Abstract
Introduction
Spheroids of mesenchymal stem cells (MSCs) in cartilage tissue engineering have been shown to enhance regenerative potential owing to their 3D structure. In this study, we explored the possibility of priming spheroids under different media to replace the use of inductive surface coatings for chondrogenic differentiation.
Methods
Rat bone marrow-derived MSCs were organized into cell spheroids by the hanging drop technique and subsequently cultured on hyaluronic acid (HA) coated or non-coated well plates under different cell media conditions. Endpoint analysis included cell viability, DNA and Glycosaminoglycan (GAG) and collagen content, gene expression and immunohistochemistry.
Results
For chondrogenic applications, MSC spheroids derived on non-coated surfaces outperformed the spheroids derived from HA-coated surfaces in matrix synthesis and collagen II gene expression. Spheroids on non-coated surfaces gave rise to the highest collagen and GAG when primed with medium containing insulin-like growth factor (IGF) for 1 week during spheroid formation. Spheroids that were grown in chondroinductive raw material-inclusive media such as aggrecan or chondroitin sulfate exhibited the highest Collagen II gene expression in the non-coated surface at 1 week.
Conclusion
Media priming by growth factors and raw materials might be a more predictive influencer of chondrogenesis compared to inductive-surfaces. Such tailored bioactivity of the stem cell spheroids in the stage of the spheroid formation may give rise to a platform technology that may eventually produce spheroids capable of chondrogenesis achieved by mere media manipulation, skipping the need for additional culture on a modified surface, that paves the way for cost-effective technologies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autologous chondrocytes are a limited resource due to donor site availability, tissue morbidity, and waiting period for expansion in culture. Several studies have illustrated the promise that multipotential mesenchymal stem cells (MSCs) hold for chondrogenesis, osteogenesis, adipogenesis and other musculoskeletal applications, including current clinical trials.4,18,22,34 Often the regenerative benefits are lost after successive passaging and expansion of cells in vitro, which relies on culturing them on a non-physiologic monolayer.32,35 3D culture overcomes some of the limitations presented by the monolayer culture system. For example, assembly of MSCs as cell spheroids by the pellet culture technique has long been employed for increasing the chondrogenic potential under in vitro conditions.1,43,46 Forming 3D cell spheroids by the conventional hanging drop method has been suggested as a means to enhance the therapeutic potential and has been shown to elicit an anti-inflammatory effect with MSCs.5,6,21,45 Recent reports have demonstrated that the use of MSC spheroids formed by hanging drop or suspension culture enhanced their therapeutic potential for cartilage regeneration in vivo. 12,21,28 Similarly, growing MSCs on surfaces with different surface stiffnesses has yielded viable spheroids with encouraging stemness and differentiation capabilities.23,26,39
Hyaluronic acid (HA) is a linear anionic polysaccharide composed of long chains of repeating disaccharide units of d-glucuronic acid and N-acetyl-d-glucosamine.11,38 In cartilage extracellular matrix (ECM), HA is the backbone of aggrecan superstructure complexes,7 and it is commonly employed as a biomaterial for cartilage tissue engineering. However, source of HA is limited, in vivo retention is short-lived, and the treatment options are expensive due to manufacturing limitations. Investigators have already explored alternatives to HA for long-term applications. For the current study, we explored the possibility of priming spheroids under different media formulations as a means to replace the use of HA for chondrogenic differentiation.
We hypothesized that MSCs that were formed into 3D spheroids, cultured in non-coated well-plates and primed under different medium compositions would out-perform in terms of survival and chondrogenic gene expression compared to spheroids in HA-coated conditions. To test this hypothesis, we examined the chondrogenic potential of MSC spheroids that were first subjected to either HA-coated or non-coated conditions. To further explore the influence of raw materials (a term our group has used to describe molecules that hold two key characteristics: (1) serve as a building block for the regenerating tissue, and (2) provide bioactive signals to cells) and growth factor under culture conditions, the spheroids were subjected to serum-rich or serum free medium with either transforming growth factor (TGF)-β3, insulin-like growth factor (IGF)-1, chondroitin sulfate (CS), and aggrecan. Lastly, an additional group was added where the medium was changed from TGF-β3 to IGF-1 after 3 days of culture (TGF ⇒ IGF), motivated by our previous work showing that switching from TGF-β3 to IGF-1 increased collagen production and collagen type II gene and protein expression.31,41 The experimental group and controls were tested for cell viability and chondrogenic applications through gene expression, immunohistochemistry and quantifying matrix content.
Materials and Methods
Cell Culture and Expansion
Rat bone marrow-derived mesenchymal stem cells (rBMSCs) were harvested from the femurs of seven young male Sprague–Dawley rats (176–200 g, Charles River) following a University of Kansas approved IACUC protocol (175–08). The IACUC protocol approved the cellular harvest procedure and usage of cells for this particular study. The cells were isolated according to a previously reported protocol.29 Briefly, isolated cells were cultured in control medium (αMEM supplemented with 10% Certified FBS and 1% Penicillin–Streptomycin (Pen-Strep)) (Invitrogen Life Technologies, Carlsbad, CA) and passaged at 80% confluence until passage 4 (P4). All of the cells from 14 different femurs were pooled together at P4 for use in the study.
Preparation of HA-Coated Well Plates
HA sodium salt of medical grade (Lifecore Biomedical, Chaska, MN, USA) was obtained as a dry powder. According to the information from the supplier, the HA molecular weight was between 200 and 350 kDa. Well plates were coated with HA according to a previously reported protocol.23 Briefly, polylysine-coated 96-well plates (Sigma-Aldrich) were cleaned ultrasonically in acetone and then rinsed with ethanol. A deionized water rinse was used to remove liquid chemicals followed by a nitrogen purge. 300 μL of 1 mg/mL aqueous solution of HA was pipetted into a single well of a 96-well plate and air-dried in a sterile environment for 3 days at 25 °C to prepare the HA-coated surfaces.
Cellular Spheroid Formation
Spheroids were generated by the hanging drop technique according to a previously reported protocol.6,44 Cellular suspensions (rBMSC) were prepared at concentration of 10 × 106 cells/mL in medium. Droplets of cell suspensions (cells with corresponding medium, see below) at a volume of 10 μL were pipetted onto the inside surface of a sterile petri dish lid in an array using a 10 μL pipette (Eppendorf, Hauppauge, NY), making sure that the droplets had a safe distance between each other to prevent mixing. The cells were allowed to aggregate overnight, with the help of gravity when the petri dish lid was reversed (Fig. 1). The sterile petri dish bottom was then filled with PBS to prevent drying of these droplets. After 24 h, the cell spheroids were collected from the dish with the help of a 1 mL pipette and placed into well plates and cultured in the same medium composition in which the spheroids were formed with a total of one spheroid per well.
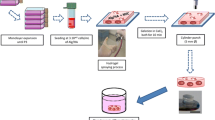
Schematic representation of the experimental procedure. rBMSCs were aggregated via the hanging drop technique as previously reported and harvested after 24 h. Subsequently, spheroids were plated onto HA-coated 96 well plates and cultured for 0, and 7 days. The individual spheroids were subjected to five different culture medium conditions, namely Dulbecco’s modified eagle medium (DMEM) with (a) 5 ng/mL TGF-β3, (b) 100 ng/mL IGF, (c) 40 μg/mL chondroitin sulfate (d) 40 μg/mL aggrecan, or (e) control medium, and (f) TGF ⇒ IGF medium.
Spheroids were created in one of six different types of medium, including control medium, and then placed on either a coated or non-coated surface, where the spheroids then continued in the same medium in which they were formed (Fig. 1). The one exception was the TGF/IGF group, where spheroids were formed in TGF medium, then cultured in TGF medium for 3 days before being switched to IGF medium. The spheroids were harvested after 24 h, and were either processed directly for different assays (i.e., the week 0, or “hanging drop” time point), or placed in well plates for additional culture. In the well plates, one set of spheroids was placed in HA-coated well plates, where the medium was changed every other day and samples were collected at 24 h and 7 days (to evaluate changes over time for the experimental coating surface). The other set of spheroids was placed under regular 96 well plates that were not coated with HA, where the medium was changed every other day and samples were collected at 7 days (providing a control surface comparison for the experimental coating surface at the final time point).
The six medium groups used for spheroid formation and culture were as follows (Fig. 1 and Table 1):
-
Control group The control medium consisted of αMEM, 10% FBS and 1% Pen-Strep.
-
TGF group TGF medium consisted of low glucose DMEM, 1% Pen-Strep, and 5 ng/mL TGF-β3 (PeproTech, Rocky Hill, NJ).
-
IGF group IGF medium consisted of low glucose DMEM, 1% Pen-Strep, and 100 ng/mL IGF-1 (PeproTech, Rocky Hill, NJ).
-
CS group CS medium consisted of low glucose DMEM, 1% Pen-Strep, and 40 μg/mL porcine CS (Catalog number: C3788, Sigma).
-
Aggrecan group Aggrecan medium consisted of low glucose DMEM, 1% Pen-Strep, and 40 μg/mL bovine aggrecan (Catalog number: A1960, Sigma).
-
TGF ⇒ IGF group Spheroids were formed in TGF medium, and then conditioned with the TGF medium in well plates for the first 3 days. After medium removal at the end of day 3, IGF was added to the spheroids for 4 additional days. Given that this group was identical to the TGF group at the hanging drop and 24 h time points, only the 7-day time point was run for this group (only coated).
Cell Viability Assay
To check the viability of the spheroids, a fluorescent live/dead viability staining kit (Invitrogen, Carlsbad, CA) was used at day 0, 24 h, and 7 days. The samples (n = 3) were washed once with phosphate-buffered saline (PBS) and incubated in live/dead staining solution (0.5 µL calcein and 2.0 µL ethidium homodimer (ETH) diluted in 1 mL PBS) for 10 min (37°C, 5% CO2). The samples were once again washed with PBS prior to imaging using an inverted epifluorescent microscope (Zeiss, Axioplus microscope). Live and dead cells were stained green and red, respectively.
DNA Analysis
Spheroids were placed in a papain digestion solution consisting of 125 mg/mL papain, 5 mL N-acetyl cysteine, 5 mL ethylenediaminetetraacetic acid and 100 mL PBS (all reagents from Sigma-Aldrich, St. Louis, MO) in distilled water overnight in a 60°C water bath. On the following day, sample digests (n = 5) were centrifuged for 5 min and stored at − 20°C until further use. DNA content was quantified for all samples utilizing a PicoGreen kit (Life-technologies, Frederick, MD) according to the manufacturer’s instructions. Previous studies from our group (unpublished) established a conversion factor of 7.7 pg/cell that may be used to convert DNA content to cell number.
Glycosaminoglycan Assay
Spheroids (n = 5 spheroids) were digested with papain digesting solution and left in a 60°C water bath overnight prior to measuring glycosaminoglycan (GAG) content. GAG content was measured with a Sircol DMMB assay kit and the manufacturer’s protocol (Biocolor B1000, Belfast, U.K.) was followed. 1.0 mL of dimethylmethylene blue dye solution was added to 100 µL of sample, standard (chondroitin sulfate from kit, Sigma), and blank. Solutions were mixed slowly for 30 min and centrifuged for 10 min. The supernatant was discarded and the pellet was resuspended, and 1.0 mL of dissociation solution was added. The solutions were vortexed and transferred to a 96-well plate and read at 656 nm in a microplate reader.
Collagen Assay
For quantifying collagen production, a Sircol soluble collagen assay kit and manufacturer’s protocol were used (Biocolor, s5000, Belfast, U.K.). Briefly, samples (n = 4) were incubated in pepsin solution (Sigma-Aldrich, St. Louis, MO) dissolved in acetic acid, and placed overnight at 4°C. 1.0 mL of Sircol dye reagent and 100 µL of each standard (collagen standards provided by the manufacturer) and sample were mixed slowly for 30 min. Solutions were centrifuged at 12,000 rpm for 10 min and the resulting supernatant discarded, keeping the pellet intact. The previous step was repeated after the addition of 750 µL of ice-cold acid salt wash. The pellet was resuspended and to this 250 µL of alkali reagent was added. Solutions were then vortexed thoroughly and 200 µL of the solution was transferred to a 96-well plate and ran at 555 nm in a microplate reader.
Gene Expression Analysis
Real-time reverse transcriptase polymerase chain reaction (RT-PCR) was used to assess gene expression levels for collagen types I, II, SOX9, and aggrecan (n = 4). To each sample 1.0 mL of lysis buffer (Qiagen, Germantown, MD) was added, and after 1 h, the solutions were added to the QIAshredder column (Qiagen 79656, Germantown, MD) to extract messenger RNA (mRNA) in accordance with the RNEasy Plus Mini Handbook (Qiagen 74136, Germantown, MD) (n = 4). A high capacity cDNA reverse transcription kit (Applied Biosystems 4368814, Foster City, CA) allowed reverse transcription of mRNA to complementary DNA (cDNA); 2 × RT Master Mix was prepared using the kit’s protocol. 10 µL of the master mix and RNA samples were combined in a 96-well plate. The well plate was then loaded into an Eppendorf Realplex Mastercycler (Eppendorf, Hamburg, Germany). cDNA concentrations were normalized with DNASE-free water, and a Taqman gene expression assay kit (Applied Biosystems 1325810, Foster City, CA) provided the primers for the above-mentioned genes. 1 µl of cDNA from each sample, 10 µl of universal fast master mix (2×), and 1 µL of a specific primer were mixed in a 96-well plate. RT-PCR reactions were run in an Eppendorff Realplex system (Eppendorff 5345 Hauppauge, NY).
Immunohistochemistry
Spheroids were fixed, cleared in xylene, dehydrated and embedded in paraffin blocks until further use. The sections were generated using a microtome and sectioned to a thickness of 4 µm. Sections (n = 3) were immersed in xylene; rehydrated in graded ethanol and rehydrated in deionized water for 5 min (all reagents from Sigma-Aldrich). The sections were exposed to 3% hydrogen peroxide in methanol for 10 min to suppress endogenous peroxidase activity and immediately the slides were incubated in proteinase K (IHCWORLD IW-1101, Woodstock, MD) at 37°C for 10 min. Sections were blocked with 3% blocking serum (Vector Laboratories S-2012, Burlingame, CA) for 30 min preceding primary antibody incubation for collagen I, collagen II, and aggrecan for 60 min (Table 2). Following primary antibody incubation, slides were exposed to biotinylated secondary antibody and ABC reagent (Vectastain ABC kit PK-6200 Vector laboratories) for 30 min each. For the negative control with the IgG isotype, the secondary antibody was directly added. Lastly, the ABC reagent was added to the sections after washing with PBS and incubated for 30 min and then washed again. Tissue staining was accomplished with ImmPact DAB peroxidase substrate (Vector laboratories SK-4105) before rinsing with distilled water and counter stained with VECTOR hematoxylin QS stain (Vector laboratories H-3404). Following staining, slides were rinsed in tap water; dehydrated in ethanol; cleared in xylene for mounting (Permount SP15-500 Fair Lawn, NJ) and viewed under an upright microscope (Zeiss, Imager A2).
Statistical Analyses
All data were expressed as mean ± standard deviation. Statistical analyses were performed using one-way ANOVA (Minitab 15, Minitab Incorporated, State College, PA) followed by a Tukey’s post hoc comparison test for repeated measurements. The statistical significance threshold was set at 0.05 for all tests (with p < 0.05).
Results
Cell Viability Assay
Cell viability was high overall for the spheroids that were isolated right after the hanging drop procedure at t = 0 h (Fig. 2). The control media group at t = 0 exhibited the largest diameter compared to other time points. At t = Day 7 on the non-coated plate, the spheroid disintegrated into a suspension of individual cells (Fig. 2) The TGF group also demonstrated a similar pattern of a decrease in spheroid diameter from t = 0 to t = Day 7, but without disintegrating. Compared to the other groups, the TGF-primed spheroids retained their 3D morphology even at Day 7 under non-coated conditions. IGF-primed spheroids exhibited a decrease in spheroid diameter from t = 0 to t = Day 7. Additionally dead cells on the spheroid periphery were observed at t = Day 7 for the coated surface groups. The aggrecan-primed spheroids also exhibited a similar decrease in spheroid diameter from t = 0 to t = Day 7. At Day 7, the coated aggrecan group retained the 3D morphology while the non-coated aggrecan group looked disintegrated. Dead cells were observed at Day 7 (both coated and non-coated). The CS group did not assume a conventional spheroid shape at t = 24 h. At t = Day 7, the CS spheroids were completely disintegrated and only small fragments were stained. Compared to the control media group, the size was visually observed to be much smaller at Day 7 (coated and non-coated). Lastly, the CS group at Day 7 under coated conditions had a rounded morphology comparable to the control media group. Dead cells were also present throughout these spheroids.
Live/dead images demonstrating cell viability at different time points. (a) control media group before spheroid formation, (b) control media spheroids 24 h after hanging drop, (c) 24 h after spheroids were placed in HA coated well plates, and 7 days after they were placed in HA-coated or non-coated surface. Scale bar = 100 μm.
DNA Content
Within each given medium group, DNA content differences over time were statistically significant between t = 0 (prior to placing on any surface) and the Day 7 time point, for both the coated and uncoated surfaces. The control medium group DNA content dropped by 67.8 and 85.7% from t = 0 to day 7 for the coated and non-coated surfaces, respectively (p < 0.05). The TGF group DNA content dropped by 69.7 and 67.4% from t = 0 to day 7 for the coated and non-coated surfaces, respectively (p < 0.05). The IGF group DNA content dropped by 84.1% and 91.7% from t = 0 to day 7 for the coated and non-coated surfaces, respectively (p < 0.05). The CS group DNA content dropped by 80.7 and 91% from t = 0 to day 7 for the coated and non-coated surfaces, respectively (p < 0.05). The DNA content of the aggrecan group dropped by 79.4 and 66.7% from t = 0 to day 7 for the coated and non-coated surfaces, respectively (p < 0.05). The TGF ⇒ IGF group DNA content dropped by 84.4% from t = 0 to day 7 for the coated surface (p < 0.05). No statistically significant differences in DNA content were noted among groups at t = 0 or 24 h.
Next, comparing the DNA contents among the groups at Day 7, the IGF group had 58.3% lower DNA content compared to the TGF group for the non-coated surface (p < 0.05). No other statistically significant differences in DNA content were noted among groups at the different time points.
GAG Content
Statistically significant increases in GAG/DNA content over time (Fig. 3) were observed in all six groups (including control medium group) for both surfaces by at Day 7, but only the control group had a significant increase by the 24 h time point. The control group had 5.3-fold, 3.4-fold, and 7.2-fold higher GAG/DNA contents at t = 24 h coated, t = Day 7 coated, and t = Day 7 non-coated, compared to the t = 0 group (p < 0.05). The TGF group had a 2.6-fold higher and 90% increase in GAG/DNA content from t = 0 h to t = Day 7 coated and non-coated groups, respectively (p < 0.05). The IGF group had a 3.1-fold, and 7.1-fold higher GAG/DNA content from t = 0 h to t = Day 7 coated and non-coated groups, respectively (p < 0.05). The CS group had 3.2-fold and 2.7-fold higher GAG/DNA content from t = 0 h to t = Day 7 coated and non-coated groups, respectively (p < 0.05). The aggrecan group had a 3.1-fold increase in GAG/DNA content from t = 0 to day 7 for the coated surface (p < 0.05). Lastly, the TGF ⇒ IGF group had a 2.6-fold increase in GAG/DNA content from t = 0 to day 7 for the coated surface (p < 0.05).
Normalized GAG content measured at 0 and 7 days, expressed as GAG/DNA. The IGF group had the highest GAG/DNA content at day 7 on the non-coated surface. Values are reported as mean ± standard deviation, n = 4. (@) represents statistically significant difference from the control group at that time point, (*) represents statistically significant difference from t = 0 h, (**) represents statistically significant difference among different media groups, (&) represents statistically significant between coated and non = coated groups (p < 0.05).
Next, the significant differences in the GAG/DNA contents of the various different groups at specific time points compared to the control group are noted. At t = 24 h, the TGF, IGF, CS, and aggrecan groups had 66.0, 66.2, 54.7, and 54.9% lower GAG/DNA contents compared to the control medium group (p < 0.05). At t = Day 7 with the coated surfaces, statistically significant differences were observed for the TGF, aggrecan and TGF ⇒ IGF groups, which had 62.8, 63.1, and 61.4% higher GAG/DNA contents than the control group (p < 0.05). In contrast, with the non-coated surfaces, the aggrecan group had a 54.9% lower GAG/DNA content than the control group at Day 7 (p < 0.05). There were no other significant differences among other groups at Day 7. Moreover, there were no significant differences in GAG content among the groups at t = 0 h.
There were significant changes in GAG/DNA content among the different test groups, but only at Day 7 for the non-coated surfaces. Specifically, the IGF group had a 99.7 and 35.8% higher GAG/DNA contents compared to the TGF group, and the aggrecan group (p < 0.05).
Finally, three significant differences in GAG/DNA content at Day 7 between coated and non-coated conditions were observed. The control group had a 2.2-fold higher, the IGF group had a 2.3-fold higher, and the aggrecan group had a 44.8% lower GAG/DNA content at Day 7 for the non-coated surface, compared to the coated surface (p < 0.05). No other comparisons between the other groups between coated and non-coated groups were statistically significant.
Collagen Content
There were no statistically significant changes in collagen content for any group from t = 0 to the 24 h time point, although the following significant increases were noted at 7 days (Fig. 4). The control group had 2.8-fold and 2.4-fold increases in collagen/DNA content from t = 0 to Day 7 with the HA-coated and non-coated surfaces, respectively (p < 0.05). The TGF group had a 3.0-fold increase in collagen/DNA from t = 0 to Day 7 with the coated surface (p < 0.05), but no significant change over time for the non-coated surface. The IGF group on the other hand displayed 4.6-fold and 10.8-fold increases in collagen/DNA from t = 0 to Day 7 for the coated and non-coated surfaces, respectively (p < 0.05). Furthermore, the CS group demonstrated 8.4-fold and 6.3-fold increases in collagen/DNA from t = 0 to Day 7 for the coated and non-coated surfaces, respectively (p < 0.05). The collagen/DNA content of the aggrecan group increased 73.9% from t = 0 to Day 7 (non-coated) (p < 0.05), but did not have any significant change for Day 7 for the coated surface. The TGF ⇒ IGF group had a 10.7-fold increase in collagen content at Day 7 for the coated surface compared to t = 0 (p < 0.05).
Normalized collagen content expressed as collagen/DNA. The IGF group at day 7 non-coated surface had the highest collagen/DNA content. Values are reported as mean ± standard deviation, n = 4. (@) represents statistically significant difference from the control group at that time point, (*) represents statistically significant difference from t = 0 h, (**) represents statistically significant difference among different media groups, (&) represents statistically significant between coated and non = coated groups (p < 0.05).
In comparing the collagen/DNA values between the control group and each of the other groups at the same time point, there were two statistically significant differences. At day 7 on the coated surface, the CS group had a 4.5-fold higher collagen/DNA content than the control group (p < 0.05). At day 7 on the non-coated surface, the IGF group had a 6.7-fold higher collagen/DNA content compared to the control group (p < 0.05).
Next, comparing the collagen/DNA values among various groups at specific time points, significant changes were observed at Day 7 as follows. At Day 7 for the coated group, the CS group had a 5.8-fold higher, and the TGF ⇒ IGF group had a 5.6-fold higher, collagen/DNA content than the aggrecan group, respectively (p < 0.05).
Finally, only one statistically significant change in collagen/DNA content between the coated and non-coated surfaces was observed. Specifically, the IGF group had a 2.1-fold higher collagen/DNA content on the non-coated surface compared to the coated surface at Day 7 (p < 0.05).
Gene Expression Analysis
Aggrecan
First, statistically significant changes in aggrecan expression over time were noted for all of the groups. In general, aggrecan gene expression tended to drop over time for all of the groups. The control group at Day 7 on the coated surface had 2.0-fold higher aggrecan gene expression than at t = 0 h, but for the non-coated surface there was a 71.4% decrease in aggrecan gene expression compared to t = 0 h (p < 0.05). There were no other significant changes over time for the control group. Coming to the TGF group, there was a 58.1, 86, and 74.8% decrease in aggrecan gene expression at 24 h, & 7 days (coated and non-coated) from t = 0 h (p < 0.05). For the IGF group, there were 74.1, 93.8 and 97.8% decreases in aggrecan gene expressions from t = 0 to 24 h and day 7 for the coated and non-coated surfaces, respectively (p < 0.05). The CS group had 89.0, 92.3, and 86.8% decreases in gene expression from t = 0 to 24 h and day 7 for the coated and non-coated surfaces, respectively (p < 0.05). The aggrecan group also had decreases of 85, 73.1, and 79.8% in aggrecan gene expression from t = 0 to 24 h and day 7 for the coated and non-coated surfaces, respectively (p < 0.05). The TGF ⇒ IGF group alone had a 63% decrease in aggrecan gene expression from t = 0 to day 7 coated surface (p < 0.05). All of the TGF ⇒ IGF comparisons were made to the TGF group.
In comparing the different groups to the control at the same time point, we observed that at t = 0, all of the medium compositions had a significantly higher aggrecan gene expression than the control group. The TGF group, IGF group, CS group, and aggrecan group had 6.8-fold higher, 5.7-fold higher, 3.5-fold higher, and 7.9-fold higher aggrecan gene expression than the control group at t = 0 (p < 0.05). Compared to the control group, there were no significant differences in gene expression among the different groups at t = 24 h. At Day 7 for the coated surface, the only significant differences from the control were for the IGF and CS groups. The IGF group had 86.5% lower, and the CS group had 83.6% lower gene expression than the control group (p < 0.05). At Day 7 for the non-coated surface, the only significant differences from the control were for the TGF and aggrecan groups. The TGF group had a 5.8-fold higher, and the aggrecan group had 5.6-fold higher aggrecan gene expression than the control medium (p < 0.05).
Next, comparisons among different groups at the same time point were made. At t = 0, the only significant differences were as follows: CS group had 38.6% lower aggrecan gene expression than the IGF group (p < 0.05), whereas the aggrecan group had a 34.4% higher aggrecan gene expression than the IGF group and 2.1-fold higher gene expression than the CS group (p < 0.05). At 24 h on the coated surface, the CS group had a 73.9% lower and the aggrecan group had a 63.7% lower aggrecan gene expression than the TGF group (p < 0.05). The aggrecan group had 38.8% higher aggrecan gene expression than the CS group on the coated surfaces (p < 0.05), and no other groups had any significant differences among each other. At Day 7 on the coated surface there were several significant differences in gene expression. The TGF ⇒ IGF group had a 2.8-fold higher aggrecan gene expression than the TGF group, 5.9-fold higher gene expression than the IGF group, and 5.4-fold higher gene expression than the IGF group (p < 0.05). In addition, the aggrecan group had a 6.2-fold higher aggrecan gene expression than the IGF group and a 4.5-fold higher gene expression than the CS group (p < 0.05). At Day 7 on the non-coated surface there were three significant differences in aggrecan gene expression. Specifically, the TGF group had a 12.8-fold higher gene expression than the IGF group, and a 3.7-fold higher gene expression than the CS group (p < 0.05). In addition, the aggrecan group had a 3.1-fold higher gene expression than the CS group (p < 0.05).
Lastly, comparing the coated and non-coated groups at Day 7, the control group on a coated surface had 86.8% higher aggrecan gene expression than on the non-coated surface (p < 0.05). All other differences between the two groups were not statistically significant.
SOX-9
Statistically significant changes in SOX-9 gene expression over time were noted for all of the groups. The control group had one significant change, a 2.9-fold increase in SOX-9 gene expression from t = 0 to Day 7 on the coated surface (p < 0.05). Similarly, the only significant increase in SOX-9 gene expression for the TGF group was at Day 7 on the non-coated surface, which had a 6.1-fold increase relative to t = 0 (p < 0.05). Similarly, for the IGF group, there was a 4.2-fold increase in SOX-9 gene expression from t = 0 to Day 7 for the non-coated surface (p < 0.05). There was a sharp decrease in SOX-9 gene expression over time for the CS group, with 93.8, 88.5, and 81.5% decreases at 24 h, and Day 7 for the coated and non-coated surfaces, respectively (p < 0.05). For the aggrecan group, there were 85.7 and 89.2% decreases in SOX-9 gene expression from t = 0 h to t = 24 h and t = Day 7 for the non-coated surface, respectively (p < 0.05).
Next, SOX-9 gene expression differences compared to the control group were made, at specific time points. At t = 0 h, the CS group had 8.8-fold higher SOX-9 gene expression than the control group (p < 0.05). At t = 24 h for the coated surface, the IGF medium group had 3.4-fold higher SOX-9 gene expression than the control medium group (p < 0.05). At t = Day 7 for the coated surface, the IGF group had 58.5% lower SOX-9 gene expression, and the TGF ⇒ IGF group had 2.6-fold higher SOX-9 gene expression compared to the control group (p < 0.05). At t = Day 7 for the non-coated surface, the aggrecan group had 84.6% lower gene expression than the control group (p < 0.05). All other comparisons between the test groups and the control group at specific time points were not statistically significant.
Next, several significant changes among different groups at the same time point were observed. At t = 0, the CS group had 10.4-fold higher SOX-9 gene expression than the TGF group, 16.3-fold higher expression than the IGF group, and 4.2-fold higher expression than the aggrecan group (p < 0.05). At t = 24 h, the TGF group had 4.5-fold and 5.0-fold higher SOX-9 gene expressions than the CS and aggrecan groups, respectively (p < 0.05). Similarly, the IGF group had a 3.5-fold and 4.6-fold higher SOX-9 gene expression than the CS and aggrecan groups, respectively (p < 0.05). At Day 7 for the coated surface, the TGF ⇒ IGF group had a 10.0-fold, 9.3-fold, 9.1-fold, and 4.4-fold higher SOX-9 gene expression than the TGF, IGF, CS, and aggrecan groups, respectively (p < 0.05). At Day 7 for the non-coated surface, there were two significant comparisons. The aggrecan group had 34.3-fold and 14.3-fold lower SOX-9 gene expression than TGF and IGF groups, respectively (p < 0.05). All the comparisons between the different media groups and specific time points were not statistically significant.
Lastly, comparing the coated and non-coated groups at Day 7, three significant differences were observed. The TGF, IGF, and aggrecan groups on the non-coated surface had 6.8-fold, 3.6-fold, and 8.0-fold higher SOX-9 gene expression than their respective coated-surface groups (p < 0.05). All other comparisons between the coated and non-coated groups were not statistically significant.
Collagen II
Statistically significant changes in collagen II gene expression over time were noted for all of the groups. The control group had one significant 60.4% decrease from t = 0 to Day 7 for the non-coated surface (p < 0.05). The TGF group had a 5.4-fold and 2.8-fold increase in collagen II gene expression from t = 0 h to 24 h and t = Day 7 on the non-coated surface, respectively (p < 0.05). On the contrary, the IGF group had a 50.7 and 52.3% decrease in collagen II expression from t = 0 h to 24 h and t = Day 7 non-coated surface, respectively (p < 0.05). One major finding was that the CS group had a significant 10.9-fold increase in collagen II gene expression from 0 h to Day 7 for the non-coated surface (p < 0.05). The aggrecan group at t = 24 h had a 6.1-fold increase in collagen II expression from t = 0 h for the non-coated surface (p < 0.05).
Next, collagen II gene expression differences between test groups and the control group were made, at specific time points. Compared to the control group at t = 0 h, there was 88.3, 62.8, 85.9, 84.7% lower collagen II gene expression in the TGF, IGF, CS, and aggrecan groups, respectively (p < 0.05). At t = 24 h, the IGF and CS groups had 87.5 and 88.7% decreases in collagen II gene expression compared to the control group (p < 0.05). Similarly, compared to the control group at t = Day 7 for the coated surface, there was 69.4, 79.7, 71.4, and 75.6% lower collagen II gene expression in the TGF, IGF, CS, and aggrecan groups, respectively (p < 0.05). At t = Day 7 for the non-coated surface, notable differences in gene expression were observed. The TGF group had a 32.4% higher, and the CS group had a 6.8-fold higher collagen II gene expression than the control group (p < 0.05). However, compared to the control group, there was a 52.8% lower and 61.8% lower collagen expression for the IGF and aggrecan groups, respectively (p < 0.05). All other differences between the different test groups and the control group at specific time points were not statistically significant.
Additionally, several significant changes among different groups at the same time point were observed. At t = 0, the IGF group had 3.1-fold higher expression of collagen II compared to the TGF group (p < 0.05). At t = 24 h, the TGF group had 5.0-fold higher expression than the CS group, and the aggrecan group had 5.6-fold higher Collagen II expression than the IGF group (p < 0.05). The TGF ⇒ IGF group had 3.0-fold higher expression than the IGF group at t = Day 7 for the coated surface (p < 0.05). At t = Day 7 on the non-coated surface, the TGF group had 3.2-fold higher and 4.0-fold higher collagen II gene expression than the IGF and aggrecan groups, respectively (p < 0.05). The CS group had a significant 4.4-fold, 14.5-fold, and 17.9-fold higher collagen II expression than the TGF, IGF and aggrecan groups, respectively, (p < 0.05). All other differences among the different media groups at specific time points are not statistically significant.
Finally, comparing the coated and non-coated groups, there were two significant changes. The control group at Day 7 on the coated surface had a 3.2-fold higher collagen II gene expression than the non-coated surface, and the CS group non-coated surface had 7.4-fold higher expression had than the coated surface, respectively (p < 0.05). All other comparisons between the two surfaces were not statistically significant.
Collagen I
Statistically significant changes in Collagen I gene expression over time were noted for all of the groups. The control group had a 2.5-fold, 2.2-fold, and 2.1-fold increase in collagen I gene expression from t = 0 to t = 24 h, and to Day 7 for the coated and non-coated surfaces, respectively (p < 0.05). Coming to the TGF group, there was 50.3 and 80.5% decrease in collagen I gene expression from t = 0 to Day 7 for the coated and non-coated surfaces, respectively (p < 0.05). The IGF group had a 2.7-fold increase in gene expression from t = 0 h to Day 7 coated surface, but 87.4% decrease in expression from t = 0 to Day 7 non-coated surface, respectively (p < 0.05). The CS group had 39.4, 59.7, and 85.0% decreases in collagen I gene expression from t = 0 to t = 24 h, and to Day 7 for the coated and non-coated surfaces, respectively (p < 0.05). The aggrecan group had 75.5%, and 90.4% decreases in collagen I gene expression from t = 0 to Day 7 for the coated and non-coated surfaces, respectively (p < 0.05). No other changes in collagen I gene expression over time were significant.
Next, gene expression changes between the test groups and the control group were made, at specific time points. There were no significant changes at t = 0. At t = 24 h, compared to the control group there was a 60.9, 59.2, 75.2, and 46.7% lower collagen I gene expression demonstrated by the TGF, IGF, CS, and aggrecan groups, respectively, compared to the control (p < 0.05). However, at Day 7 on the coated surface, the IGF and TGF ⇒ IGF groups had 68% and 33.6% higher collagen I gene expressions compared to the control group, respectively (p < 0.05). The TGF, CS, and aggrecan groups had 63.4, 81.2 and 87.7% lower collagen I gene expression than the control group respectively (p < 0.05). Compared to the control group at Day 7 non-coated surface, the TGF, IGF, CS, and aggrecan groups evidenced 85.1, 91.9, 92.7 and 95.0% lower gene expression, respectively (p < 0.05). No other changes in collagen gene expression between the test groups and the control group at a given time points were significant.
Several significant changes among different groups at a given time point were observed. At t = 0, the TGF group had a 46.4% higher collagen I gene expression than the CS group (p < 0.05). At t = 24 h, the TGF, IGF, and aggrecan groups had 57.9% higher, 64.9% higher, and 2.1-fold higher collagen I gene expression compared to the CS group, respectively (p < 0.05). At Day 7 on the coated surface, the IGF group had 4.6-fold, 8.9-fold, 13.7-fold, and 3.4-fold higher collagen I gene expression compared to the TGF, CS, aggrecan, and TGF ⇒ IGF groups, respectively (p < 0.05). Additionally, the TGF ⇒ IGF group had 2.6-fold and 3.9-fold higher expression than the CS and aggrecan groups, respectively (p < 0.05). There were no significant differences among groups at Day 7 for the non-coated surface.
Lastly, there were two significant difference between the coated and non-coated surfaces. Specifically, the TGF group had a 2.5-fold higher collagen I gene expression on the coated surface than on the non-coated surface (p < 0.05). Similarly, the IGF group on the coated surface had a 21.9-fold higher collagen I gene expression than on the non-coated surface (p < 0.05). There were no other statistically significant comparisons between the coated and non-coated surfaces.
Immunohistochemistry
IHC staining of the control spheroids revealed that the diameter of the spheroids decreased over the one-week period, specifically from t = 0 to the 7 day time point on the non-coated surface. Figure 5a represents the collagen I immunostaining for representative groups. At t = 0, aggrecan group had the highest collagen I staining intensity followed by the CS and TGF groups at 24 h. The 7-day HA-coated (TGF) spheroid started to show disintegration and the 7D HA-coated IGF spheroids showed complete degradation in spheroid morphology. Figure 5b represents the collagen II immunostaining for select groups. The hanging drop group with CS medium showed highly intense staining for collagen II compared to the TGF and aggrecan medium. Figure 5c shows aggrecan immunostaining for representative groups. Cell clusters from TGF-primed medium stained after hanging drop and after 24 h on the coated surface showed intense aggrecan staining. Though the 24 h coated control spheroid group showed negligible collagen I staining, by day 7 it stained intensely throughout the spheroid.
Discussion
For the first time, the current study demonstrated that including raw materials in the medium composition may provide a quick and cost-effective method to differentiate MSCs toward the cartilage phenotype. One way of exploiting MSCs is organizing them into 3D stem cell spheroids or spheroids that have shown good regeneration and anti-inflammatory properties. Spheroids have recently been shown to be more effective than single cells for defect filling and better cartilage regeneration.15,24
Previous in vitro studies have shown that microenvironment and media compositions plays an important role in shaping the cell migration and eventual differentiation of the MSC spheroids.2,26 HA is a commonly explored potentially chondroinductive ECM component used both as a surface and as a scaffold.8,19 Results of the best-performing groups are summarized in Table 3. For the current study, we used a M w of 360 kDa that accounted for an intermediate range of stiffness. Compared to the previous studies, we have for the first time used MSC spheroids of higher cell densities (1 × 105 cells per spheroid) directed at chondrogenic applications. The effect of media compositions can clearly be seen in the GAG and collagen content of the spheroids (Figs. 3 and 4); however, the long-term effects cannot be elucidated from the current study. The non-coated groups primed under IGF medium conditions had a much higher GAG and collagen content compared to the control and HA-coated groups. In general, the combination of non-coated surface with IGF/CS medium composition had a much higher collagen II gene expression and collagen content compared to the HA-coated and control groups. Although the average size of the spheroids varied among the groups, from the live-dead assay (Fig. 2) we can infer that the spheroids at the time of being harvested from the hanging drop petri dishes possessed the highest diameter compared to the spheroids after 24 h and 7 day culture. Due to a shortage of cells, we prioritized on the HA-coated group and did not include the 24 h non-coated group for the current study. While our results still support the conclusion that media supplementation enhanced spheroid formation, the current study neglected to focus on inner necrosis and future improvements to the approach would benefit from consideration of spheroid size/diameter to achieve desirable cumulative viability. The cells near the deep interior of the spheroids would not be expected to respond as the cells at the surface that interact with the culture substrate surface.3,25 Moreover HA-coated surfaces often suffer from surface stability that may affect long-term cultures further supporting the rationale for placing primary emphasis on the medium composition during spheroid formation than on the culture surface.
New medium recipes are constantly being explored to enable provision of the best culture conditions for cellular differentiation. In particular, growth factors and bioactive peptides as medium supplements are being extensively investigated for musculoskeletal applications.20,33,42 Several studies have shown the therapeutic advantages of using TGF-β in scaffold fabrication for controlled release over time.9, 13 During the last decade, IGF-1 has also gained a lot of interest as it has been shown to be misregulated playing an anabolic role in cartilage degeneration.30,36 Several approaches have thus employed IGF-1 in their scaffold design or in the medium for regenerating cartilage.17,40,41 At t = 0, the DNA content of the IGF group was higher than what we have seen so far (Fig. 6),41 thus reiterating the importance of cellular presentation as three-dimensional spheroids compared to cellular suspensions. When transferred from a 3D format to a 96-well plate there was significant cell death and decrease in DNA content due to decreased cell-to-cell interactions. Thus, there is an increased interest in the field to directly move from 3D spheroids to in vivo setting that would eliminate intermittent cell death and promote increased rate of survival; thereby completely skipping the culture on the substrate step altogether.37 Of particular interest was the TGF ⇒ IGF group, where the spheroids were primed with TGF-β for the first three days and then changed to IGF-1 for the last 4 days. This additional group was specifically included to observe the effect of IGF following early differentiation, as previously observed.41 The IGF non-coated group exhibited the highest GAG/DNA content. Although several groups have explored IGF for chondrogenesis,10,27 the current study for the first time showed that IGF may be leveraged as a priming formulation for use in stem cell spheroids. One of the main directions of our research group has been to explore the chondroinductive properties of so-called ‘raw materials,’ and heretofore we have assessed raw materials such as aggrecan, CS, decellularized cartilage, and bioactive glass in scaffold fabrication. To take it one step further, in the current study we directly added CS and aggrecan in the medium during the spheroid formation step.14,16 Although the concentrations are different under in vivo conditions, premature priming of the spheroids to raw materials may preemptively induce GAG and collagen production that can be complemented when placed in the body. In the current study, we found that the CS medium primed at t = 0 showed the highest SOX-9 gene expression (Fig. 7). The paramount finding of this paper is that the CS group at Day 7 on the non-coated surface displayed the highest collagen II gene expression and immunostaining, demonstrating that the stem cell spheroids are indeed able to respond to raw-material cues in the media, thus providing a simpler and more-efficient way to elicit chondrogenesis. Moreover, the collagen I gene expression was enhanced at Day 7 for the HA-coated surface with the highest expression evidenced by the IGF group. The increased collagen I gene expression only further corroborates the idea that HA coating and growth factor priming may play a complementary role at late-stage chondrogenesis but further long-term studies must be performed to dissect the molecular pathways. The current study utilizes the complementary approach that opens a new path to look at medium formulations, as raw materials heretofore have exclusively been used only for scaffold fabrication. If the addition of CS in the medium under non-coated surface could yield results comparable to that of growth factors, it is an easy and efficient approach from a financial and regulatory standpoint and cannot be ignored for future applications. Although there have been several studies that have independently explored surface stiffness on spheroid properties, and different media formulations; there is a lack of published techniques that conjointly explore the effect of both the parameters on spheroid differentiation.
(a) DNA content analysis of the different groups using the picogreen assay measured at 0 and 7 days. Please note the decrease in DNA content for all of the groups after 7 days of culture. Values are reported as mean ± standard deviation, n = 4. (@) represents statistically significant difference from the control group at that time point and (*) represents statistically significant difference from hanging drop (t = 0) time point (p < 0.05). There was no significant difference in values between the coated and non-coated groups.
Gene expression of aggrecan, SOX9, collagen I, and collagen II for all the groups. While aggrecan gene expression was enhanced fort = 0 time point, the collagen II and SOX9 gene expression was highest at the day 7 coated or non-coated surface. Values are reported as mean ± standard deviation, n = 4. (@) represents statistically significant difference from the control group at that time point, (*) represents statistically significant difference from t = 0 h, (**) represents statistically significant difference among different media groups, (&) represents statistically significant between coated and non = coated groups (p < 0.05).
Although spheroids present several advantages over single cell suspensions, the main drawback when it comes for clinical applications is the time required for spheroid formation. Secondly, there was a genuine attempt to vigorously perform immunohistochemistry testing for all the groups, but the spheroids are extremely small after 1 week of culture and in many cases disintegrated and hence were lost to sample processing. Future studies that focus on better spheroid retention in paraffin molds and look at the regeneration from a different testing paradigm may benefit the entire field. Finally, the experiment presented here only serves as a preliminary in vitro approach to support the different capacities of the spheroids subjected to different surface chemistry/stiffness and medium compositions.
Upon establishment of the benefits of spheroid priming, future studies could look at other medium raw material/growth factor combinations to find a winning formulation for cartilage-like tissue spheroids. The next step along the way is spheroid implantation into animal models and exploring flexible biomaterials for spheroid encapsulation would also be worthwhile.37
Conclusion
In the current study, we successfully primed the spheroids under different medium conditions with exposure to either HA-coated or non-coated well plates and observed the chondrogenesis of the spheroids. Although the current setup only considers chondrogenic applications, ultimately different medium composition should be explored for different applications. The key finding of the study is that the medium composition during the spheroid formation is most important parameter. Concurrently we observed that all the groups at t = 0 demonstrated a high cell viability, and high aggrecan and SOX-9 gene expression compared to the other time points. This finding is impactful as skipping in vitro culture following spheroid formation saves time and cost, thus making the process highly reproducible, and thus standardizing the protocol from a regulatory standpoint. Additionally, raw materials (i.e., CS and aggrecan) alone had the capability to induce SOX-9 and collagen II gene expression and collagen II immunostaining, compared to the growth factor-primed groups, suggesting that raw material addition in the medium could be a new economic and efficient way of looking at medium formulation for chondrogenic applications. Even the TGF ⇒ IGF media showed an increased collagen II gene expression compared to the control group at 7 days, thus showcasing the importance of the close interplay of relevant growth factors during the early stages of stem cell differentiation. The priming exercise gave new insights into 3D spheroid behavior under a diverse microenvironment, revealing the complex interplay between surface modification and medium composition (i.e., containing growth factors or raw materials). While the HA-coated surface may help in collagen I specific tissue-differentiation, the non-coated surface outperformed other groups for collagen II gene expression thus signifying the minimal conditions necessary to commence chondrogenesis. The inclusion of raw materials in the medium may be a promising path to generate cost-effective chondromimetic tissue for cartilage regeneration in the future.
References
Arufe, M., A. De la Fuente, I. Fuentes-Boquete, F. J. De Toro, and F. J. Blanco. Differentiation of synovial CD-105 + human mesenchymal stem cells into chondrocyte-like cells through spheroid formation. J. Cell. Biochem. 108:145–155, 2009.
Baraniak, P. R., M. T. Cooke, R. Saeed, M. A. Kinney, K. M. Fridley, and T. C. McDevitt. Stiffening of human mesenchymal stem cell spheroid microenvironments induced by incorporation of gelatin microparticles. J. Mech. Behav. Biomed. Mater. 11:63–71, 2012.
Baraniak, P. R., and T. C. McDevitt. Scaffold-free culture of mesenchymal stem cell spheroids in suspension preserves multilineage potential. Cell Tissue Res. 347:701–711, 2012.
Barry, F. P., and J. M. Murphy. Mesenchymal stem cells: clinical applications and biological characterization. Int. J. Biochem. Cell Biol. 36:568–584, 2004.
Bartosh, T. J., and J. H. Ylostalo. Preparation of anti‐inflammatory mesenchymal stem/precursor cells (MSCs) through sphere formation using hanging‐drop culture technique. Curr. Protocols Stem Cell Biol. 28:2B. 6.1–2B. 6.23, 2014.
Bartosh, T. J., J. H. Ylöstalo, A. Mohammadipoor, N. Bazhanov, K. Coble, K. Claypool, R. H. Lee, H. Choi, and D. J. Prockop. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc. Natl. Acad. Sci. 107:13724–13729, 2010.
Bulpitt, P., and D. Aeschlimann. New strategy for chemical modification of hyaluronic acid: preparation of functionalized derivatives and their use in the formation of novel biocompatible hydrogels. J. Biomed. Mater. Res. 47:152–169, 1999.
Burdick, J. A., and G. D. Prestwich. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 23:H41–H56, 2011.
Cals, F. L. J., C. A. Hellingman, W. Koevoet, R. J. B. de Jong, and G. J. V. M. van Osch. Effects of transforming growth factor-ss subtypes on in vitro cartilage production and mineralization of human bone marrow stromal-derived mesenchymal stem cells. J. Tissue Eng. Regen. Med. 6:68–76, 2012.
Cucchiarini, M., and H. Madry. Overexpression of human IGF-I via direct rAAV-mediated gene transfer improves the early repair of articular cartilage defects in vivo. Gene Ther. 21:811–819, 2014.
Fan, H., Y. Hu, L. Qin, X. Li, H. Wu, and R. Lv. Porous gelatin–chondroitin–hyaluronate tri-copolymer scaffold containing microspheres loaded with TGF-β1 induces differentiation of mesenchymal stem cells in vivo for enhancing cartilage repair. J. Biomed. Mater. Res. Part A. 77:785–794, 2006.
Fennema, E., N. Rivron, J. Rouwkema, C. van Blitterswijk, and J. de Boer. Spheroid culture as a tool for creating 3D complex tissues. Trends Biotechnol. 31:108–115, 2013.
Grimaud, E., D. Heymann, and F. Rédini. Recent advances in TGF-β effects on chondrocyte metabolism: potential therapeutic roles of TGF-β in cartilage disorders. Cytokine Growth Factor Rev. 13:241–257, 2002.
Gupta, V., N. Mohan, and M. S. Detamore. Microsphere-based scaffolds carrying opposing gradients of chondroitin sulfate and tricalcium phosphate. Frontiers Bioeng. Biotechnol. 2015. 10.3389/fbioe.2015.00096.
Hatsushika, D., T. Muneta, T. Nakamura, M. Horie, H. Koga, Y. Nakagawa, K. Tsuji, S. Hishikawa, E. Kobayashi, and I. Sekiya. Repetitive allogeneic intraarticular injections of synovial mesenchymal stem cells promote meniscus regeneration in a porcine massive meniscus defect model. Osteoarthr Cartil. 22:941–950, 2014.
Ingavle, G. C., A. W. Frei, S. H. Gehrke, and M. S. Detamore. Incorporation of aggrecan in interpenetrating network hydrogels to improve cellular performance for cartilage tissue engineering. Tissue Eng. Part A. 19:1349–1359, 2013.
Jaklenec, A., A. Hinckfuss, B. Bilgen, D. M. Ciombor, R. Aaron, and E. Mathiowitz. Sequential release of bioactive IGF-I and TGF-β 1 from PLGA microsphere-based scaffolds. Biomaterials. 29:1518–1525, 2008.
Jo, C. H., Y. G. Lee, W. H. Shin, H. Kim, J. W. Chai, E. C. Jeong, J. E. Kim, H. Shim, J. S. Shin, and I. S. Shin. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells. 32:1254–1266, 2014.
Kim, I. L., R. L. Mauck, and J. A. Burdick. Hydrogel design for cartilage tissue engineering: A case study with hyaluronic acid. Biomaterials. 32:8771–8782, 2011.
Kock, L. M., R. M. Schulz, C. C. van Donkelaar, C. B. Thümmler, A. Bader, and K. Ito. RGD-dependent integrins are mechanotransducers in dynamically compressed tissue-engineered cartilage constructs. J. Biomech. 42:2177–2182, 2009.
Kramer, J., F. Böhrnsen, U. Lindner, P. Behrens, P. Schlenke, and J. Rohwedel. In vivo matrix-guided human mesenchymal stem cells. Cell. Mol. Life Sci. 63:616–626, 2006.
Krampera, M., G. Pizzolo, G. Aprili, and M. Franchini. Mesenchymal stem cells for bone, cartilage, tendon and skeletal muscle repair. Bone. 39:678–683, 2006.
Lai, J.-Y., and I-H Tu Adhesion. phenotypic expression, and biosynthetic capacity of corneal keratocytes on surfaces coated with hyaluronic acid of different molecular weights. Acta Biomater. 8:1068–1079, 2012.
Lee, J., M. Sato, H. Kim, and J. Mochida. Transplantation of scaffold-free spheroids composed of synovium-derived cells and chondrocytes for the treatment of cartilage defects of the knee. Eur Cell Mater. 22:275–290, 2011.
Lewis, N. S., E. E. Lewis, M. Mullin, H. Wheadon, M. J. Dalby, and C. C. Berry. Magnetically levitated mesenchymal stem cell spheroids cultured with a collagen gel maintain phenotype and quiescence. J. Tissue Eng. 8:2041731417704428, 2017.
Loessner, D., K. S. Stok, M. P. Lutolf, D. W. Hutmacher, J. A. Clements, and S. C. Rizzi. Bioengineered 3D platform to explore cell–ECM interactions and drug resistance of epithelial ovarian cancer cells. Biomaterials. 31:8494–8506, 2010.
Madry, H., G. Kaul, D. Zurakowski, G. Vunjak-Novakovic, and M. Cucchiarini. Cartilage constructs engineered from chondrocytes overexpressing IGF-I improve the repair of osteochondral defects in a rabbit model. Eur Cell Mater. 25:229–247, 2013.
Martinez, I., J. Elvenes, R. Olsen, K. Bertheussen, and O. Johansen. Redifferentiation of in vitro expanded adult articular chondrocytes by combining the hanging-drop cultivation method with hypoxic environment. Cell Transplant. 17:987–996, 2008.
Mellott, A. J., M. E. Godsey, H. E. Shinogle, D. S. Moore, M. L. Forrest, and M. S. Detamore. Improving viability and transfection efficiency with human umbilical cord Wharton’s jelly cells through use of a ROCK inhibitor. Cell. Reprogramm. 16:91–97, 2014.
Miller, M. J., S. Ahmed, P. Bobrowski, and T. M. Haqqi. The chrondoprotective actions of a natural product are associated with the activation of IGF-1 production by human chondrocytes despite the presence of IL-1β. BMC Complement Altern. Med. 6:13, 2006.
Mohan, N., V. Gupta, B. Sridharan, A. Sutherland, and M. S. Detamore. The potential of encapsulating “raw materials” in 3D osteochondral gradient scaffolds. Biotechnol. Bioeng. 111:829–841, 2014.
Murphy, K. C., S. Y. Fang, and J. K. Leach. Human mesenchymal stem cell spheroids in fibrin hydrogels exhibit improved cell survival and potential for bone healing. Cell Tissue Res. 357:91–99, 2014.
Ng, K., J. DeFrancis, L. Kugler, T.-A. Kelly, M. Ho, C. O’Conor, G. Ateshian, and C. Hung. Amino acids supply in culture media is not a limiting factor in the matrix synthesis of engineered cartilage tissue. Amino Acids. 35:433–438, 2008.
Orozco, L., A. Munar, R. Soler, M. Alberca, F. Soler, M. Huguet, J. Sentís, A. Sánchez, and J. García-Sancho. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: A pilot study. Transplantation. 95:1535–1541, 2013.
Siddappa, R., R. Licht, C. van Blitterswijk, and J. de Boer. Donor variation and loss of multipotency during in vitro expansion of human mesenchymal stem cells for bone tissue engineering. J. Orthop. Res. 25:1029–1041, 2007.
Spiller, K. L., Y. Liu, J. L. Holloway, S. A. Maher, Y. Cao, W. Liu, G. Zhou, and A. M. Lowman. A novel method for the direct fabrication of growth factor-loaded microspheres within porous nondegradable hydrogels: Controlled release for cartilage tissue engineering. J. Controlled Rel. 157:39–45, 2012.
Sridharan, B., A. D. Laflin, M. A. Holtz, D. M. Pacicca, N. K. Wischmeier, and M. S. Detamore. In vivo evaluation of stem cell aggregates on osteochondral regeneration. J. Orthop. Res. 35(8):1606–1616, 2016.
Tan, H., C. R. Chu, K. A. Payne, and K. G. Marra. Injectable in situ forming biodegradable chitosan–hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials. 30:2499–2506, 2009.
Vinardell, T., E. J. Sheehy, C. T. Buckley, and D. J. Kelly. A comparison of the functionality and in vivo phenotypic stability of cartilaginous tissues engineered from different stem cell sources. Tissue Eng. Part A. 18:1161–1170, 2012.
Wang, L., and M. S. Detamore. Effects of growth factors and glucosamine on porcine mandibular condylar cartilage cells and hyaline cartilage cells for tissue engineering applications. Archives of oral biology 54:1–5, 2009.
Wang, L., and M. S. Detamore. Insulin-like growth factor-I improves chondrogenesis of predifferentiated human umbilical cord mesenchymal stromal cells. J. Orthop. Res. 27:1109–1115, 2009.
Wilson, R., D. Belluoccio, C. B. Little, A. J. Fosang, and J. F. Bateman. Proteomic characterization of mouse cartilage degradation in vitro. Arthritis Rheum. 58:3120–3131, 2008.
Winter, A., S. Breit, D. Parsch, K. Benz, E. Steck, H. Hauner, R. M. Weber, V. Ewerbeck, and W. Richter. Cartilage-like gene expression in differentiated human stem cell spheroids: A comparison of bone marrow–derived and adipose tissue–derived stromal cells. Arthritis Rheum. 48:418–429, 2003.
Xu, F., B. Sridharan, S. Wang, U. A. Gurkan, B. Syverud, and U. Demirci. Embryonic stem cell bioprinting for uniform and controlled size embryoid body formation. Biomicrofluidics. 5:022207, 2011.
YlÖstalo, J. H., T. J. Bartosh, K. Coble, and D. J. Prockop. Human mesenchymal stem/stromal cells cultured as spheroids are self-activated to produce prostaglandin E2 that directs stimulated macrophages into an anti-inflammatory phenotype. Stem Cells. 30:2283–2296, 2012.
Yoon, H. H., S. H. Bhang, J.-Y. Shin, J. Shin, and B.-S. Kim. Enhanced cartilage formation via three-dimensional cell engineering of human adipose-derived stem cells. Tissue Eng. Part A. 18:1949–1956, 2012.
Compliance with Ethical Standards
The National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R01 AR056347 supported research reported in this publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We would also like to recognize support from the Kansas Bioscience Authority Rising Star Award. BanuPriya Sridharan, Amy D. Laflin, and Michael S. Detamore, have no financial or intellectual property conflicts to disclose. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Michael R. King oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Sridharan, B., Laflin, A.D. & Detamore, M.S. Generating Chondromimetic Mesenchymal Stem Cell Spheroids by Regulating Media Composition and Surface Coating. Cel. Mol. Bioeng. 11, 99–115 (2018). https://doi.org/10.1007/s12195-017-0517-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-017-0517-4