Abstract
Objectives
To investigate the behaviors of aggregates of human mesenchymal stem cells (hMSCs) on chondrogenesis and chondrocyte hypertrophy using spatiotemporal expression patterns of chondrogenic (type II collagen) and hypertrophic (type X collagen) markers during chondrogenesis.
Results
hMSCs were cultured on either a polystyrene surface or polyamidoamine dendrimer surface with a fifth generation (G5) dendron structure in chondrogenic medium and growth medium. At day 7, cell aggregates without stress fibers formed on the G5 surface and triggered differentiation of hMSCs toward the chondrogenic fate, as indicated by type II collagen being observed while type X collagen was undetectable. In contrast, immunostaining of hMSCs cultured on polystyrene, which exhibited abundant stress fibers and did not form aggregates, revealed no evidence of either type II and or type X collagen. At day 21, the morphological changes of the cell aggregates formed on the G5 surface were suppressed as a result of stress fiber formation. Type II collagen was observed throughout the aggregates whereas type X collagen was detected only at the basal side of the aggregates. Change of cell aggregate behaviors derived from G5 surface alone regulated chondrogenesis and hypotrophy, and this was enhanced by chondrogenic medium.
Conclusions
Incubation of hMSCs affects the expression of type II and X collagens via effects on cell aggregate behavior and stress fiber formation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human mesenchymal stem cells (hMSCs) are promising cell source for clinical applications because of their multipotency and capacity for self-renewal (Giuliani et al. 2013). They can differentiate into osteoblasts, adipocytes, or chondrocytes when exposed to appropriate conditions (Herlofsen et al. 2011; Westhrin et al. 2015). For chondrogenic differentiation, hMSCs are typically grown in three-dimensional (3D) culture in serum-free chondrogenic medium supplemented with transforming growth factor (TGF)-β. hMSCs cultured in this manner rapidly lose their fibroblast-like morphology, begin to express cartilage-specific markers, and assume a compact shape, rather than spreading in a monolayer culture (Barry et al. 2004; Cooke et al. 2011; Oldershaw et al. 2010). The cartilage-specific marker expression that occurs during hMSC chondrogenesis has been partially explained by RhoA signaling that regulates the formation of stress fibers (Chi et al. 2013). Prevention of stress fiber formation in 3D culture, by inhibition of RhoA signaling, results in not only changes in cell shape to a spherical structure but also increases in the levels of chondrogenic markers (Woods et al. 2006). Moreover, type II collagen upregulation is activated by the transcription factor SOX9 via the Smad pathway when cells are cultured in chondrogenic medium containing TGF-β3 (Hardingham et al. 2006). Although hMSCs tend to simultaneously acquire hypertrophic properties during chondrogenic induction, the process of the shift in the chondrocyte phenotype toward hypertrophic differentiation is unclear.
Chondrogenic lineage commitment of MSCs can be induced at the periphery of cell aggregates on a polyamidoamine dendrimer surface with a fifth generation (G5) dendron structure in growth medium without any differentiation-inducing factors (Kim and Kino-oka 2014). On the G5 surface, cells exhibit active migration due to transient contraction caused by matrix metalloproteinase activation-induced fibronectin aggregation, followed by transient active extension due to paxillin phosphorylation (Ogawa et al. 2015). Active cell migration on the G5 surface promotes cell aggregate formation through the formation of cell–cell interactions (Ogawa et al. 2016). The resultant restriction of cell spreading inside the cell aggregates promotes directed differentiation of hMSCs toward a chondrogenic fate, as indicated by expression of type II collagen, which is characteristic of chondrogenesis (Singh et al. 2012). The behaviors of the observed aggregate are accompanied by dynamic cytoskeletal formation in aggregates on the culture surface. Endogenous Rho family GTPase signaling pathways accompanied by changes in aggregate behaviors are an intrinsic cue for modulation of transcription factors.
In the present study, we employed a G5 surface as a substrate for promotion of cellular aggregation to examine chondrogenesis and hypertrophy in response to aggregate behaviors of hMSCs in growth medium and chondrogenic medium. Based on our findings related to spatiotemporal expression pattern of type II and X collagens in response to aggregate behavior, we discuss possible mechanisms that may contribute to the shift of the chondrocyte phenotype toward hypertrophic differentiation during the chondrogenic differentiation of hMSCs.
Materials and methods
Cell culture
Bone marrow-derived hMSCs were from Lonza (Walkersville, MD, USA). Maintenance of hMSCs was on a conventional polystyrene culture surface in Dulbecco’s modified Eagle’s medium, 10% (v/v) fetal bovine serum (FBS), and 1% (v/v) antibiotics at 37 °C in a humidified atmosphere containing 5% CO2. The cells were passaged by enzymatic treatment at 80% confluence. hMSCs at less than passage 5 were used in all experiments.
hMSCs were cultured in growth medium or chondrogenic medium consisting of chondrogenic differentiation medium, hMSC chondrogenic SingleQuots supplement (Lonza), and 10 ng TGF-β3/ml (Wako Pure Chemical Industries, Tokyo, Japan). Seeding was at 5 × 103 cells/cm2, and the media were changed every 3 days. For hMSC attachment in chondrogenic medium, the cells were cultured in 10% (v/v) chondrogenic medium (CM) + FBS for 3 days and then in serum-free CM for day 4–21. As a control, a low binding culture surface (Sumitomo Bakelite, Tokyo, Japan) was used to generate floating hMSC aggregates.
Preparation of the dendrimer-immobilized surface
The polystyrene surface of square 8-well plates (Nunc) was used to prepare a G5 dendrimer surface as described previously (Ogawa et al. 2016). Briefly, hydroxyl groups were displayed on the surface by addition of a potassium tert-butoxide solution. Next, dendron structures were generated by reactions with glutaraldehyde and tris (2-aminoethyl) amine solutions, which was performed five times to establish the fifth generation dendrimer. To display terminal ligands, glucose solution was added to the culture plates. Then, the plates were immersed in a sodium borohydride solution without removal of the glucose solution. Finally, the plates were washed with sterile water before use.
Dynamic observation of cell aggregate behaviors
Time-lapse images were obtained every 10 min at several positions using a BioStation imaging system (Nikon).
Immunofluorescence staining
Visualization of F-actin and collagen was carried out as described previously (Ogawa et al. 2016). The cells were fixed with 4% (v/v) paraformaldehyde. Then, the samples were permeabilized with 0.5% (v/v) polyoxyethylene (10) octylphenyl ether in phosphate-buffered saline. Non-specific binding was masked by incubation with Block Ace solution (Dainippon Sumitomo Pharma, Osaka, Japan) for 90 min. The cells were then incubated with primary antibodies against human type II or X collagens (Santa Cruz Biotechnology) at 4 °C overnight. The cells were washed with Tris-buffered saline and labeled with a fluorescent secondary antibody (Alexa Fluor 488 donkey anti-goat IgG; ThermoFisher) for 1 h. Finally, the cells were stained with 4′,6-diamidino-2-phenylindole (ThermoFisher) and Rhodamine/phalloidin (ThermoFisher) for 40 min to visualize the nucleus and F-actin, respectively. Images were obtained using a confocal laser scanning microscope.
Gene expression analysis
Cells were collected from the culture surfaces by enzymatic treatment, and RNA was extracted using an RNeasy Mini Kit (Qiagen). Total RNA was reverse transcribed into cDNA using a PrimeScript RT reagent kit (Takara). Quantitative real-time PCR was performed using SYBR Green (SYBR Premix Ex Taq; Takara Bio) and a StepOnePlus real-time PCR system (ThermoFisher). The primers used in this study were as follows: type II collagen (COL2A1), forward 5′-GGC AAT AGC AGG TTC ACG TAC A-3′, reverse 5′-CGA TAA CAG TCT TGC CCC ACT T-3′ (Oldershaw et al. 2010); type X collagen (COL10A1), forward 5′-GCT TCA GGG AGT GCC ATC ATC-3′, reverse 5′-CTC ACA TTG GAG CCA CTA GGA ATC-3′ (Mulller et al. 2008); glyceraldehyde-3-phosphate dehydrogenase (GAPDH), forward 5′-CAA CGG ATT TGG TCG TAT TGG-3′, reverse 5′-GCC ATG GGT GGA ATC ATA TTG-3′ (Kim and Kino-oka 2014). The target genes were amplified by 40 amplification cycles of 95 °C for 3 s and 60 °C for 30 s. Relative expression levels were calculated using the \( 2^{{ - \Delta {\text{C}}_{t} }} \) method and normalized to GAPDH expression.
Statistical analysis
All experiments were performed at least three times, and data are expressed as means with standard deviations. The Tukey–Kramer method was used to determine statistical significance among the data sets. p < 0.01 was considered as significant.
Results
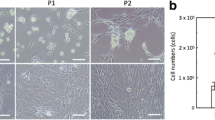
Dynamic behaviors of hMSC aggregates during chondrogenic differentiation hMSCs were cultured on G5 and polystyrene surfaces in growth medium and chondrogenic medium, and morphological variations were investigated on day 7 and 21 after seeding. As shown in Fig. 1, cells on G5 surfaces were spherical dominantly on both day 7 and 21. Some cells at the basal side of aggregates cultured on the G5 surface were stretched to a larger size. Conversely, cells cultured on polystyrene were elongated without aggregate formation in both culture media throughout the whole culture period.
To clarify the dynamic behavior of cells on G5 and polystyrene surfaces, we conducted time-lapse observations of representative cell aggregates. In an early phase of culture from day 6–7, cell aggregates on the G5 surface in both growth medium and chondrogenic medium showed dynamic changes in morphology associated with repetitive stretching and contracting during migration (Supplementary Movie 1). However, in a late phase of culture from day 20–21, these migratory behaviors of the cell aggregates slowed down on the G5 surface. Some cells undergoing morphological changes were also seen, usually at the edges of aggregates, where the cells had a flattened appearance. These aggregates on the G5 surface underwent spontaneous transition from spherical to flattened morphology at the basal side through decreased migration in the following 21 days of culture. However, cells on the polystyrene surface were flat and demonstrated continuous stretching.
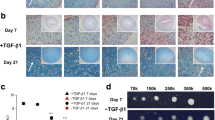
Cytoskeletal organization and collagen localization in hMSCs during chondrogenic differentiation. To examine the effect of surface-mediated aggregate behaviors of hMSCs on chondrogenic differentiation, fluorescence staining of F-actin, and type II and X collagens was performed in cells cultured on G5 and polystyrene surfaces in growth medium and chondrogenic medium. In cells cultured in growth medium on the G5 surface at an early phase of culture (day 7), a linear structure of F-actin was observed adjacent to the plasma membrane at the basal side of the aggregates on the culture surface, whereas less F-actin was present at the middle of the aggregates (Fig. 2a, c). The cells on the basal side of aggregates attached to the G5 surface were elongated with active filopodia exhibiting short, thin, and fibrous F-actin structures at all edges. Conversely, cells cultured on the polystyrene surface showed distinct long, thick, and fibrous stress fibers. At day 21, cells cultured on both G5 and polystyrene surfaces exhibited distinct stress fibers, although stress fibers remained absent in cells at the middle of the aggregates (Fig. 2a, c).
Immunofluorescence staining of F-actin (red), type II and X collagen (green), and nuclear staining (blue) in hMSCs cultured on G5 surface (a–h) and polystyrene surface (i–p) in growth medium at days 7 (a–d and i–l) and 21 (e–h and m–p) after the start of the experimental treatment. Scale bar = 50 μm
After immunostaining of type II and X collagens (chondrocyte markers), cells in a monolayer or spheroid showed expression differences in time- and location-dependent manners (Supplementary Movie 2). Type II collagen was produced exclusively around aggregated cells on the G5 surface in growth medium, although its expression was detected partially in the uppermost layer of cell aggregates at day 7 (Fig. 2b). However, type II collagen was seldom observed in monolayer cells cultured on the polystyrene surface (Fig. 2j). At day 21, the expression of type II collagen was markedly enhanced in the cells of whole cell aggregates on the G5 surface (Fig. 2f), whereas its expression in monolayer cells on polystyrene surfaces occurred only after culture in growth medium (Fig. 2n).
In contrast to the type II collagen in cells, notable differences were seen in the distribution of type X collagen (a hypertrophy marker). Type X collagen was not detected under any culture condition at day 7 (Fig. 2d and Supplementary Movie 3), but its expression appeared in morphologically large and flat cells at day 21 (Fig. 2h). Interestingly, the staining signal appeared to intensify at the basal side of aggregated cells on the G5 surface. By comparing the middle and basal side of aggregates on G5 surfaces, its expression was much more intense in aggregated cells at the basal than in the attached cells at the middle side of aggregates (Fig. 2h). Cells on the polystyrene surface were dispersed in a confluent monolayer with appreciable amounts of type X collagen, and the expression levels in cells on these surfaces were higher than those in cells on the G5 surface (Fig. 2n, p).
Similar patterns of F-actin and type II and X collagens on G5 surface were obtained in cultures kept in chondrogenic medium for 21 days (Fig. 3, Supplementary Movies 4 and 5). Staining for type II collagen was visible within the aggregates cultured in chondrogenic medium at day 7, and this pattern was detectable between cells throughout the aggregate aggregates at day 21 of differentiation (Fig. 3b, f). Furthermore, type X collagen was almost undetectable in cell aggregates at day 7 but it was strongly expressed in the attached cells at the basal side of aggregates at day 21 (Fig. 3d, h). These results indicate that changes of migratory behavior on G5 surface induce time- and location-dependent differences of type II and X collagens during chondrogenic differentiation or vice versa, regardless of the type of media.
Immunofluorescence staining of F-actin (red), type II and X collagen (green), and nuclear staining (blue) in hMSCs cultured on G5 surface (a–h) and polystyrene surface (i–p) in chondrogenic medium at days 7 (a–d and i–l) and 21 (e–h and m–p) after the start of the experimental treatment. Scale bar = 50 μm
Changes in collagen gene expression of hMSCs during chondrogenic differentiation. To measure mRNA expression of chondrogenesis- and hypertrophy-associated genes, hMSC aggregates were subjected to real-time quantitative PCR analyses. As shown in Fig. 4, the mRNA level of type II collagen was decreased slightly from day 7 to day 21 while increasing expression of type X collagen. The mRNA level of type II and X collagens on G5 surface followed similar trends over time in growth medium and chondrogenic medium, but they was a significantly higher expression level of type II and X collagens than that in growth medium. These results indicated that active aggregate migratory behavior on G5 surface can lead to increased type II collagen expression at early phase of differentiation, and reduced aggregate migratory behavior leads to chondrogenic differentiation toward a hypertrophic phenotype at late phase of differentiation.
Discussion
Aggregate formation of hMSCs in a dynamic manner induces initiation of lineage commitment toward a chondrogenic fate. The possible mechanisms underlying chondrocyte differentiation and hypertrophy in response to aggregate behaviors of MSCs on a G5 surface are described. From the viewpoint of hMSC aggregates generated on the G5 surface in the growth medium without any differentiation-related supplement, the most important aspect for migratory behavior of aggregates is the balance between cell–cell and cell–substrate interactions that induce spatial differences in their morphological and functional characteristics (Fig. 5). Time-lapse microscopy demonstrated that the hMSC aggregates exhibited repetitive morphological changes with stretching and contracting during migration when cultured on the G5 surface at the early stage of culture from day 6–7 in both growth medium and chondrogenic medium (Supplementary Movie 1). Cells at the basal side of aggregates failed to develop actin stress fibers, enabling them to migrate actively and undergo protrusion and contraction in the aggregates (Fig. 2). Cytoskeletal staining of F-actin indicated loss of stress fibers in aggregates, which was associated with stimulated migration on the G5 surface at the early phase, suggesting that the G5 surface permits moderate activation of Rho family GTPases associated with stimulating migration (Kim et al. 2010; Ogawa et al. 2015). Compared with monolayer cells on the polystyrene surface, hMSCs in aggregates on the G5 surface showed major changes in morphology and cytoskeleton organization that are known to regulate hMSC properties and contribute to functional enhancement. Cells at the basal side of aggregates on the G5 surface showed a flattened cellular structure with the development of mature stress fibers in growth medium (Fig. 2). A similar observation was made on the polystyrene surface. It was considered that chondrogenic phenotypes on the G5 surface are regulated by the actin cytoskeletal architecture through cell–cell and cell–substrate interactions that in turn elicit differential contractile forces and adhesion to stimulate cell migration. These results suggest that this migratory behavior of aggregates on the G5 surface was responsible for the suppression of integrin expression in hMSC-derived chondrocytes during differentiation, thereby facilitating the development of chondrogenic phenotypes with abundant expression of type II collagen.
Altered migratory behavior of MSC aggregates induces transition from chondrogenesis to the hypertrophic phenotype during chondrogenic differentiation.
Chondrocytes in monolayer culture have reduced cell–cell interactions and enter a hypertrophic state (Hirsch et al. 1997; Tanaka et al. 2007). Expression of integrins in chondrocytes changes along with the stages of differentiation (Tanaka et al. 2007). In the early stages of differentiation, there are changes in the extracellular matrix (ECM), such as deposition of proteoglycans and type II collagen, and hence alterations in the cell–substrate interactions. Increased ECM secretion and retention in aggregates also promote autocrine signaling, presumably by local growth factor enrichment via ECM binding (Yang et al. 2016). These features have been thought to form flattened aggregates that attach to the substratum through the secretion of ECM components, such as collagen, proteoglycans, and fibronectin, by resident cells at a late phase of culture (Ogawa et al. 2015, 2016).
These findings lead us to suggest that, during the process of hypertrophic chondrocyte differentiation, a shift in production from type I to type II and IX collagens occurs when chondrogenesis begins, and a shift from type II to type X collagen occurs when chondrocytes become hypertrophic (Djouad et al. 2007; Hamid et al. 2012). These shifts occur through cooperative signaling between cadherin- and integrin-linked kinases within the signaling cascade to regulate chondrogenesis (Wood et al. 2007). We have found that the aggregate morphology of hMSCs associated with active migration on the G5 surfaces was responsible for the coordinated regulation of the balance between cell–cell and cell–substrate interactions, thereby switching their transition from a hMSC-derived chondrogenic lineage to a hypertrophic phenotype. According to immunostaining, type II collagen was produced exclusively around the aggregated cells on the G5 surface (Figs. 2, 3), although expression of type II collagen was undetectable in cells at the basal side of aggregates. However, similar to cells on the polystyrene surface, type X collagen expression was detected in cells at the basal side of aggregates on the G5 surface at day 21 (Figs. 2, 3). It was considered that the suppressed migratory behavior on the G5 surface at the late phase of chondrogenic induction was responsible for the promotion of integrin expression in hMSC-derived chondrocytes during differentiation, thereby switching from chondrogenic phenotypes with type II collagen expression to hypertrophic phenotypes with type X collagen expression. These findings provide important insights to understand the processes of development of the hypertrophic phenotype from the differentiated state of hMSC aggregates on the G5 surface, suggesting that a delicate interaction balance between adjacent cells and their substrate along with suppressed migratory behaviors are closely related to expression of a hypertrophic phenotype in MSC-derived chondrocytes.
In conclusion, Migratory behavior of cell aggregates on chondrogenesis and hypertrophy during hMSC differentiation. Active migration is responsible for the aggregation while maintaining the chondrogenic phenotypes with abundant formation of type II collagen at the early phase. After suppression of the morphological changes and migration of cell aggregates on the G5 surface at the late phase, stretched cells at the basal side of the aggregates express type X collagen. This approach has led to a new understanding of the establishment of a supportive niche in hMSC differentiation-inducing cultures and the dynamic interplay between and within hMSC aggregates. In addition to its use in studying the mechanism of chondrocyte hypertrophy, the in vitro hMSC model studied here is thus a potentially useful model to screen bioactive factors with the aim of inhibiting the hypertrophic differentiation of tissue-engineered cartilage.
References
Barry FP, Murphy JM (2004) Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol 36:568–584
Chi X, Wang S, Huang Y, Stamnes M, Chen JL (2013) Roles of Rho GTPases in intracellular transport and cellular transformation. Int J Mol Sci 14:7089–7108
Cooke ME, Allon AA, Cheng T, Kuo AC, Kim HT, Vail TP, Marcucio RS, Schneider RA, Lotz JC, Alliston T (2011) Structured three-dimensional co-culture of mesenchymal stem cells with chondrocytes promotes chondrogenic differentiation without hypertrophy. Osteoarthr Cartil 19:1210–1218
Djouad F, Delorme B, Maurice M, Bony C, Apparailly F, Louis-Plence P, Canovas F, Charbord P, Noel D, Jorgensen C (2007) Microenvironmental changes during differentiation of mesenchymal stem cells towards chondrocytes. Arthritis Res 9:R33
Giuliani N, Lisignoli G, Magnani M, Racano C, Bolzoni M, Dalla Palma B, Spolzino A, Manferdini C, Abati C, Toscani D, Facchini A, Aversa F (2013) New insights into osteogenic and chondrogenic differentiation of human bone marrow mesenchymal stem cells and their potential clinical applications for bone regeneration in pediatric orthopaedics. Stem Cells Int 2013:312501
Hamid AA, Idrus RBH, Saim AB, Sathappan S, Chua KH (2012) Characterization of human adipose-derived stem cells and expression of chondrogenic genes during induction of cartilage differentiation. Clinics 67:99–106
Hardingham TE, Oldershaw RA, Tew SR (2006) Cartilage, SOX9 and Notch signals in chondrogenesis. J Anat 209:469–480
Herlofsen SR, Kuchler AM, Melvik JE, Brinchmann JE (2011) Chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells in self-gelling alginate discs reveals novel chondrogenic signature gene clusters. Tissue Eng Part A 17:1003–1013
Hirsch MS, Lunsford LE, Trinkaus-Randall V, Svoboda KK (1997) Chondrocyte survival and differentiation in situ are integrin mediated. Dev Dyn 210:249–263
Kim MH, Kino-oka M (2014) Switching between self-renewal and lineage commitment of human induced pluripotent stem cells via cell-substrate and cell-cell interactions on a dendrimer-immobilized surface. Biomaterials 35:5670–5678
Mueller MB, Tuan RS (2008) Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum 58:1377–1388
Ogawa Y, Kim MH, Kino-oka M (2015) Changes in human mesenchymal stem cell behaviors on dendrimer-immobilized surfaces due to mediation of fibronectin adsorption and assembly. J Biosci Bioeng 120:709–714
Ogawa Y, Kim MH, Kino-Oka M (2016) Migration-driven aggregate behaviors of human mesenchymal stem cells on a dendrimer-immobilized surface direct differentiation toward a cardiomyogenic fate commitment. J Biosci Bioeng 122:627–632
Oldershaw RA, Baxter MA, Lowe ET, Bates N, GradyLM Soncin F, Brison DR, Hardingham TE, Kimber SJ (2010) Directed differentiation of human embryonic stem cells toward chondrocytes. Nat Biotechnol 28:1187–1194
Singh P, Schwarzbauer JE (2012) Fibronectin and stem cell differentiation: lessons from chondrogenesis. J Cell Sci 125:3703–3712
Tanaka K, Yokosaki Y, Higashikawa F, Saito Y, Eboshida A, Ochi M (2007) The integrin alpha5beta1 regulates chondrocyte hypertrophic differentiation induced by GTP-bound transglutaminase 2. Matrix Biol 26:409–418
Westhrin M, Xie M, Olderoy MO, Sikorski P, Strand BL, Standal T (2015) Osteogenic differentiation of human mesenchymal stem cells in mineralized alginate matrices. PLoS ONE 10:e0120374
Wood A, Wang G, Beier F (2007) Regulation of chondrocyte differentiation by the actin cytoskeleton and adhesive interactions. J Cell Physiol 213:1–8
Woods A, Beier F (2006) RhoA/ROCK signaling regulates chondrogenesis in a context-dependent manner. J Biol Chem 281:13134–13140
Yang SS, Jin LH, Park SH, Kim MS, Kim YJ, Choi BH, Lee CT, Park SR, Min BH (2016) Extracellular matrix (ECM) multilayer membrane as a sustained releasing growth factor delivery system for rhTGF-β3 in articular cartilage repair. PLoS ONE 11:e0156292
Acknowledgements
The authors gratefully acknowledge financial support from the Thailand Research Fund through the Royal Golden Jubilee Ph.D. Program (Grant No. PHD/0016/2555). This work was also supported by the project “Development of cell manufacturing and processing system for industrialization of regenerative medicine” (No. P14006) commissioned by the Japan Agency for Medical Research and Development (AMED).
Supporting information
Supplementary Movie 1—Behavior of hMSC aggregates on G5 and polystyrene surfaces in growth medium and chondrogenic medium. The analysis was performed at an early phase of culture from day 6 to 7 and from day 20 to 21.
Supplementary Movie 2—Fluorescence images of type II collagen (green), nuclei (blue), and F-actin (red) in hMSCs cultured on G5 and polystyrene surfaces in growth medium at day 7 and 21.
Supplementary Movie 3—Fluorescence images of type X collagen (green), nuclei (blue), and F-actin (red) in hMSCs cultured on G5 and polystyrene surfaces in growth medium at day 7 and 21.
Supplementary Movie 4—Fluorescence images of type II collagen (green), nuclei (blue), and F-actin (red) in hMSCs cultured on G5 and polystyrene surfaces in chondrogenic medium at day 7 and 21.
Supplementary Movie 5—Fluorescence images of type X collagen (green), nuclei (blue), and F-actin (red) in hMSCs cultured on G5 and polystyrene surfaces in chondrogenic medium at day 7 and 21.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wongin, S., Ogawa, Y., Kim, MH. et al. Chondrogenesis and hypertrophy in response to aggregate behaviors of human mesenchymal stem cells on a dendrimer-immobilized surface. Biotechnol Lett 39, 1253–1261 (2017). https://doi.org/10.1007/s10529-017-2339-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-017-2339-9