Abstract
Objectives
The utilization of biotechnology in leather sector has more extensive in modern years; more particular to proteolytic enzymes and employed in several steps of the leather making such as soaking, dehairing, bating, solid waste management etc. The current study evaluates the performance of alkaline protease from Bacillus crolab MTCC 5468 in single soaking of goat skins matrix by comparing with the conventional multiple soaking processes.
Results
According to the obtained results, the optimum concentration for maximum rehydration of goat skins was accomplished at 1.0% (v/w) of alkaline protease at duration of 3 h over traditional rehydration method (4–6 h). The moisture level, total protein, chloride content and total organic carbon of enzymatic rehydration was superior to that of conventional rehydration and it was also used to measure the effectiveness of rehydration process. Scanning electron microscopic images of enzymatically processed leather exhibits enhanced opening of fiber bundles and smooth grain surface than conventional method. Furthermore, the alkaline protease treated leather exhibited improved moisture uptake, removal of chlorides and suppleness because of hydrolysis of non-collagenous proteins as indicated by well opened up fiber bundles in histological analysis.
Conclusions
The application of alkaline protease in rehydration operation of leather production confirmed scope for diminishing water quantity around 66.6%, soaking duration at 50%, minimizing use of harmful dehairing chemicals at 50–60%, thereby, eliminating the bating operation during pre-tanning. These outcomes suggest that alkaline protease have potential application in rehydration of skins for immense environmental concerns of leather tanning sectors.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The worldwide environment is increasingly deteriorating with period as a result of the socio-economic activities of manhood. The manufacturing industries more particularly leather tanning industry, cause contrary differences in the instantaneous environment and therefore, being dared by society. The leather processing industry has instigated an adverse effect on the environment. In beam house processes, leather making comprises a sequential unit processes that can be categorized into three groups: (i) pre-tanning, which clean and remove non-leather making proteins from the hide/skin; (ii) tanning process, which stabilize the hides/skins; and (iii) post-tanning and finishing procedures for camouflage properties to the leather products (Thanikaivelan et al. 2005). Generally, leather manufacturing includes numerous steps and several chemicals are added and a variety of components are expelled (Saikia et al. 2017). The major problems that arise in leather making industry are availability of good quality water and management of effluent. Approximately 30–35 m3 wastewater is disposed to environment during the manufacturing of every 1 ton of raw hides/skins by world leather industry (Ahmed Basha et al. 2009). It is estimated that over 600 kg of wastes is produced for every ton of wet-salted hide, which results in 200–300 kg of leather (Ozgunay et al. 2007).
The leather making comprises of altering the raw hides/skins, a greatly perishable material, into stable leather and is appropriate for a wide variety of end applications. The raw hides/skins is made up of about 60% moisture content, 35% proteins (both fibrous and non-fibrous) fats, carbohydrate moieties and mineral salts (Zambare et al. 2013; Buljan et al. 1997). The fibrous protein collagen is the leather creating material. The different constituents of the animal hides/skins except the collagen are separated often damaging to the creation of good quality leathers. Conventionally, sodium chloride i.e., common salt (30–40%) is used for dehydration of hides/skins. These dehydrated hides/skins are sent to leather industries for further processing.
In leather making, the first unit operation performed in the leather industries is the soaking or rehydration in which the salt is removed and the goods are rehydrated to their original condition. Rehydration is generally accomplished either in drums or paddles. Typically, 300–400% water is used in each stage of rehydration along with small quantities of non-ionic wetting agents to hasten the soaking and a bactericide to prevent bacterial attack. The first change of water is commonly stated to as dirt soaking which removes adhering blood, dung and partial removal of salt. Second or third changes of water are necessary to bring back the raw material to the original form which eliminates complete salt and inter-fibrillar proteins such as albumin, globulin etc. The soak liquor discharged from traditional rehydration process which comprises a substantial concentration of soluble protein, 500–1000 mg/L; fats, 80–155 mg/L; muco-polysaccharides, 120–230 mg/L; and TDS, 30,000–70,000 mg/L (Maharaja et al. 2019). Traditionally, the rehydration takes about 8–20 h for wet salted skins (Sharphouse 1983) and 24–48 h for dried skins (Sarkar 1991).
Microbial enzymes have been broadly used in leather making such as soaking, dehairing, bating and degreasing processes. Currently, enzyme-mediated leather processing is being employed in several tanning industries due to its better environmental performance (Kandasamy et al. 2012; Senthilvelan et al. 2012). As an alternative to conventional rehydration the eco-benign enzymatic soaking was studied. In earlier studies, the proteolytic enzymes obtained from A. flavus and B. subtilis were used for soaking and some amylolytic enzymes were also seen to exhibit rehydration properties (Kamini et al. 1999). Benefits of enzymes during rehydration are that, this method loosens the scud, initiates the opening up of fiber bundles, produces wrinkle free grain surface, improves softness, improves pliability, increases area yield and reduces the processing time. Thus, this work focuses on the application of alkaline protease from a novel Bacillus crolab MTCC 5468 in single effective rehydration of goat skins with reduced water quantity, time duration and eliminating the bating process in pre-tanning; thereby providing good quality leather with environmental benefits.
Materials and methods
Materials
-
1.
Alkaline protease acquired from novel bacteria named Bacillus crolab MTCC 5468 was formerly isolated in the laboratory with an accession number HM446165 from IMTECH, Chandigarh, India.
-
2.
The crude alkaline protease was prepared by solid state fermentation using single solid substrate (wheat bran alone).
-
3.
Raw salted goat skins for rehydration operation were processed from local traders.
-
4.
All the chemicals used in this work were of analytical and commercial grade.
Methods
Alkaline protease by solid state fermentation (SSF)
The optimization parameters for maximum production of enzyme were formerly standardized in the laboratory. The alkaline protease was obtained by solid state fermentation using 500 g wheat bran alone was moistened with 1000 mL double distilled water (i.e. 1.0:2.0 ratio) and it was autoclaved at a temperature 120 °C at 15 lbs for 15 min. The sterilized wheat bran in tray was then inoculated with 30% (v/w) of 24 h old bacterial culture containing a cell count of 1.9 × 10−8 cells/mL prepared in nutrient broth. This was then incubated at 37 °C for 96 h. At the end of fermentation process, the enzyme was extracted from fermented wheat bran using homogenization in tenfold quantity of 0.2 M carbonate-bicarbonate buffer at pH 9.0 followed by centrifugation to obtain the crude alkaline protease. The enzyme activity and protein content of alkaline protease was calculated using standard methods (Ranjithkumar et al. Ranjithkumar et al. 2017a, b).
Protease assay
Proteolytic activity was measured by the Kunitz method (1947). Concisely, to 0.9 mL of 0.2 M carbonate- bicarbonate buffer, pH 9.0, 0.1 mL of crude alkaline protease and 1 mL of 1% casein were added. A 3.0 mL of 10% trichloroacetic acid (TCA) was added to enzyme blank before adding the substrate. The enzyme samples and the blank were incubated for 10 min at 37 °C followed by deactivating the reaction of enzyme sample by TCA addition. Then the samples were filtered through Whatman No. 1 filter paper. In a separate tube, a 0.1 mL aliquot of this filtrate was made up to 1 mL using double distilled water and to this was added 0.2 mL of copper sulphate (5 mM), 1 mL sodium hydroxide solution (0.245 N), 1 mL sodium carbonate (4.9%) and 0.2 mL sodium potassium tartrate (0.25%) and kept at room temperature for 15 min after vortexing. To this 0.5 mL of Folin’s phenol reagent was mixed and incubated in dark for 20 min. After incubation, the blue color developed was then read at 640 nm using UV-spectrophotometer. One unit of proteolytic activity (U) was defined as microgram of tyrosine liberated per minute per mL of the crude enzyme under standard assay conditions.
Protein content of alkaline protease
Protein content of the alkaline protease was estimated by Lowry’s method (1951). A 0.2 mL of alkaline protease was made up to 1.0 mL using double distilled water. To this diluted alkaline protease (crude), 0.2 mL of copper sulphate and 1.0 mL of sodium hydroxide solution was added. After vortexing, 1.0 mL of sodium bicarbonate and 0.2 mL of sodium potassium tartrate were added and incubated at room temperature for 15 min. To the tubes 0.5 mL Folin’s phenol reagent was added and incubated in dark for 20 min and water was used as blank. The blue color developed was read at 640 nm in spectrophotometer. The amount of protein present in the crude alkaline protease was determined using bovine serum albumin (BSA) as standard.
Application of alkaline protease in rehydration
The rehydration ability of the crude alkaline protease was assessed at laboratory scale. Two sets of experiments were carried out as shown in the Table.
S. No | Experiments | Process | Soaking agents |
|---|---|---|---|
Control | Three soaking | 1st soaking + 300% water | |
I | 2nd soaking + 300% water | ||
3rd soaking + 300% water | |||
II | Experiment | One soaking | 0.5% enzyme alone + 300% water |
III | 1.0% enzyme alone + 300% water | ||
IV | 1.5% enzyme alone + 300% water | ||
V | 2.0% enzyme alone + 300% water |
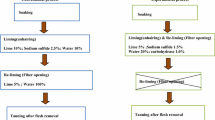
Salt preserved raw goat skins were taken and cut into five pieces of approximately 300 g (v/w) weight each, marked as I, II, III, IV, V and was taken for rehydration studies. Goat skin piece (I) was taken for conventional soaking (Control) and remaining (II, III, IV, V) for enzymatic rehydration method (Experiment). Three soaking was carried out for control. In the first soaking, the skins were soaked in 300% (v/w) water for one hour and the same was repeated for additional soaking process at every 1 h interval, in the case of control.
One time soaking was carried out for enzymatic rehydration, where the remaining goat skins were soaked in 300% (v/w) water with different concentrations of alkaline protease from Bacillus crolab MTCC 5468 (0.5, 1.0, 1.5 and 2.0% (v/w) alone) without any chemical additives. The percentages of inputs (protease enzyme) were calculated based on the mass of the skin and soaking experiment was performed at room temperature. Rehydration was conducted by pit method for both control and experimental goat skins and their action on goat skin was assessed at 1 h interval.
All pieces of goat skins were observed periodically for its rehydration, water uptake and softness of the goat skins during soaking (up to 7 h). At the end of every 1 h, the samples were collected and subjected to moisture analysis, chloride and protein content. Finally, the soaked goat skins were dehaired, limed and tanned using standard methods given in “Appendix 1”. The chrome tanned skins were further converted into crust leather as per the standard methods given in “Appendix 2” and were evaluated for their physical and organoleptic properties.
Moisture analysis
The moisture analysis of both control and experimental rehydrated skin samples was collected up to 7 h at 1 h interval were determined by A.P.H.A method (1985). 1 g of soaked skin was taken on previously weighed concave glass and was kept in a hot air oven at 105 °C for about 5 h. After drying, it was cooled in a desiccator and weighed.
The % of moisture content of the soaked goat skin samples was calculated using the following formula
.
Chloride (Cl−) content
The salinity profile of control and experimental soak liquor was collected at every 1 h interval and studied by Argentometric method. A 10 mL of processed liquor sample were taken and neutralized using dilute H2SO4 (pH 7.0–8.0). To this add 1.0 mL of potassium dichromate (K2Cr2O7) indicator and mix well and titrate against standard silver nitrate (AgNo3) solution till AgCrO4 starts precipitating as pale red precipitate at end point.
where A is the AgNO3 required for sample, B is AgNO3 required for blank, N is Normality of AgNO3.
Total organic carbon (TOC)
During soaking experiments, the soak liquor was collected at every 1 h intervals and investigated the concentration of total organic carbon (TOC) using a TOC elementar (Model Vario SELECT 39101002 Instrument). The purpose of these assays was to detect the elimination of organic matter (lipids, keratin and non-collagenous proteins from the extracellular matrix) from the goat skins and to recognize a more effectual process for skin cleaning (De Souza and Gutterres 2012).
Analysis of total protein content of soak liquors
After soaking, the spent liquor was collected at every 1 h interval from control and experiment soak liquor and centrifuged at 5000 rpm for 5 min. Then the total protein content in supernatant was estimated by Lowry’s method (1951) using BSA as a standard.
Chrome content of tanned leathers
The chromium content of both conventional and experimental wet blue leather was determined by standard IUC method to estimate their chromium uptake ability of conventional and enzymatically processed leathers. The results were expressed on dry weight basis of respective wet blue leathers (Mclaughlin and Theis 1945; IUC 8 1998).
Thermal stability of leathers
The chrome tanned control and experimental leathers were measured for shrinkage temperature according to the standard method (IUP 2 2000; IUP 16 2000).
Water vapour permeability
The crust leather from control and experiment were analysed for water vapour permeability and water absorption (IULTCS/IUP15 2012; Ranjithkumar et al. 2017a).
Capillary flow porometry analysis
The crust leathers were subjected to PMI capillary flow porometer to analyze the pore size and its distribution (Chen and Penumadu 2006; Fernando and Chung 2002).
Scanning electron microscopic analysis and EDX
The crust leather from control and experimental samples were cut from the official sampling position (IUP 2 2000) washed and fixed in formalin solution. Then samples were dehydrated using ethyl alcohol series and were dried. The dehydrated samples were then gold coated using Edwards E 306 sputter coater and were observed using FEI Quanta 200 High-Resolution Scanning Electron Microscope. Correspondingly, the control and enzyme treated crust leathers were subjected to energy-dispersive X-ray spectroscopy (EDX) analysis to reveal the presence of chromium and other elements. A JEOL JSM 5300 LV (Bruker) SEM with EDX was used to study the elemental distribution of the leather samples.
Histological studies
After soaking, the goat skins were conventionally dehaired and approximately 5 cm2 sizes were cut from official sampling portions and were prepared for histological analysis using standard method (Bancroft and Gamble 2004). Briefly, the pelt samples were washed thoroughly with double distilled water followed by 10% (v/w) formaldehyde fixation. After fixation, the pelt were consecutively dehydrated with 10%, 30%, 50%, 70%, 90% and 100% (v/v) of ethyl alcohol followed by xylene action and then dehydrated samples were fixed in paraffin wax. The samples were then sectioned (6 µm thickness) using microtome instrument (Leica). Thus, sectioned samples were stained using hematoxylin and eosin. The slides were then microscopically observed for histological structures pattern.
Analysis of spent liquors
At the end of rehydration operation, the spent soak liquors from both conventional and experimental rehydration processes were collected separately and were studied for their pollution parameters by measuring different parameters such as Biological Oxygen Demand, Chemical Oxygen Demand, Total Dissolved Solids and Total Suspended Solids as per the standard procedures (Clesceri et al. 1989).
Physical and organoleptic properties of crust leathers
The samples from control and experimental crust leathers were conditioned for 24 h at 20 °C and 65% relative humidity as per the standard procedure. The physical properties such as tear strength, tensile strength and % elongation at break and grain crack strength were measured (IUC 8, 1998). The bulk properties of crust leathers, such as grain appearance, grain smoothness, fullness and softness were evaluated by hand and visual examination by three experienced leather technologists.
Results and discussion
Alkaline protease from Bacillus crolab MTCC 5468
The proteolytic activity of alkaline protease from Bacillus crolab MTCC 5468 by solid state fermentation was found in the range of 38,000–42,000 U/gds (Fig. 1) and protein content was in the range of 75–92 mg/mL (Fig. 2). The crude alkaline protease was formerly studied at different temperatures (25–50 °C) and pH (6–12). The temperature for both alkaline protease production and bacterial growth was found to be in the range of 30–45 °C, while optimum enzyme production was perceived at 37 °C. With respect to pH, strain growth and production of alkaline protease was better over a wide range of pH 8–11, with maximal growth and enzyme activity is found at pH 9.0 (Ranjithkumar et al. 2017a, b).
Moisture content of leathers
The soaking of salted animal skins require longer periods (5–6 h) for maximum rehydration of skins. To reduce the soaking duration and water quantity, alkaline proteases have been employed in this study. The wet salted goat skins were soaked in 300% (v/w) water (one soaking) with various quantities of enzyme without any chemical additives and 300% (v/w) water alone was carried out for conventional soaking (Three soaking) and were observed intermittently for its moisture uptake capability as well as the softening of the goat skins. Results obtained showed that, the % of moisture content was improved for 0.5% (v/w) and 1.0% (v/w) protease (Fig. 3). Higher enzyme concentrations causes major removal of soluble proteins and looseness which may lead to decrease in the physical strength of goat skins. The better moisture level is due to the removal of non-leather forming proteins mainly globular proteins present in the skin matrix (Zambare et al. 2013). So, the most appropriate concentration of maximum rehydration was attained at 1.0% (v/w) alkaline protease and softening of goat skins was achieved at 3 h.
One time rehydration of goat skin (Experiment) with alkaline protease (1.0% (v/w)) results in the better removal of inter-fibrillar materials and clean pelt (elimination of salt, dirt, blood from the goat skin), because of the efficient enzymatic action, paving way for effective rehydration at 3 h, compared to conventional method with numerous soakings with longer duration. According to Jianzhong et al. (2013) the rehydration conditions have been maintained at pH 9.0, with added chemical additives for effective rehydration, while in this present study the enzymes solely was employed without any chemical additives and there is no separate soaking conditions to be maintained as aforementioned. Therefore, significant reduction of water quantity and duration of the rehydration process was observed when alkaline protease was employed.
Chlorides content in soak liquors
The soaking efficacy of the alkaline protease from Bacillus crolab MTCC 5468 on rehydration was evaluated by chloride analysis in the spent soak liquor. Usually, sodium chloride is used to protect the fresh goat skins from decay immediately after they are flayed in the slaughter house (Senthilkumar et al. 2011). The salt present in preserved skins has to be removed before further processing, which is accomplished by rehydration, using more water in traditional multiple soaking, thereby producing the first source of wastewater. The salinity profile of both control and enzymatic soak liquor are shown in Fig. 4. The results of the salt removal of single soaking with 1.0% (v/w) enzyme treated soak liquor was comparable to that of 1.5 and 2.0% (v/w). Control and 0.5% (v/w) enzyme treatment did not show significant removal of salt, compared to other enzyme concentrations. Therefore, the suitable concentration for maximum salt removal was attained at 1.0% (v/w) protease enzyme.
Protein content in soak liquors
In leather processing, alkaline proteases are used to remove elastic fibers and globular proteins. The effectiveness of the protease enzyme can be considered by determining the hydrolysis of non-leather making proteins from the skins (Fig. 5). The protein content of the enzymatically treated soak liquor is higher than that in the conventionally soak liquor. The protein content of the experimental soak liquor gradually increased up to 1.0% (v/w) enzyme treated liquor. Protein content of the soak liquor which is treated with 0.5% (v/w) enzyme is lesser when compared with other concentrations (1.0, 1.5 and 2.0% (v/w)). The protein content showed increasing tendency up to 3 h; beyond which no significant increase was observed. The maximum removal of protein was observed in 1.0% (v/w) of enzyme treated liquor at 3 h. The reason for the increased level of protein content in the enzymatic soaking is due to the removal of non-collagenous materials, facilitating better rehydration of the goat skin matrix.
Total organic carbon
The values of total organic content (TOC) in the spent soaking liquor were shown in the graphs (Fig. 6). Differences in organic matter removal were observed in the control and experiment. At the beginning (after 1 h), the enzymatic treatments showed superior performance than the conventional soaking. The organic content removal slowly increased when enzyme concentration increased from 0.5 to 1.0% (v/w) at every 1-h interval of enzymatic soaking than the control. The profile indicates a correlation between processing time and removal of soluble protein. Increasing the enzyme concentration (2.0% (v/w)) led to an insignificant increase of organic matter content in the spent soak liquor. This means that the excess of enzyme does not improve the release of organic matter. Durga et al. (2019) reported that use of carbohydrases (1.0% (v/w)) on rehydration of goat skin resulted in removal of TOC ~ 11,000 mg/L in 3 h. While in the present study, the better removal of TOC ~ 14,000 mg/L at the end of 3 h. The maximum value of total organic carbon is due to significant removal of organic matter present in the skin matrix; thereby, obtaining clean goat skin when alkaline protease was employed during rehydration. Hence, TOC analysis indicated substantial removal non-collagenous proteins from skin matrix in the enzymatic soaking (1.0% (v/w)) within shorter duration than conventional methods.
Chrome content of tanned leathers
The chrome content and shrinkage temperature of the tanned leathers of both control and experimental was determined and is shown in the Table 1. The results obtained from chromium analysis of enzymatically soaked skins were better when compared with control skins. 1.0% (v/w) enzyme treated wet blue leathers were comparable to that of remaining of enzyme treated wet blue leathers. This may be due to the opened up collagen bundles, better pore size, wherein more number of collagen cross linking sites were accessible for fixation with tanning operations. The chrome content in the conventionally soaked skins was less compared to enzymatic soaking.
Effluent analysis
The pollution parameters of both control and experimental soak liquor are presented in Table 2. As seen from the enzymatic rehydration, a substantial reduction was observed in pollution parameters such as chemical oxygen demand (COD), Total suspended solids and total dissolved solids over conventional rehydration. Furthermore, the soak liquors of enzymatic method have shown a decrease of total solids and COD approximately 80% and 55% of pollution load respectively and possible to reduce 60–65% water usage in rehydration leads to significant decrease of effluent discharge. Additionally, the practice of alkaline protease in rehydration process causes the marginal reduction of dehairing compounds such as Ca(OH)2 and sodium sulfide, leading to a major reduction in the environmental pollution parameters. So the results obtained from enzymatic method of leather making can be considered as a preeminent way for eco-benign process for environment.
Water vapour permeability of crust leathers
The water vapour permeability (WVP) of both conventional and experimental crust leathers are shown in Table 3. The result indicates that WVP of the enzymatically soaked goat skins was better than that of the conventionally soaked skins. In three enzyme concentrations such as 1.0, 1.5, 2.0% (v/w), similar WVP values were observed and there are no significant differences between them. Increased WVP of the enzymatically soaked leather is due to the reason that it results in a complete removal of non-collagenous proteins, and well opened fiber structure leading to the free flow of water vapour through the collagen fibers, while avoiding the water diffusion through the crust leather, facilitating better comfort for footwear user. The values of WVP of enzymatically soaked leather (1.0% (v/w) enzyme concentration) was slightly higher than other concentrations of enzyme. WVP of the 0.5% (v/w) enzyme treated leather and conventional crust leather was significantly low unlike other experimental crust leathers which may be due to lesser pore size and insufficient opening of fibers bundles.
Capillary flow porometry analysis
Capillary flow porometry (CFP) measurements have been carried out for both conventional and enzymatically processed crust leathers. The mean flow pore diameter values are shown in the Table 4. It is evident from Fig. 7 the experimental goat crust leather (0.5%, 1.0% 1.5% and 2% (v/w) enzyme) have pore size in the range of 0.10–0.52 µm, 0.1–1.1 µm, 0.1–1.0 µm, 0.1–0.52 µm and 0.1–1.0 µm; whereas the conventionally soaked skins showed less pore size in the range 0.1–0.35 µm. These results indicate that the enzyme treated leathers has better pore size over conventional multiple soaking; probably due to the better opening up of collagen fibres by hydrolysis of non-collagenous proteins.
Scanning electron micrograph and EDX
The surface and cross section of control and enzyme treated crust leathers were examined at a magnification of × 250 and micrographs are given in Figs. 8, 9. The morphological analysis of enzymatically soaked goat skins were comparable or slightly better than that of conventionally soaked skins. In the conventionally soaked skins, the grain surface was slightly rough with moderate opening of fibre bundles. The 1.0%, 1.5% and 2.0% (v/w) enzyme treated skins displayed smooth grain surface and better opened up fibre structure than 0.5% (v/w) enzyme treated skins. From the SEM images it can be concluded that the fibre bundles are loosened well and uniformly spread in enzyme treated leather that confirmed the data obtained from histological assessment (Fig. 9).The alkaline protease from Bacillus crolab MTCC 5468 was able to act efficiently on skin matrix and facilitate better removal of non-collagenous proteins and loosening of fibre structure. Paul et al. (2001) described that protease is well known for hydrolyzing the inter-fibrillary non-collagenous proteins and loosening of fibre bundles. So this is considered as a benefit of enzymatic rehydration method over the conventional numerous soaking processes.
The SEM–EDX investigation of control and experimental leather samples were shown in Fig. 10. From the results, the 1% (v/w) enzyme treated sample, weight % of Cr is 28.73 while atomic % is 15.94 indicating relatively higher percentage of Cr as present in the sample, when compared to control leather which relatively absorbed lower amount of Cr, exhibiting weight % of Cr is 17.11 while atomic % is 9.68. EDX results expose that, enhanced chromium absorption maybe due elimination of non-leather forming proteins and well opened collagen fiber bundles within the skin matrix; in which more number chromium uptake in goat skin during tanning operation.
Histological analysis
The rehydration capability of the alkaline protease from Bacillus crolab MTCC 5468 was assessed histologically for sections of dehydrated goat skins. The histological analysis of control and experimental pelts are shown in Fig. 11. The opening of collagen fibre bundles in the enzymatically soaked pelt was better to that of conventionally soaked pelt. Additionally, enzymatic rehydration facilitates the loosening of hair. The enzymatic soaked goat skin specimens showed empty follicles whereas the control showed left over disintegrated hair follicles. From the results obtained from histological analysis the 1.0% (v/w) enzyme concentration sufficient for optimum opening of fiber matrix of goat skins. The result implies that alkaline protease can work as a very good soaking aid in leather making process. The application enzymatic rehydration facilitates also dehairing or liming operation of leather making which reduces the need for dehairing chemicals by 30–60% (Zambare et al. 2013; Nielsen 2006).
Physical and organoleptic properties of leathers
The tear strength, tensile strength, % of elongation at break and grain crack of crust leathers from both control and experiment are shown in Table 5. The properties of the enzymatically soaked goat skins (0.5, 1.0, 1.5 and 2.0 (v/w)) were comparable to that of conventionally soaked goat skins. The properties of enzyme treated (both 1.0% and 1.5%) and control sample crust leather show that there was no significant difference between them. However, tear strength, % elongation at break and shrinkage temperature of enzyme treated leather was considerably better when compared to conventionally soaked goat skins. By the results of SEM and histological analysis, on using enzyme in the rehydration process, a better opening of collagen bundles was observed. The evaluation of organoleptic properties of crust leathers showed that the rating for softness, grain flatness, smooth and fullness of enzymatically soaked leather was comparable to the conventionally soaked leather; the grain flatness and smoothness of enzyme treated skins are slightly better than the control (Fig. 12). Therefore, using enzyme in soaking is likely to improve the leather grain quality compared to that of control leathers.
Conclusion
The alkaline protease from Bacillus crolab MTCC 5468 has a nature to hydrolyze non-collagenous skin proteins in the skin matrix. The enzymatic processes work faster than the chemical processes. From the results, it may be concluded that when 1.0% (v/w) enzyme was used for soaking of goat skins could be completed in 3 h duration with single soaking. By adopting the enzymatic method, it is possible to reduce 60–65% water in soaking operation. No major differences in the SEM images of the goat skins were observed between the various concentrations of enzymes applied in soaking. However, enzymatic soaking showed significant differences in the analysis of the TOC and proteins in the residual baths than conventional process. It is also found by adopting this system of soaking, that there is no deterioration in physical or functional properties in the resultant leathers. Application of alkaline protease during soaking reduced the soaking time, promoted hair loosening, and assisted dehairing process by minimizing the use of chemicals.
References
Ahmed Basha C, Soloman PA, Velan M, Balasubramanian N, Roohil Kareem L (2009) Participation of electrochemical steps in treating tannery wastewater. Ind Eng Chem Res 48:9786–9796
American Public Health Association (APHA) (1985) Standard methods for the examination of water and wastewater, 16th edn. Washington, DC, APHA
Bancroft JD, Gamble M (2004) Theory and practice of histological techniques. In: Jones ML, Totty BA (eds) Connective tissues and stains, 15th edn. Churchill Livingstone Publications, London, pp 139–200
Buljan J, Reich G, Ludvik J (1997) Mass balance in leather processing. IULTCS (International Union of Leather Technologists and Chemists Societies). Proceeding of a Conference on 11th–14th September 1997, London
Chen X, Penumadu D (2006) Review Characterizing microstructure of refractory porous materials. J Mater Sci 41:3403–3415
Clesceri LS, Greenberg AE, Trussell RR (1989) Standard methods for the examination of water and wastewater, 17th edn. American public health association, Washington, DC
De Souza FR, Gutterres M (2012) Application of enzymes in leather processing: a comparison between chemical and co-enzymatic processes. Braz J Chem Eng 29(03):473–481
Durga J, John Sundar V, Rose C, Muralidharan C (2019) Green processing: minimising harmful substances in leather making. Environ Sci Pollut Res 26(7):6782–6790
Fernando JA, Chung DDL (2002) Pore structure and permeability of an alumina fiber filter membrane for hot gas filtration. J Porous Mater 9:211–219
ISO:14268: (IULTCS/IUP15) (2012) Leather—physical and mechanical tests. Determination of water vapor permeability
IUC 8 (1998) Determination of chromic oxide content. J Soc Leather Technol Chem 82:200–208
IUP 16 (2000) Determination of shrinkage temperature. J Soc Leather Technol Chem 84:359
IUP 2 (2000) Sampling. J Soc Leather Technol Chem 84:303–309
Jianzhong M, Xueyan H, Dangge G, Bin L, Jing J (2013) Greener approach to efficient leather soaking process: role of enzymes and their synergistic effect. J Clean Prod 78(1):226–232
Kamini NR, Hemachander C, Geraldine J, Mala S, Puvanakrishnan R (1999) Microbial enzyme technology as an alternative to conventional chemical in leather industry. Curr Sci 77:80–86
Kandasamy N, Velmurugan P, Sundarvel A, Jonnalagadda RR, Bangaru C, Palanisamy T (2012) Eco-benign enzymatic dehairing of goat skins utilizing a protease from Pseudomonas fluorescens species isolated from fish visceral waste. J Clean Prod 25:27–33
Kunitz M (1947) Crystalline soybean trypsin inhibitor. J Gen Physiol 30:231–291
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with folin—phenol reagent. J Biol Chem 193:265–273
Maharaja P, Swarnalatha S, Saravanan P, Sekaran G (2019) Removal of fat components in high TDS leather wastewater by saline-tolerant lipase-assisted nanoporous-activated carbon. Appl Biochem Biotechnol 187:474–492
Mclaughlin GD, Theis ZR (1945) The chemistry of leather manufacture, vol 8. Reinhold publihing, Newyork, pp 158–160
Nielsen PH (2006) Environmental assessment of enzyme application in the tanning industry. Leather International August–September, pp 18–24
Ozgunay H, Colak S, Mutlu MM, Akyuz F (2007) Characterization of leather industry wastes. Pol J Environ Stud 16(6):867–873
Paul RG, Mohammed I, Davighi D, Addy VL, Covington AD (2001) The use of neutral protease in enzymatic unhairing. J Am Leather Chem Assoc 96:180–185
Ranjithkumar A, Durga J, Ramesh R, John sundar V, Rose C, Muralidharan C (2017a) Studies on alkaline protease from Bacillus crolab MTCC 5468 for applications in leather making. J Am Leather Chem Assoc 112:232–239
Ranjithkumar A, Durga J, Ramesh R, Rose C, Muralidharan C (2017b) Cleaner processing: a sulphide-free approach for depilation of skins. Environ Sci Pollut Res 24:180–188
Saikia P, Goswami T, Dutta D, Dutta NK, Sengupta P, Neog P (2017) Development of a flexible composite from leather industry waste and evaluation of their physico-chemical properties. Clean Technol Environ Policy 19(8):2171–2178
Sarkar KT (1991) Preparation of hides and skins for tanning. Theory and practice of leather manufacture. Oxford University Press, Oxford, p 99
Senthilkumar S, Balaji D, Surianarayanan M (2011) Optimization studies on production of a salt-tolerant protease from pseudomonas aeruginosa strain bc1 and its application on tannery saline wastewater treatment. Braz J Microbiol 42:1506–1515
Senthilvelan T, Kanagaraj J, Mandal AB (2012) Application of enzymes for dehairing of skins: cleaner leather processing. Clean Technol Environ Policy 14:889–897
Sharphouse JH (1983) Leather technician’s handbook. Leather Producers Association, Northampton, p 64
Thanikaivelan P, Raghava Rao J, Unni Nair B, Ramasami T (2005) Recent Trends in Leather Making: processes, Problems, and Pathways. Crit Rev Environ Sci Technol 35:37–79
Zambare VP, Nilegaonkar SS, Kanekar PP (2013) Protease production and enzymatic soaking of salt-preserved buffalo hides for leather processing. IIOAB Lett 3:1–7
Acknowledgements
The authors gratefully acknowledge the Council of Scientific and Industrial Research (CSIR), New Delhi for funding this research work and are thankful to the Director, CSIR—Central Leather Research Institute for his support. The author A. Ranjithkumar thank University Grants Commission—Rajiv Gandhi National Fellowship F1-17.1/2016-17/RGNF-2015-17-SC-TAM-17696/(SA-III/Website).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1
The soaked goat skins from conventional were subjected to unhairing using 3% (w/w) sodium sulphide and lime 10% (w/w) whereas, partial unhairing chemicals (sodium sulphide 1.5% (w/w) and lime 5%(w/w)) were used for experimental skins. After unhairing all goat skins were relimed using 10% lime and 150% water for 48–72 h. These relimed goat skins were defleshed (sub-cutaneous tissues) using fleshing machine. After defleshing, the skins were delimed using 1% (w/w) ammonium chloride with 100% water (v/w) for 45 min in rotating drum, followed by treatment with 1% (w/w) alkali bate for 30 min in conventional method. After bating operation, the goat skins were washed two times and drained out. The washed skins were pickled using 8% (w/w) common salt and 80% (v/w) fresh water and run for 10 min followed by acidification using 0.5% (v/w) formic acid was mixed with 10% (v/w) water and given for 2 feeds at 10 min interval and run for 15 min, then 1% (v/w) sulphuric acid was mixed with 10% (v/w) water and given for 4 feeds at every 10 min intermission and the drum was additional run for 60 min. The final pH of pickled goat skin was 2.8 and the pickled skins were tanned using 8% (w/w) basic chromium sulphate (BCS) for 2 feed in half of the pickle bath for 90 min, then 50% (v/w) water was added and run for 30 min. After two feed of BCS, was basified using 1% (w/w) sodium formate was mixed with 10% (v/w) water and added to the rotating drum and then 1% (w/w) sodium bicarbonate was mixed with 10% (v/w) water and fed in 3 parts at 10 min intervals. Then, drum was continuously run for more than 60 min for additional fixing of tanning material. After chrome tanning operation, the pH of the both liquor and wet blue was observed to be 3.8.
Appendix 2
The wet blue leather was washed with 100% (v/w) water in a drum for 10 min, the water was drained out and wet blue leathers were treated with neutralizing syntan 1% (v/w) in 100% (v/w) float in a drum and run for 20 min. After this, 0.5% (w/w) sodium formate and 0.5% (w/w) sodium bicarbonate were added to the running drum in 3 feeds at 10 min time interval. After the liquor accomplished a pH of 5.0, the wet blue leathers were washed twice with water 200% (v/w) for 10 min. After completion of washing, 100% (v/w) water with 3% (w/w) resin syntan were added to the drum and run for 20 min. Followed by this, dying was carried out using 2% (w/w) acid dye for 30 min and fat liquoring was carried out with synthetic fat liquor 4% (w/w) in the drum for 30 min. Subsequently, melamine based re-tanning syntan 4% (w/w) was added and run for 40 min followed by the addition of synthetic fat liquor 4% (w/w), polymeric fat liquor 3% (w/w) and natural fat liquor oil 4% (w/w) and further running the drum for 40 min. Finally, the auxiliaries were leather was fixed using 2% (v/w) formic acid diluted with 20% (v/w) water and added at 3 feeds at every 10 min interval and the drum was further run for 30 min. After this, the leather was piled overnight and were subjected to setting and hooked for drying. The dried crust leathers were staked and buffed using 400 grit emery papers.
Rights and permissions
About this article
Cite this article
Ammasi, R., Victor, J.S., Chellan, R. et al. Alkaline protease for an efficacious rehydration of skin matrix by a novel Bacillus crolab MTCC 5468 in sustainable leather production: a green approach. Biotechnol Lett 42, 249–267 (2020). https://doi.org/10.1007/s10529-019-02769-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-019-02769-0