Abstract
Objective
To reveal the shifts of microbial communities along ammonium gradients, and the relationship between microbial community composition and the anaerobic digestion performance using a high throughput sequencing technique.
Results
Methane production declined with increasing ammonium concentration, and was inhibited above 4 g l−1. The volatile fatty acids, especially acetate, accumulated with elevated ammonium. Prokaryotic populations showed different responses to the ammonium concentration: Clostridium, Tepidimicrobium, Sporanaerobacter, Peptostreptococcus, Sarcina and Peptoniphilus showed good tolerance to ammonium ions. However, Syntrophomonas with poor tolerance to ammonium may be inhibited during anaerobic digestion. During methanogenesis, Methanosarcina was the dominant methanogen.
Conclusion
Excessive ammonium inhibited methane production probably by decoupling the linkage between acidification process and methanogenesis, and finally resulted in different performance in anaerobic digestion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anaerobic digestion is widely applied to treat organic wastes (Bouallagui et al. 2004). It involves three main steps: substrate hydrolysis, acidification and methanogenesis. The conversion of organic matter to methane is dependent on the syntrophic interactions of functionally distinct microorganisms (Franke-Whittle et al. 2014). Decoupling of acidification and methanogenesis might result in the failure of anaerobic digestion.
Ammonium is an end-product of anaerobic digestion of proteins, urea and nucleic acids. High ammonium concentrations may severely inhibit anaerobic digestion (Yenigün and Demirel 2013). Free ammonia is the cause of the inhibition, which can passively diffuse into cells, causing proton imbalance and potassium deficiency (Chen et al. 2008; Niu et al. 2013). Ammonia also affects microbial communities in anaerobic digesters. Increased ammonium would select Firmicutes but inhibit syntrophic metabolism performed by specific species (Li et al. 2015).
Although the effect of ammonium on microbial communities has been studied (Abouelenien et al. 2010), many studies used low-resolution microbial profiling methods, such as terminal-restriction fragment length polymorphism (T-RFLP) and denaturing gradient gel electrophoresis (DGGE). These methods are unlikely to discern the response of sensitive particular taxonomic groups to ammonium inhibition (Kim et al. 2014). Moreover, the interaction of chemical properties are usually complex in samples collected from environment, e.g., full-scale or household anaerobic digesters, which makes it difficult to evaluate the direct influence of ammonium over microbial communities. Here we set up a series of anaerobic digesters with different concentration of ammonium, and analyzed microbial communities by high throughput sequencing techniques. The objectives were (i) to examine the direct effect of different concentration of ammonium on methane and volatile fatty acids production; (ii) to reveal the relationship between microbial communities and the anaerobic digestion performance.
Methods
Setup of fermentation system
The anaerobic digesters used the following conditions: total solids (from swine manure) 6 % (w/v), initial pH 7 ± 0.1, 35 ± 2 °C and a hydraulic retention time (HRT) of 8 days. After incubation for 6 days, NH4Cl was added to reactors at 2.5, 4, 5.5 and 7 g l−1 (labeled as R1, R2, R3 and R4, respectively); the control received only 1 g l−1. They were incubated for a further 15 days. All the experiments were conducted in triplicate. The reactors were manually mixed daily. The biogas production and methane content were daily monitored. The volatile fatty acids (VFAs) and microbial community composition were analyzed using samples collected at day 21.
PCR amplification, high throughput sequencing, sequencing data processing and statistical analysis
Genomic DNA was extracted using a kit (Sangon Biotech, China). PCR amplification was conducted as previously described (Li et al. 2014). PCR products were prepared for sequencing on the Illumina Miseq platform using MiSeq Reagent Kit v2.
Amplicon sequences were analyzed using the QIIME Pipeline (Caporaso et al. 2010). All sequence reads were sorted by their unique barcodes. Uchime algorithm was used to remove chimera sequences (Edgar et al. 2011). A 97 % identity of cut-off was used to cluster sequences into operational taxonomic units. Each sample was randomly resampled at 9190 reads. The phylogenetic affiliation of each sequence was assigned by the Ribosomal Database Project classifier (Wang et al. 2007). The original sequence data are available at the European Nucleotide Archive by Accession No. PRJEB14682.
Microbial community structure were assessed by principal coordinates analysis (PCoA) in Fast UniFrac (http://bmf.colorado.edu/fastunifrac/). Differences in the relative abundances of taxonomic units between samples were tested by one-way-analysis of variance (ANOVA). The linear or non-linear correlations between microbial diversity, species abundance and environmental factors were analyzed using SPSS 18.0 software.
Results and discussion
Effect of ammonium on methane and volatile fatty acids (VFAs) production
Daily methane production decreased once NH4Cl was added at day 6. Reactors R3 (with 5.5 g NH4Cl l−1) and R4 (with 7 g NH4Cl l−1) showed faster declines than R1 (2.5 g NH4Cl l−1) and R2 (Fig. 1a). From day 12 to 21, methane production in R1 and R2 started to recover to the condition of the control, while R3 and R4 recovered to <60 % of the original level at day 6. Eventually, the accumulative methane production of R1, R2, and control were higher than that of R3 and R4 (Fig. 1b). Both the daily and accumulative methane production suggested that anaerobic digestion system can tolerate a certain level of ammonium. However, digestion process was inhibited at >4 g NH4Cl l−1, since excessive ammonia would impose an inhibition effect on microbial activity.
Changes of daily and cumulative methane production. a Daily production rate of methane; b cumulative production of methane. The data was shown as means ± standard deviation (n = 3). The arrow indicates the time for NH4Cl addition. The concentration of NH4Cl for R1, R2, R3 and R4 was 2.5, 4, 5.5 and 7 g l−1, respectively
At the end of the process (day 21), acetic acid was the most abundant VFA in all the reactors (Fig. 2a) at 40, 69 and 72 mM in R1, R2 and the control, respectively. In contrast, 128 mM acetate was detected in both R3 and R4. Formic acid and propionic acid were both <40 mM. The variation of VFAs concentration may reflect a kinetic uncoupling between acid producers and consumers (Franke-Whittle et al. 2014). The much higher level of acetate in R3 and R4 suggested that NH4Cl >4 g l−1 decreased the transformation efficiency of the organic acids into methane. Usually, a high VFA concentration causes the pH value to decrease. However, due to the buffering capacity of high ammonium and \( {\text{HCO}}_{3}^{ - } \) contents (Sterling et al. 2001; Walter et al. 2015), the pH values of all digesters during the digestion process were relatively constant, from 6.7 to 7.1 (Fig. 2b). The optimum range is 6.8–7.4 for methane production (Khan et al. 2016). So, ammonium should be the main causative inhibitor.
Inhibition of the anaerobic digestion process is usually indicated by biogas production, accumulation of volatile fatty acids (VFAs), and the variation of pH values. This study suggested that anaerobic digestion system can tolerate a certain level of ammonium but inhibition occurs when beyond this threshold. Based on methane production and environmental variable dynamics of the system, we collected slurry samples at the end of fermentation for further microbial community analysis.
Effect of ammonium on microbial communities
The Miseq sequencing technique provides increased resolution to reveal microbial communities in anaerobic digesters (Vanwonterghem et al. 2014) and is used to study the shift of microbial community composition and structure in response to environmental variables (Li et al. 2014, 2015). Using the Miseq sequencing, we found that the microbial community diversity varied along the ammonium gradients. In general, observed operational taxonomic units, Shannon’s diversity and Simpson’s diversity indices increased with ammonium from 2.5 to 7 g l−1 (Supplementary Table 1). R1 treatment showed significant (p < 0.05) difference with the other treatments. Principal coordinate analysis showed that samples from different reactors were divided into three distinct groups (Fig. 3), suggesting that ammonium was the key driver to structure the microbial communities.
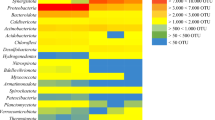
Among all the reactors, the relative abundances of representative phyla changed with the ammonium concentration implying that ammonium concentration changes by 1 g l−1 could dramatically shift microbial communities at phylum level (Table 1). The members of Firmicutes, such as Clostridium, Tepidimicrobium, Sporanaerobacter, Peptostreptococcus, Sarcina and Peptoniphilus, significantly and positively correlated with the concentrations of ammonium and acetic acid (Table 2), suggesting a high tolerance of these species to ammonium. Since Firmicutes mainly utilize cellulose, xylan, mono- and di-saccharides, and play important roles in the hydrolysis and acid formation during anaerobic digestion (Lin et al. 2016), the inhibition exerted by excessive ammonium may not influence these stages in anaerobic digestion. This is supported by the VFAs accumulation in our experiments. However, Syntrophomonas was negatively correlated with ammonium, pH and acetic acid, but positively correlated with methane, which suggested that Syntrophomonas is vulnerable to ammonium. Syntrophomonas performs syntrophic metabolism in association with hydrogenotrophic methanogens during anaerobic digestion (Shen et al. 2014). Thus it is vital for the transformation of butyrate to acetate and H2. In this study, excessive ammonium in R4 resulted in the decline of Syntrophomonas and, hence, the syntrophic metabolism may be inhibited.
Methanogens were mainly Methanosarcina, Methanobrevibacter, Methanoculleus, Methanosphaera and Methanomassillcoccus (Table 1). These methanogens produce methane through mixotrophic and hydrogenotrophic pathways. Methanogens related to different methanogenesis pathways have different tolerances to ammonium, with mixotrophic methanogens (Methanosarcina) > aceticlastic methanogens (Methanosaeta) (Lu et al. 2013). In this study, Methanosarcina dominated in all reactors, which is in line with the high level of acetate in the reactors. However, the accumulated acetate in R3 and R4 failed to be transformed by Methanosarcina, suggesting that excessive ammonium did inhibit methanogenic activity. Additionally, there was a discrepancy between the relative abundance of methanogens and methane production (Table 2). It is possible that the remnant DNA could still be detectable after a long time of cell death (Lu et al. 2013). Thus, further metatranscriptome analysis is needed.
Overall, anaerobic reactors fed with swine manure can tolerate a certain level of ammonium but inhibition occurs when beyond this threshold. Ammonium exerted a strong effect on the microbial communities. Excessive ammonium decouples the linkage between acidification and methanogenesis in anaerobic digestion. The shift of specific taxa under ammonium gradients may reflect their adaptations to different niches in anaerobic digestion process, which finally results in different efficiency in anaerobic digestion.
References
Abouelenien F, Fujiwara W, Namba Y, Kosseva M, Nishio N, Nakashimada Y (2010) Improved methane fermentation of chicken manure via ammonia removal by biogas recycle. Biores Technol 101:6368–6373
Bouallagui H, Torrijos M, Godon JJ, Moletta R, Cheikh RB, Touhami Y, Delgenes JP, Hamdi M (2004) Microbial monitoring by molecular tools of a two-phase anaerobic bioreactor treating fruit and vegetable wastes. Biotechnol Lett 26:857–862
Caporaso JG, Kuczynski J, Stombaugh J (2010) QIIME allows analysis of highthroughput community sequencing data. Nature Methods 7:335–336
Chen Y, Cheng JJ, Creamer KS (2008) Inhibition of anaerobic digestion process: a review. Biores Technol 99:4044–4064
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27:194–200
Franke-Whittle IH, Walter A, Ebner C, Insam H (2014) Investigation into the effect of high concentrations of volatile fatty acids in anaerobic digestion on methanogenic communities. Waste Manag 34:2080–2089
Khan MA, Ngo HH, Guo WS, Liu Y, Nghiem LD, Hai FI, Deng LJ, Wang J, Wu Y (2016) Optimization of process parameters for production of volation fatty acid, biohydrogen and methane from anaerobic digestion. Biores Technol 219:738–748
Kim SY, Pramanik P, Bodelier PL, Kim PJ (2014) Cattle manure enhances methanogens diversity and methane emissions compared to swine manure under rice paddy. PLoS ONE 9:593–611
Li X, Rui J, Mao Y, Yannarell A, Mackie R (2014) Dynamics of the bacterial community structure in the rhizosphere of a maize cultivar. Soil Biol Biochem 68:392–401
Li J, Rui J, Yao M, Zhang S, Yan X, Wang Y, Yan Z, Li X (2015) Substrate type and free ammonia determine bacterial community structure in full-scale mesophilic anaerobic digesters treating cattle or swine manure. Front Microbiol 6:1330–1337
Lin Q, He G, Rui J, Li X (2016) Microorganism-regulated mechanisms of temperature effects on the performance of anaerobic digestion. Microb Cell Factor 15:1–18
Lu F, Hao L, Guan D, Qi Y, Shao L, He P (2013) Synergetic stress of acids and ammonium on the shift in the methanogenic pathways during thermophilic anaerobic digestion of organics. Water Res 47:2297–2306
Niu Q, Qiang W, Qiang H, Li Y (2013) Microbial community shifts and biogas conversion computation during steady, inhibited and recovered stages of thermophilic methane fermentation on chicken manure with a wide variation of ammonia. Biores Technol 146:223–233
Shen P, Han F, Su S, Zhang J, Chen Z, Li J, Gan J, Feng B, Wu B (2014) Using pig manure to promote fermentation of sugarcane molasses alcohol wastewater and its effects on microbial community structure. Biores Technol 155:323–329
Sterling MC, Lacey RE, Engler CR, Ricke SC (2001) Effects of ammonia nitrogen on H2 and methane production during anaerobic digestion of dairy cattle manure. Bioresour Technol 77:9–18
Vanwonterghem I, Jensen P, Ho D, Batstone D, Tyson GW (2014) Linking microbial community structure, interactions and function in anaerobic digesters using new molecular techniques. Curr Opin Biotechnol 27:55–64
Walter A, Franke-Whittle IH, Wagner AO, Insam H (2015) Methane yields and methanogenic community changes during co-fermentation of cattle slurry with empty fruit bunches of oil palm. Biores Technol 175:619–623
Acknowledgements
The authors thank the support by National Key Technology Support Program (2014Banaerobic digestion02B04) and National Basic Research Program of China (2013CB733502).
Supplementary information
Supplementary Table 1—Microbial diversity indices at 97 % sequence similarity.
Supplementary Table 2—Variation partitioning analysis of environmental variables for the prokaryotic community structure.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, M., Lin, Q., Rui, J. et al. Ammonium inhibition through the decoupling of acidification process and methanogenesis in anaerobic digester revealed by high throughput sequencing. Biotechnol Lett 39, 247–252 (2017). https://doi.org/10.1007/s10529-016-2241-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-016-2241-x