270. https://doi.org/10.1101/gr.091777.109
Auesukaree C, Damnernsawad A, Kruatrachue M et al (2009) Genome-wide identification of genes involved in tolerance to various environmental stresses in Saccharomyces cerevisiae J Appl Genet 50:301–310. https://doi.org/10.1007/BF03195688
Avanzi IR, Gracioso LH, Baltazar M, PG et al (2017) Rapid bacteria identification from environmental mining samples using MALDI-TOF MS analysis. Environ Sci Pollut Res 24:3717–3726. https://doi.org/10.1007/s11356-016-8125-8
Azhar AF, Bery MK, Colcord AR et al (1981) Factors affecting alcohol fermentation of wood acid hydrolysate. Biotechnol Bioeng 11:293–300
Basso LC, de Amorim HV, de Oliveira AJ, Lopes ML (2008) Yeast selection for fuel ethanol production in Brazil. FEMS Yeast Res 8:1155–1163. https://doi.org/10.1111/j.1567-1364.2008.00428.x
Basso TO, Gomes FS, Lopes ML et al (2014) Homo- and heterofermentative lactobacilli differently affect sugarcane-based fuel ethanol fermentation. Antonie van Leeuwenhoek Int J Gen Mol Microbiol 105:169–177. https://doi.org/10.1007/s10482-013-0063-6
Benjaphokee S, Hasegawa D, Yokota D et al (2012) Highly efficient bioethanol production by a Saccharomyces cerevisiae strain with multiple stress tolerance to high temperature, acid and ethanol. N Biotechnol 29:379–386
Bosma EF, Forster J, Nielsen AT (2017) Lactobacilli and pediococci as versatile cell factories—evaluation of strain properties and genetic tools. Biotechnol Adv 35:419–442. https://doi.org/10.1016/J.BIOTECHADV.2017.04.002
Boyer LJ, Vega JL, Klasson KT et al (1992) The effects of furfural on ethanol production by Saccharomyces cerevisiae in batch culture. Biomass Bioenergy 3:41–48. https://doi.org/10.1016/0961-9534(92)90018-L
Caspeta L, Nielsen J (2015) Thermotolerant yeast strains adapted by laboratory evolution show trade-off at ancestral temperatures and preadaptation to other stresses. MBio. https://doi.org/10.1128/mBio.00431-15
Caspeta L, Castillo T, Nielsen J (2015) Modifying yeast tolerance to inhibitory conditions of ethanol production processes. Front Bioeng Biotechnol 3:184. https://doi.org/10.3389/fbioe.2015.00184
Collograi KC, da Costa AC, Ienczak JL (2019) Effect of contamination with Lactobacillus fermentum I2 on ethanol production by Spathaspora passalidarum Appl Microbiol Biotechnol 103:5039–5050. https://doi.org/10.1007/s00253-019-09779-y
Costa VMM, Basso TOO, Angeloni LHPHP et al (2008) Production of acetic acid, ethanol and optical isomers of lactic acid by Lactobacillus strains isolated from industrial ethanol fermentations. Cienc E Agrotecnol 32:503–509
Cunha JT, Aguiar TQ, Romaní A, Oliveira C, Domingues L (2015) Contribution of PRS3, RPB4 and ZWF1 to the resistance of industrial Saccharomyces cerevisiae CCUG53310 and PE-2 strains to lignocellulosic hydrolysate-derived inhibitors. Bioresour Technol 191:7–16
De Angelis M, Gobbetti M (2011) Lactic Acid bacteria – Lactobacillus spp.: general characteristics, encyclopedia of dairy sciences, 2nd edn. pp 78–90
Della-Bianca BE, Basso TO, Stambuk BU et al (2013) What do we know about the yeast strains from the Brazilian fuel ethanol industry? Appl Microbiol Biotechnol 97:979–991. https://doi.org/10.1007/s00253-012-4631-x
Della-Bianca BE, Gombert AK (2013) Stress tolerance and growth physiology of yeast strains from the Brazilian fuel ethanol industry. Antonie van Leeuwenhoek Int J Gen Mol Microbiol 104:1083–1095. https://doi.org/10.1007/s10482-013-0030-2
Kammerer D, Claus A, Carle R, Schieber A (2004) Polyphenol screening of pomace from red and white grape varieties (Vitis vinifera L.) by HPLC-DAD-MS/MS. J Agric Food Chem. https://doi.org/10.1021/JF049613B
Klinke HB, Thomsen AB, Ahring BK (2004) Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl Microbiol Biotechnol 66:10–26. https://doi.org/10.1007/s00253-004-1642-2
Lam FH, Ghaderi A, Fink GR, Stephanopoulos G (2014) Engineering alcohol tolerance in yeast. Science. https://doi.org/10.1126/science.1257859
Larsson S, Palmqvist E, Hahn-Hägerdal B et al (1999) The generation of fermentation inhibitors during dilute acid hydrolysis of softwood. Enzyme Microb Technol 24:151–159. https://doi.org/10.1016/S0141-0229(98)00101-X
Liu ZL, Ma M, Song M (2009) Evolutionarily engineered ethanologenic yeast detoxifies lignocellulosic biomass conversion inhibitors by reprogrammed pathways. Mol Genet Genomics 282:233–244. https://doi.org/10.1007/s00438-009-0461-7
Luttik MAH, Kötter P, Salomons FA, van der Klei IJ, van Dijken JP, Pronk JT (2000) The Saccharomyces cerevisiaeICL2 gene encodes a mitochondrial 2-methylisocitrate lyase involved in propionyl-coenzyme a metabolism. J Bacteriol 182(24):7007–7013
Navarro AR (1994) Effects of furfural on ethanol fermentation by Saccharomyces cerevisiae: mathematical models. Curr Microbiol 29:87–90. https://doi.org/10.1007/BF01575753
Olsson L, Nielsen J (1997) On-line and in situ monitoring of biomass in submerged cultivations. Trends Biotechnol 15:517–522. https://doi.org/10.1016/S0167-7799(97)01136-0
Palmqvist E, Hahn-Hägerdal B (2000) Fermentation of lignocellulosic hydrolysates. I: inhibition and detoxification. Bioresour Technol 74:17
Pampulha ME, Loureiro-Dias MC (1990) Activity of glycolytic enzymes of Saccharomyces cerevisiae in the presence of acetic acid. Appl Microbiol Biotechnol 34:375–380. https://doi.org/10.1007/BF00170063
Reis VR, Bassi APG, Cerri BC et al (2018) Effects of feedstock and co-culture of Lactobacillus fermentum and wild Saccharomyces cerevisiae strain during fuel ethanol fermentation by the industrial yeast strain PE-2. AMB Express 8:23. https://doi.org/10.1186/s13568-018-0556-9
Russell JB (1992) Another explanation for the toxicity of fermentation acids at low pH: anion accumulation versus uncoupling. J Appl Bacteriol 73:363–370. https://doi.org/10.1111/j.1365-2672.1992.tb04990.x
Skerker JM, Leon D, Price MN et al (2013) Dissecting a complex chemical stress: chemogenomic profiling of plant hydrolysates. Mol Syst Biol 9:674. https://doi.org/10.1038/msb.2013.30
Soni ML, Meehrotra RN, Kapoor RC (1982) Dissociation constant and some thermodynamic parameters of leavulinic acid. Indian J Chem 21:1960197
van den Broek M, Bolat I, Nijkamp JF et al (2015) Chromosomal copy number variation in Saccharomyces pastorianus is evidence for extensive genome dynamics in industrial lager brewing strains. Appl Environ Microbiol 81:6253–6267. https://doi.org/10.1128/AEM.01263-15
van der Pol EC, Bakker RR, Baets P, Eggink G (2014) By-products resulting from lignocellulose pretreatment and their inhibitory effect on fermentations for (bio)chemicals and fuels. Appl Microbiol Biotechnol 98:9579–9593. https://doi.org/10.1007/s00253-014-6158-9
van Dijken JP, Bauer J, Brambilla L et al (2000) An interlaboratory comparison of physiological and genetic properties of four Saccharomyces cerevisiae strains. Enzyme Microb Technol 26:706–714
van Maris AJA, Abbott DA, Bellissimi E et al (2006) Alcoholic fermentation of carbon sources in biomass hydrolysates by Saccharomyces cerevisiae: current status. Antonie Van Leeuwenhoek Int J Gen Mol Microbiol 90:391–418. https://doi.org/10.1007/S10482-006-9085-7
Wahlbom CF, Hahn-Hägerdal B (2002) Furfural, 5-hydroxymethyl furfural, and acetoin act as external electron acceptors during anaerobic fermentation of xylose in recombinant Saccharomyces cerevisiae Biotechnol Bioeng 78:172–178. https://doi.org/10.1002/bit.10188
Zetty-Arenas AM, Alves RF, Portela CAF et al (2019) Towards enhanced n-butanol production from sugarcane bagasse hemicellulosic hydrolysate: Strain screening, and the effects of sugar concentration and butanol tolerance. Biomass Bioenergy 126:190–198. https://doi.org/10.1016/j.biombioe.2019.05.011
Acknowledgements
We would like to express our gratitude to Dr. Maria Anita Mendes and Dr. Claudio Augusto Oller do Nascimento for equipment and infrastructure, as well as for performing the identification of the bacterial isolates. We are very grateful to the excellent technical assistance performed by Thamiris Giacon. P.C. and D.P.P would like to thank the scholarship provided by CAPES and FAPESP, respectively. I.S.V and L.R.C. would like to thank the scholarship provided by CNPq.
Funding
This work was supported by Fundação de Apoio à Pesquisa do Estado de São Paulo (Grant Nos. 2015/50612-8; 2018/01759-4; 2018/17172-2) and also by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001.
Author information
Authors and Affiliations
Contributions
Conceptualization: [PC, DPP, TOB]; Methodology: [PC, BLVC, IVS]; Formal analysis and investigation: [PC, DPP, ATCA, LRC, TOB]; Writing—original draft preparation: [PC, DPP, TOB]; Writing—review and editing: [DPP, BLCV, TOB]; Funding acquisition: [TOB]; Resources: [TOB]; Supervision: [BLVC, TOB].
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cola, P., Procópio, D.P., Alves, A.T.C. et al. Differential effects of major inhibitory compounds from sugarcane-based lignocellulosic hydrolysates on the physiology of yeast strains and lactic acid bacteria. Biotechnol Lett 42, 571–582 (2020). https://doi.org/10.1007/s10529-020-02803-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-020-02803-6