Abstract
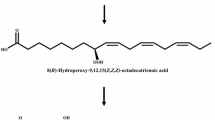
The optimal conditions for the production of 5,8-dihydroxy-9(Z)-octadecenoic acid (5,8-diHOME) from oleic acid by whole recombinant Escherichia coli cells expressing diol synthase from Aspergillus nidulans were 40 °C, pH 7.5, 10 % (v/v) dimethyl sulfoxide, 35 g cells l−1, and 12 g oleic acid l−1 at 250 rpm in a 250 ml baffled flask. Under these conditions, whole recombinant cells produced 5.2 g 5,8-diHOME l−1 together with 1 g l−1 of the intermediate 8-hydroperoxy-9(Z)-octadecenoic acid (8-HPOME) after 60 min. This corresponded to a conversion yield of 43 % (w/w), a volumetric productivity of 5.2 g l−1 h−1, and a specific productivity of 148 mg g cells−1 h−1. This is the first report of the biotechnological production of 5,8-diHOME from oleic acid.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydroxyfatty acids exhibit higher reactivity, viscosity, solvent miscibility, and hydrophilicity than free fatty acids (Metzger and Bornscheuer 2006; Hou 2009). These reactive properties have facilitated hydroxyfatty acids to be exploited as starting materials for resins, waxes, nylons, plastics, lubricants, and cosmetics (Naughton 1974; Ogunniyi 2006), and as precursors in the synthesis of the flavor lactones (An et al. 2013; An and Oh 2013). Dihydroxyfatty acids reduce surface tension and have antimicrobial properties against a wide range of bacteria and fungi (Parra et al. 1990; Paul et al. 2010). Thus, they have been used in the manufacture of polyurethane rigid foam and skin care products (Hou 2008).
Hydroxyfatty acids containing the different positions of hydroxy groups and double bonds exhibited different activities. For examples, 9S-hydroxy-10,12(E,Z)-octadecadienoic acid increased atherosclerosis and rheumatoid arthritis, whereas 13S-hydroxy-9,11(Z,E)-octadecadienoic acid reduced artherogenesis and inhibited cancer cell adhesion (Kim and Oh 2013). Thus, the biological activities of hydroxyfatty acids seems depend on the positions of hydroxy groups and double bonds.
The biotechnological production of dihydroxyfatty acids from oleic acid has so far been limited to Pseudomonas aeruginosa PR3. This strain has produced 7,10-dihydroxy-8(E)-octadecenoic acid (7,10-diHOME) from oleic acid or substrates containing oleic acid, such as triolein and olive oil (Hou et al. 1991; Kuo et al. 1998; Chang et al. 2008; Suh et al. 2011). 7,10-DiHOME has antibacterial activity of against plant pathogenic bacteria (Sohn et al. 2013). 5,8-Dihydroxy-9(Z)-octadecenoic acid (5,8-diHOME), a precocious sexual inducer (psi) factor, regulates the sexual and asexual life cycles of filamentous fungi (Mazur et al. 1991; Brodhun et al. 2009). However, other biological properties of 5,8-diHOME are unknown. Moreover, to the best of our knowledge, the biotechnological production of 5,8-diHOME from oleic acid has not yet been reported.
Before investigating the biological properties of 5,8-diHOME, we have achieved its production from oleic acid using whole recombinant Escherichia coli cells expressing diol synthase from Aspergillus nidulans (Fig. 1). The reaction conditions, such as pH, temperature, solvent, agitation speed, and concentrations of cells and substrate, were then optimized.
Materials and methods
Preparation of the hydroxyfatty acid standards
Whole recombinant cells expressing the wild-type or double-site variant (H1004A-C1006S) diol synthase from A. nidulans were used for the production of 5,8-diHOME or 8-hydroperoxy-9(Z)-octadecenoic acid (8-HPOME), respectively (Brodhun et al. 2009; Seo et al. 2014). After the reactions, these compounds were purified from the reaction solutions by solvent fractional crystallization at low temperature and the use of semi-prep HPLC system as previously described (Seo et al. 2014). The dihydroxyfatty acids obtained were purified to >99 % purity and were used as standards in subsequent analyses.
Microorganisms, plasmids, gene cloning, and culture conditions
Aspergillus nidulans ATCC 10074, E. coli ER2566, and pET-21a(+) plasmid were used as the sources of diol synthase gene, host cells, and expression vector, respectively. Pseudomonas aeruginosa PR3 was kindly provided by Professor Hak-Ryul Kim (Kyungpook National University, Daegu, South Korea), and was used for converting the published OD610 values (Chang et al. 2008; Suh et al. 2011) to the cell mass. Gene cloning was performed using the DNA sequence of A. nidulans diol synthase (GenBank accession number AY502073) as previously described (Seo et al. 2014). For protein expression, recombinant E. coli ER2566 cells were cultivated at 200 rpm in 2 l flask containing 500 ml LB medium at 37 °C with 20 μg ampicillin ml−1 until the OD600 reached 0.6. IPTG was then added at 0.1 mM, and culture was incubated at 16 °C for 16 h. The cells were harvested by centrifugation at 13,000×g for 20 min at 4 °C and then washed twice with saline to prepare a concentrated cell suspension for 5,8-diHOME production.
Optimization of reaction conditions
Unless otherwise stated, all reactions were performed in 50 mM HEPES (pH 7.5) buffer containing 0.14 g oleic acid l−1, 2 g cells l−1, and 10 % (v/v) dimethyl sulfoxide (DMSO) at 40 °C for 10 min. To determine the effects of pH and temperature on the production of 5,8-diHOME from oleic acid, the pH was varied from 6.0 to 8.5 using 50 mM HEPES buffer (pH 6.0–8.0) and 50 mM EPPS buffer (pH 8.0–8.5) at 40 °C; and the temperature was varied from 30 to 55 °C at pH 7.5. The thermal stability of the recombinant cells was determined after incubating the cells at temperatures ranging from 25 to 50 °C for 1 h. The solvents, including ethanol, methanol, ethyl acetate, propanol, iso-octane, DMSO, hexanol, dimethyl ether, hexane, and butanol, at 5 and 10 % (v/v) were tested in 50 mM HEPES buffer (pH 7.5) at 40 °C for 5,8-diHOME production. To determine the optimal concentration of DMSO for the production of 5,8-diHOME, its concentration was varied from 0 to 16 % (v/v). The optimal agitation speed for 5,8-diHOME production was determined from 0 to 270 rpm. The reactions were performed in 50 mM HEPES buffer (pH 7.5) containing 20 g cells l−1, 3 g oleic acid l−1, and 10 % (v/v) DMSO at 40 °C for 60 min. Cells varied from 5 to 55 g l−1 at a constant substrate concentration of 12 g oleic acid l−1 and the substrate was varied from 2 to 16 g l−1 at a constant concentration of 35 g cells l−1. The reactions were performed in 50 mM HEPES buffer (pH 7.5) and 10 % DMSO at 40 °C for 60 min.
Analytical methods
The cell mass was determined from OD600 values. Oleic acid and dihydroxyfatty acids were analyzed using an HPLC system with a reversed phase Nucleosil C18 column. The eluate was monitored at 202 nm and eluted at 40 °C at 0.4 ml min−1 using a gradient of solvent A (acetonitrile/water/acetic acid, 50/50/0.1, by vol.) and solvent B (acetonitrile/acetic acid, 100/0.1, v/v) as follows: 75 % solvent A for 0–3 min; 30 % solvent A for 3–8 min; 100 % solvent B for 8–15 min; and 75 % solvent A for 15–25 min. LC–MS/MS analysis was performed using an ESI interfaced with a Thermo-Finnigan LCQ Deca XP plus ion trap mass spectrometer (Thermo Scientific, Pittsburgh, PA, USA) at the NICEM facility (Seoul National University, Seoul, South Korea).
Results and discussion
Identification of the product and intermediate obtained from the conversion of oleic acid using whole recombinant cells expressing diol synthase from A. nidulans
The reaction product from the conversion of oleic acid using recombinant cells expressing the wild-type diol synthase from A. nidulans was analyzed by LC–MS/MS. The total molecular mass of the product was represented by a peak at m/z 313 (Fig. 2). The peak at m/z 295 was formed by loss of H2O from the total molecular mass. The peaks at m/z 115 and 173 resulted from the cleavage of the hydroxyl groups at the C5 and C8 positions from the α-carbon of the hydroxyfatty acid, respectively. These peaks indicated that the product was 5,8-diHOME. The reaction intermediate obtained from oleic acid by recombinant cells expressing the double-site variant (H1004A-C1006S) of diol synthase was identified as 8-HPOME using LC–MS/MS (Supplementary Fig. 1).
Optimization of the reaction conditions for the production of 5,8-diHOME from oleic acid using whole recombinant cells expressing diol synthase from A. nidulans
The reaction conditions for 5,8-diHOME production were optimized. Maximal conversion was at pH 7.5 and at 40 °C (Supplementary Figs. 2 and 3). The maximal conversion of 5,8-diHOME from linoleic acid by the same strain was at pH 7.5 and 35 °C (Seo et al. 2014). To evaluate the thermal stability of the recombinant cells, the residual enzyme activity was determined after 1 h pre-incubation at temperatures ranging from 25 to 50 °C (Supplementary Fig. 4). The enzyme activity decreased above 30 °C, and it was nearly abolished at 50 °C.
The effect of solvents on the production of 5,8-diHOME from oleic acid was also investigated. 5,8-DiHOME production was highest when using DMSO compared to the other solvents tested (Supplementary Fig. 5), with an optimal concentration of 10 % (v/v) (Supplementary Fig. 6). The production of 5,8-diHOME from linoleic acid by the same strain increased by adding 20 % (v/v) DMSO (Seo et al. 2014). To increase the production of 13-hydroxyoctadecadienoic acid from linoleic acid using immobilized soybean lipoxygenase, 10 % (v/v) DMSO was used (Omar et al. 2003).
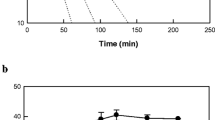
The production of 5,8-diHOME from oleic acid was improved by increasing the agitation speed (Fig. 3). However, above 250 rpm, the production began to plateau. Therefore, the agitation speed was set at 250 rpm. The optimal cell concentration was 35 g cells l−1 at 250 rpm (Fig. 4a). The effect of the substrate concentration on 5,8-diHOME production was assessed over 60 min. The conversion yield decreased as oleic acid increased above 4 g l−1. However, the maximum production of 5,8-diHOME required 12 g oleic acid l−1 (Fig. 4b). Therefore, the optimal concentration of the substrate was determined to be 12 g l−1, and the conversion yield was approx. 43 % (w/w).
Effect of agitation speed on the production of 5,8-diHOME from oleic acid using whole recombinant cells expressing diol synthase from A. nidulans. The reactions were performed in 50 mM HEPES buffer (pH 7.5) containing 20 g cells l−1, 5 g oleic acid l−1, and 10 % (v/v) DMSO at 40 °C for 60 min. Data present the means of three experiments and error bars represent the standard deviation
Effects of the concentrations of cells and substrate on the production of 5,8-diHOME from oleic acid using whole recombinant cells expressing diol synthase from A. nidulans. a Effect of cell concentration. The reactions were performed at 40 °C for 60 min at 250 rpm by varying the cell concentration from 5 to 55 g l−1 in 50 mM HEPES buffer (pH 7.5) containing 12 g oleic acid l−1, and 10 % (v/v) DMSO. b Effect of substrate concentration. 5,8-DiHOME (filled circle) and oleic acid (open square). The reactions were performed at 40 °C for 60 min at 250 rpm by varying the substrate concentration from 2 to 16 g l−1 in 50 mM HEPES buffer (pH 7.5) containing 35 g cells l−1, oleic acid, and 10 % (v/v) DMSO. Data present the means of three experiments and error bars represent the standard deviation
Production of 5,8-diHOME from oleic acid using whole recombinant cells expressing diol synthase from A. nidulans under the optimized conditions
The optimal reaction conditions for the production of 5,8-diHOME from oleic acid were 40 °C, pH 7.5, 10 % (v/v) DMSO, 35 g cells l−1, and 12 g oleic acid l−1 at 250 rpm in a 250 ml baffled flask. Under these conditions, whole recombinant cells produced 5.2 g 5,8-diHOME l−1 from 12 g oleic acid l−1 together with 1 g l−1 of the intermediate 8-hydroperoxy-9(Z)-octadecenoic acid after 60 min (Fig. 5). This corresponded to a conversion yield of 43 % (w/w), a volumetric productivity of 5.2 g l−1 h−1, and a specific productivity of 148 mg g-cell−1 h−1.
Time-course reactions for the production of 5,8-diHOME (filled circle) from oleic acid (open circle) via the 8-HPOME intermediate (filled triangle) using whole recombinant cells expressing diol synthase from A. nidulans under the optimized conditions. The reactions were performed at 40 °C for 70 min at 250 rpm in 50 mM HEPES buffer (pH 7.5) containing 35 g cells−1, 12 g oleic acid l−1, and 10 % (v/v) DMSO. Data present the means of three experiments and error bars represent the standard deviation
The production of dihydroxyfatty acids from oleic acid using whole recombinant E. coli expressing diol synthase from A. nidulans was compared with P. aeruginosa PR3 (Table 1). P. aeruginosa PR3 produced 9.1 g 7,10-diHOME l−1 from 10.2 g oleic acid l−1 with a conversion yield of 89 % (w/w) and a volumetric productivity of 0.19 g l−1 h−1 (Kuo et al. 1998). This was the highest reported concentration, volumetric productivity, and conversion yield. The production of 7,10-diHOME from olive oil by P. aeruginosa PR3 showed the highest specific productivity of 47 mg g-cell−1 h−1 (Suh et al. 2011). The volumetric and specific productivities of 5,8-diHOME production in this study were 28- and threefold higher than those obtained by whole P. aeruginosa PR3, respectively. However, the concentration and conversion yield obtained in this study were about 50 % less.
In summary, the production of 5,8-diHOME from oleic acid was optimized using whole recombinant cells expressing diol synthase from A. nidulans. Under these optimized conditions, whole recombinant cells produced 5.2 g 5,8-diHOME l−1 from 12 g oleic acid l−1 over 60 min. To the best of our knowledge, there are the highest volumetric and specific productivities reported in the production of dihydroxyfatty acid from oleic acid. This is the first report on the biotechnological production of 5,8-diHOME from oleic acid.
References
An JU, Oh DK (2013) Increased production of γ-lactones from hydroxyfatty acids by whole Waltomyces lipofer cells induced with oleic acid. Appl Microbiol Biotechnol 97:8265–8272
An JU, Joo YC, Oh DK (2013) New biotransformation process for production of the fragrant compound γ-dodecalactone from 10-hydroxystearate by permeabilized Waltomyces lipofer cells. Appl Environ Microbiol 79:2636–2641
Brodhun F, Gobel C, Hornung E, Feussner I (2009) Identification of PpoA from Aspergillus nidulans as a fusion protein of a fatty acid heme dioxygenase/peroxidase and a cytochrome P450. J Biol Chem 284:11792–11805
Chang IA, Bae JH, Suh MJ, Kim IH, Hou CT, Kim HR (2008) Environmental optimization for bioconversion of triolein into 7,10-dihydroxy-8(E)-octadecenoic acid by Pseudomonas aeruginosa PR3. Appl Microbiol Biotechnol 78:581–586
Hou CT (2008) New bioactive fatty acids. Asia Pac J Clin Nutr 17(1):192–195
Hou CT (2009) Biotechnology for fats and oils: new oxygenated fatty acids. New Biotechnol 26:2–10
Hou CT, Bagby MO, Plattner RD, Koritala S (1991) A novel compound, 7,10-dihydroxy-8(E)-octadecenoic acid from oleic acid by bioconversion. J Am Oil Chem Soc 68:99–101
Kim KR, Oh DK (2013) Production of hydroxyfatty acids by microbial fatty acid-hydroxylation enzymes. Biotechnol Adv 31:1473–1485
Kuo TM, Manthey LK, Hou CT (1998) Fatty acid bioconversions by Pseudomonas aeruginosa PR3. J Am Oil Chem Soc 75:875–879
Mazur P, Nakanishi K, El-Zayat AAE, Champe SP (1991) Structure and synthesis of sporogenic psi factors from Aspergillus nidulans. J Chem Soc, Chem Commun 20:1486–1487
Metzger JO, Bornscheuer U (2006) Lipids as renewable resources: current state of chemical and biotechnological conversion and diversification. Appl Microbiol Biotechnol 71:13–22
Naughton FC (1974) Production, chemistry, and commercial applications of various chemicals from castor oil. J Am Oil Chem Soc 51:65–71
Ogunniyi D (2006) Castor oil: a vital industrial raw material. Bioresour Technol 97:1086–1091
Omar MN, Moynihan H, Hamilton R (2003) Scaling-up the production of 13S-hydroxy-9Z,11E-octadecadienoic acid (13S-HODE) through chemoenzymatic technique. Bull Korean Chem Soc 24:397–399
Parra JL, Pastor J, Comelles F, Manresa MA, Bosch MP (1990) Studies of biosurfactants obtained from olive oil. Tenside Surf Det 27:302–306
Paul S, Hou CT, Kang SC (2010) α-Glucosidase inhibitory activities of 10-hydroxy-8(E)-octadecenoic acid: an intermediate of bioconversion of oleic acid to 7,10-dihydroxy-8(E)-octadecenoic acid. New Biotechnol 27:419–423
Seo MJ, Shin KC, Oh DK (2014) Production of 5,8-dihydroxy-9,12(Z,Z)-octadecadienoic acid from linoleic acid by whole recombinant Escherichia coli cells expressing diol synthase from Aspergillus nidulans. Appl Microbiol Biotechnol. doi:10.1007/s00253-014-5709-4
Sohn HR, Bae JH, Hou CT, Kim HR (2013) Antibacterial activity of a 7,10-dihydroxy-8(E)-octadecenoic acid against plant pathogenic bacteria. Enzyme Microb Tech 53:152–153
Suh MJ, Baek KY, Kim BS, Hou CT, Kim HR (2011) Production of 7,10-dihydroxy-8(E)-octadecenoic acid from olive oil by Pseudomonas aeruginosa PR3. Appl Microbiol Biotechnol 89:1721–1727
Acknowledgments
This study was supported by Grants from the Bio-industry Technology Development Program, Ministry for Agriculture, Food and Rural Affairs (No. 112002-3) and the Korea Healthcare Technology R&D Project, Ministry for Health & Welfare, Republic of Korea (No. 2012-009).
Supporting information
Supplementary Fig. 1—LC–MS/MS spectrum of 8-HPOME obtained from conversion of α-linolenic acid using diol synthase from A. nidulans double-site (H1004A-C1006S) variant
Supplementary Fig. 2—Effect of temperature on the production of 5,8-diHOME from oleic acid using whole recombinant cells expressing diol synthase from A. nidulans
Supplementary Fig. 3—Effect of pH on the production of 5,8-diHOME from oleic acid using whole recombinant cells expressing diol synthase from A. nidulans
Supplementary Fig. 4—Effect of enzyme stability on the production of 5,8-diHOME from oleic acid using whole recombinant cells expressing diol synthase from A. nidulans
Supplementary Fig. 5—Effect of solvents on the production of 5,8-diHOME from oleic acid using whole recombinant cells expressing diol synthase from A. nidulans
Supplementary Fig. 6—Effect of dimethyl sulfoxide concentration on the production of 5,8-diHOME from oleic acid using whole recombinant cells expressing diol synthase from A. nidulans
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Seo, MJ., Shin, KC., Jeong, YJ. et al. Production of 5,8-dihydroxy-9(Z)-octadecenoic acid from oleic acid by whole recombinant cells of Aspergillus nidulans expressing diol synthase. Biotechnol Lett 37, 131–137 (2015). https://doi.org/10.1007/s10529-014-1650-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-014-1650-y