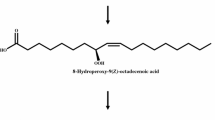
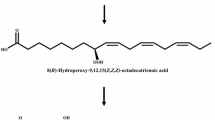
Abstract
Objective
To increase the production of 10S-hydroxy-8(E)-octadecenoic acid from oleic acid by whole recombinant Escherichia coli cells expressing Nostoc punctiforme 10S-dioxygenase with the aid of a chaperone.
Results
The optimal conditions for 10S-hydroxy-8(E)-octadecenoic acid production by recombinant cells co-expressing chaperone plasmid were pH 9, 35 °C, 15 % (v/v) dimethyl sulfoxide, 40 g cells l−1, and 10 g oleic acid l−1. Under these conditions, recombinant cells co-expressing chaperone plasmid produced 7.2 g 10S-hydroxy-8(E)-octadecenoic acid l−1 within 30 min, with a conversion yield of 72 % (w/w) and a volumetric productivity of 14.4 g l−1 h−1.
Conclusion
The activity of recombinant cells expressing 10S-dioxygenase was increased by 200 % with the aid of a chaperone, demonstrating the first biotechnological production of 10S-hydroxy-8(E)-octadecenoic acid using recombinant cells expressing 10S-dioxygenase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydroxy unsaturated fatty acids have higher viscosity, solubility, hydrophilicity, reactivity, and antibacterial activity than non-hydroxy unsaturated fatty acids. Owing to their functionality, hydroxy unsaturated fatty acids have been widely used as starting materials for the synthesis of chemical compounds and are key ingredients in antimicrobial food additives, antibacterial herbs, cosmetics, and medicines (Hou 2000; Zheng et al. 2005; Kim and Oh 2013; Han et al. 2016).
Pseudomonas aeruginosa (Kuo et al. 2008) and P. aeruginosa 42A2 (Culleré et al. 2001; Bódalo et al. 2005; Martin-Arjol et al. 2013), which are wild-type bacteria containing 10S-dioxygenase, convert oleic acid to 10S-hydroperoxy-8(E)-octadecenoic acid, which is reduced to 10S-hydroxy-8(E)-octadecenoic acid. 10S-Dioxygenase from Nostoc punctiforme PCC 73102 was cloned and expressed in Escherichia coli (Brash et al. 2014). However, the quantitative production of 10S-hydroxy-8(E)-octadecenoic acid by recombinant cells has not been attempted.
Overproduction of recombinant proteins in host cells often leads to their misfolding and aggregation (Liberek et al. 2008). Chaperones can assist in the refolding of misfolded and aggregated proteins to their native states, and chaperone-aided expression is associated with increased production of active proteins. The most abundant and physiologically important chaperones in E. coli are known to be GroES/GroEL, DnaK/DnaJ/GrpE, and DnaK/DnaJ/GrpE/GroES/GroEL (Nishihara et al. 1998).
In the present study, the activity of recombinant E. coli cells expressing 10S-dioxygenase from N. punctiforme PCC 73102 was increased with the aid of a chaperone, and recombinant cells co-expressing chaperone plasmid were used to produce 10S-hydroxy-8(E)-octadecenoic acid. The reaction conditions, including pH, temperature, and the concentrations of dimethyl sulfoxide, cells, and substrate were optimized. Under the optimized conditions, increased production of 10S-hydroxy-8(E)-octadecenoic acid from oleic acid was achieved.
Materials and methods
Preparation of hydroxy fatty acid standards
Recombinant cells expressing 10S-dioxygenase from N. punctiforme PCC 73102 were used to prepare 10S-hydroxy-8(E)-octadecenoic acid, 10S-hydroxy-8,12(E,Z)-octadecadienoic acid, and 10S-hydroxy-8,12,15(E,Z,Z)-octadecatrienoic acid from unsaturated fatty acids including oleic acid, linoleic acid, and α-linolenic acid, respectively. The reactions were performed in 50 mM Tris/HCl (pH 7.0) containing 5 g cells l−1, 2 g unsaturated fatty acid l−1, and 5 % (v/v) dimethyl sulfoxide at 35 °C with agitation of 250 rpm for 60 min. After the reactions 10S-hydroperoxy fatty acid in the supernatant was reduced to 10S-hydroxy fatty acid with 100 mM l-cysteine on ice for 20 min. It was then purified as described previously (Seo et al. 2014). Purified hydroxy unsaturated fatty acids (purity > 99 %) were used as standards.
Bacterial strains, plasmids, and culture conditions
Escherichia coli BL21(DE3) and the pET15b plasmid (Novagen) were used as the host cells and expression vector, respectively. The 10S-dioxygenase gene of N. punctiforme PCC 73102 (GenBank accession number ACC83776.1; 1692 base pairs) was synthesized by Cosmogenetech. The synthesized gene was cloned and expressed in E. coli BL21(DE3). Each chaperone plasmid, pGro7 (groES-groEL), pKJE7 (dnaK-dnaJ-grpE), or pG-KJE8 (dnaK-dnaJ-grpE-groES-groEL) (Takara), was co-expressed in the host cells. Cells co-expressing chaperone plasmid were grown in a 2 l flask containing 500 ml lysogeny broth (LB) at 37 °C with shaking at 200 rpm. LB contains 35 μg ampicillin ml−1 and 30 μg chloramphenicol ml−1; 2 mg l-arabinose ml−1; or 2 mg l-arabinose ml−1 and 5 ng tetracycline ml−1, was used for pET15b; pGro7 or pKJE7; or pG-KJE8 selection, respectively. IPTG at 0.1 mM was added to induce 10S-dioxygenase expression when the OD600 value reached 0.8. The culture was incubated at 16 °C with shaking at 150 rpm for 18 h.
Enzyme purification
Cells were harvested from culture broth, resuspended in 50 mM phosphate buffer (pH 7.0) containing 300 mM NaCl and 1 mg lysozyme ml−1, and disrupted by sonication on ice. Unbroken cells and cell debris were removed by centrifugation at 13,000×g for 20 min, and the pellet and supernatant (crude enzyme) were subjected to SDS-PAGE analysis to identify 10S-dioxygenase and chaperone proteins. The supernatant was applied to a His-Trap HP affinity chromatography column equilibrated with 50 mM phosphate buffer (pH 7) on an FPLC system. The bound protein was eluted using the same buffer that contained a linear gradient of 10–250 mM imidazole at 1 ml min−1. The active fractions were collected and dialyzed against 50 mM CHES buffer (pH 9) for 16 h. After dialysis, the resulting solution was used as the purified enzyme. All protein purification steps proceeded at 4 °C.
Determination of specific activities of purified enzyme and recombinant cells
To determine the specific activities of purified 10S-dioxygenase and recombinant cells expressing 10S-dioxygenase from N. punctiforme with or without a chaperon toward unsaturated fatty acids, the reactions with oleic acid, linoleic acid, and α-linolenic acid were performed in 50 mM CHES buffer (pH 9.0) containing 0.14 g unsaturated fatty acid l−1, 0.05 g enzyme l−1 or 0.1 g cells l−1, and 5 % (v/v) dimethyl sulfoxide at 35 °C for 5 min.
Production of 10S-hydroxy-8(E)-octadecenoic acid from oleic acid
The optimal concentrations of cells and substrate for 10S-hydroxy-8(E)-octadecenoic acid production were determined by varying the cell concentration from 5 to 60 g l−1 with 10 g oleic acid l−1 and varying the substrate concentration from 2.5 to 18 g l−1 with 40 g cells l−1 for 60 min. The time-course reactions for 10S-hydroxy-8(E)-octadecenoic acid production by recombinant cells co-expressing the 10S-dioxygenase gene of N. punctiforme with pGro7 were conducted in 50 mM CHES buffer (pH 9.0) containing 10 g oleic acid l−1, 40 g cells l−1, and 15 % (v/v) dimethyl sulfoxide at 35 °C with agitation at 250 rpm for 40 min.
Analytical methods
The cell mass was determined from OD600 values. The concentrations of unsaturated fatty acids and hydroxy unsaturated fatty acids were determined using an HPLC system, a reverse phase Nucleosil C18 column and with detection at 202 nm. The eluate was obtained using a gradient of solvent A (acetonitrile/water/acetic acid, 50:50:0.1, by vol.) and solvent B (acetonitrile/acetic acid, 100:0.1, v/v) at 35 °C. LC–MS/MS analysis of hydroxy unsaturated fatty acids was performed using a Thermo-Finnigan LCQ Deca XP plus ion trap mass spectrometer at the NICEM facility (Seoul National University). The concentrations of hydroxy unsaturated fatty acids were determined using linear calibration curves relating the peak areas to the concentrations of authentic standards as determined by LC–MS/MS methods.
Results and discussion
Identification of the products obtained from the conversion of unsaturated fatty acids by recombinant cells expressing 10S-dioxygenase from N. punctiforme
The reaction products obtained from the conversion of unsaturated fatty acid by recombinant cells expressing 10S-dioxygenase from N. punctiforme was analyzed by LC–MS/MS. The total molecular mass of the product was represented by a peak at m/z 297 in LC–MS/MS (Supplementary Fig. 1a). Fragments are formed by cleavage of the hydroxylated carbon (Oliw et al. 1998). The peak at m/z 184 resulted from α-cleavage of the hydroxyl group at the C10 position. The peak at m/z 279 was formed by the loss of H2O from the total molecular mass. The product chirality of 10S-dioxygenase from N. punctiforme was reported as the S-form (Brash et al. 2014). Therefore, the product was 10S-hydroxy-8(E)-octadecenoic acid. The LC–MS/MS spectra of the products formed from linoleic acid and α-linolenic acid by recombinant cells expressing 10S-dioxygenase from N. punctiforme also showed the same peaks at m/z 184 fragment, indicating that these compounds had a hydroxyl group at the C10 position (Supplementary Fig. 1b, c). The total molecular masses of these products were represented by peaks at m/z 295 and 293 fragment, respectively. These peaks indicated that the products were 10S-hydroxy-8,12(E,Z)-octadecadienoic acid and 10-hydroxy-8,12,15(E,Z,Z)-octadecatrienoic acid, respectively.
Substrate specificity of purified 10S-dioxygenase and recombinant cells expressing 10S-dioxygenase from N. punctiforme toward unsaturated fatty acids
The levels of specific activities of purified 10S-dioxygenase and recombinant cells expressing 10S-dioxygenase from N. punctiforme toward unsaturated fatty acids followed the order oleic acid > linoleic acid > α-linolenic acid (Table 1). The k cat values of purified N. punctiforme 10S-dioxygenase followed the same order as the specific activities (Brash et al. 2014). Oxygenases, including 5,8-linoleate diol synthase from Aspergillus nidulans (Seo et al. 2014), 13-lipoxygenase from Burkholderia thailandensis (Sim et al. 2015), and 10R-dioxygenase from A. nidulans (Han et al. 2016), are more stable in recombinant cells than purified oxygenases for the conversion of unsaturated fatty acids to hydroxy unsaturated fatty acids. Therefore, instead of using purified 10S-dioxygenase, recombinant cells expressing 10S-dioxygenase from N. punctiforme were used to produce 10S-hydroxy-8(E)-octadecenoic acid.
Effect of chaperone co-expression on the activity of recombinant cells and the expression of soluble 10S-dioxygenase from N. punctiforme in recombinant cells
The effect of chaperone co-expression in E. coli on the activity of recombinant cells expressing 10S-dioxygenase from N. punctiforme was investigated with aid of plasmid pGro7, pKJE7, or pG-KJE8. The level of activity of recombinant cells with the chaperone plasmid followed the order pGro7 > pG-KJE8 > pKJE7 (Fig. 1a). The activity of recombinant cells co-expressing pGro7, pG-KJE8, or pKJE7 was 200, 182, or 55 % higher than that of cells expressing the 10S-dioxygenase gene without chaperone plasmid (control). The DnaK chaperone already existed in the expressed proteins of E. coli BL21 (DE3), the host cells used for this study. The chaperone system makes it possible to stabilize the overexpressed genes. Thus, the DnaK was expressed in E. coli BL21 (DE3) without chaperone and overexpressed in the host with the chaperone plasmid pKJE7 or pG-KJE8. The molecular weights of DnaK, 10S-dioxygenase, and GroEL were 70, 63, and 60 kDa, respectively. Due to a little difference of molecular weights, the two overexpressed bands from top in SDS-PAGE of crude extract of pGro7 were seemed to be DnaK and 10S-dioxygenase. However, they were 10S-dioxygenase and GroEL (Fig. 1b). SDS-PAGE showed that the soluble 10S-dioxygenase content in the crude extract of recombinant cells co-expressing the 10S-dioxygenase gene with pGro7 was higher than that of recombinant cells co-expressing pKJE7 or pG-KJE8. In another study, the co-expression of pGro7 was also used to increase the solubility of carotenoid cleavage dioxygenase from Nostoc sp. PCC7120 (Marasco et al. 2006). Thus, recombinant cells co-expressing the 10S-dioxygenase gene of N. punctiforme with pGro7 were used in subsequent experiments.
Effect of chaperone co-expression on recombinant cells containing 10S-dioxygenase from N. punctiforme. The control was recombinant cells expressing the 10S-dioxygenase gene without the chaperone plasmid. a Effect of chaperone co-expression on the activity of recombinant cells. The reactions were performed in 50 mM CHES buffer (pH 9.0) containing 0.14 g oleic acid l−1, 0.1 g cells l−1, and 5 % (v/v) dimethyl sulfoxide for 5 min. At 100 % relative activity, 0.057 g 10S-hydroperoxy-8(E)-octadecenoic acid l−1 was produced. Data represent the means of three separate experiments and error bars represent the standard deviation. b Effect of chaperone co-expression on the soluble expression of 10S-dioxygenase from N. punctiforme in recombinant cells. The pellet (P) and crude enzyme (CE) fractions of recombinant cells expressing 10S-dioxygenase with or without chaperone in SDS-PAGE. 10S-Dioxygenase (molecular mass 63 kDa) and chaperone genes such as DnaK (70 kDa), GroEL (60 kDa), DnaJ (40 kDa), GrpE (25 kDa), and GroES (10 kDa) are marked with arrows
Optimization of reaction conditions for the production of 10S-hydroxy-8(E)-octadecenoic acid from oleic acid by recombinant cells co-expressing chaperone plasmid
The maximal conversion yield of oleic acid to 10S-hydroxy-8(E)-octadecenoic acid by whole recombinant E. coli cells co-expressing the 10S-dioxygenase gene of N. punctiforme with pGro7 was at pH 9 (Supplementary Fig. 2a), 35 °C (Supplementary Fig. 2b), and 15 % (v/v) dimethyl sulfoxide (Supplementary Fig. 3). The production of 10S-hydroxy-8(E)-octadecenoic acid from oleic acid as a substrate by P. aeruginosa 42A2 was performed at pH 8 or 7 and 30 °C (Bódalo et al. 2005; Kuo et al. 2008). The production of 10S-hydroxy-8(E)-octadecenoic acid from oleic acid as a substrate was investigated by varying the concentration of cells and substrate (Fig. 2). Below 40 g cells l−1 and 10 g oleic acid l−1, 10S-hydroxy-8(E)-octadecenoic acid production increased as the concentrations of cells and oleic acid increased. However, above these concentrations, 10S-hydroxy-8(E)-octadecenoic acid production reached a plateau. Therefore, the optimal concentrations of cells and oleic acid were 40 and 10 g l−1, respectively.
Effects of the concentrations of cells and substrate on the production of 10S-hydroxy-8(E)-octadecenoic acid from oleic acid by whole recombinant cells co-expressing the 10S-dioxygenase gene of N. punctiforme with pGro7. a Effects of cell concentration. The reactions were performed in 50 mM CHES (pH 9.0) containing 10 g oleic acid l−1, cells, and 15 % (v/v) dimethyl sulfoxide at 35 °C with agitation at 250 rpm for 60 min. b Effect of substrate concentration. The reactions were performed in 50 mM CHES (pH 9.0) containing oleic acid, 40 g cells l−1, and 15 % (v/v) dimethyl sulfoxide at 35 °C with agitation at 250 rpm for 60 min. After the reactions, 10S-hydroperoxy-8(E)-octadecenoic acid in the reaction solutions was reduced to 10S-hydroxy-8(E)-octadecenoic acid with 100 mM l-cysteine on ice for 20 min. 10S-Hydroxy-8(E)-octadecenoic acid production (filled circles) and conversion yield (empty circles). Data represent the means of three separate experiments, and error bars represent the standard deviation
Production of 10S-hydroxy-8(E)-octadecenoic acid from oleic acid by recombinant cells co-expressing chaperone plasmid under optimized conditions
The optimal reaction conditions for the production of 10S-hydroxy-8(E)-octadecenoic acid from oleic acid were pH 9.0, 35 °C, 15 % (v/v) dimethyl sulfoxide, 40 g cells l−1, and 10 g oleic acid l−1. Under these conditions, whole recombinant cells co-expressing the 10S-dioxygenase gene with pGro7 produced 7.2 g 10S-hydroxy-8(E)-octadecenoic acid l−1 after 30 min (Fig. 3), with a conversion yield of 72 % (w/w) and a volumetric productivity of 14.4 g l−1 h−1.
Time course reactions for the production of 10S-hydroxy-8(E)-octadecenoic acid (filled circles) from oleic acid (open circles) by recombinant cells co-expressing the 10S-dioxygenase gene of N. punctiforme with pGro7 under the optimized conditions. The reactions were performed in 50 mM CHES (pH 9.0) containing 10 g oleic acid l−1, 40 g cells l−1, and 15 % (v/v) dimethyl sulfoxide at 35 °C with agitation at 250 rpm for 40 min. After reactions, 10S-hydroperoxy-8(E)-octadecenoic acid in the reaction solutions was reduced to 10S-hydroxy-8(E)-octadecenoic acid with 100 mM l-cysteine on ice for 20 min
The production of 10S-hydroxy-8(E)-octadecenoic acid from oleic acid by fermentation and cell conversions is summarized in Table 2. P. aeruginosa 42A2 NCIMB 40045 converted 20 g oleic acid l−1 to 8.6 g 10S-hydroxy-8(E)-octadecenoic acid l−1, with a yield of 43 %, a volumetric productivity of 0.29 g l−1 h−1, and a specific productivity of 41 mg g−1 h−1 (Martin-Arjol et al. 2013). These are the highest concentration, volumetric productivity, and specific productivity that have been reported for 10S-hydroxy-8(E)-octadecenoic acid production prior to the results being reported here. P. aeruginosa 42A2 NCIMB 40045 gave 49 % (w/w) conversion yield, which was previously the highest conversion recorded. The concentration, conversion yield, and volumetric and specific productivities for 10S-hydroxy-8(E)-octadecenoic acid achieved in this study were 84, 147, 4970, and 880 %, respectively, relative to the previously highest results.
In summary, the activity of recombinant E. coli cells expressing the 10S-dioxygenase gene of N. punctiforme was increased by 200 % by co-expression of the chaperone plasmid pGro7. To the best of our knowledge, this is the first biotechnological production of 10S-hydroxy-8(E)-octadecenoic using recombinant cells. Moreover, the production of 10S-hydroxy-8(E)-octadecenoic acid from oleic acid by whole recombinant cells co-expressing 10S-dioxygenase gene with the chaperone plasmid gave the highest conversion yield and volumetric and specific productivities for 10S-hydroxy-8(E)-octadecenoic acid reported thus far.
References
Bódalo A, Bastida J, Máximo M, Hidalgo A, Murcia M (2005) Production of (E)10-hydroxy-8-octadecenoic acid with lyophilized microbial cells. Am J Biochem Biotechnol 1:1–4
Brash AR, Niraula NP, Boeglin WE, Mashhadi Z (2014) An ancient relative of cyclooxygenase in cyanobacteria is a linoleate 10S-dioxygenase that works in tandem with a catalase-related protein with specific 10S-hydroperoxide lyase activity. J Biol Chem 289:13101–13111
Culleré J, Durany O, Busquets M, Manresa A (2001) Biotransformation of oleic acid into (E)-10-hydroxy-8-octadecenoic acid and (E)-7,10-dihydroxy-8-octadecenoic acid by Pseudomonas sp. 42A2 in an immobilized system. Biotechnol Lett 23:215–219
Han JE, Seo MJ, Shin KC, Oh DK (2016) Production of 10R-hydroxy unsaturated fatty acids from hempseed oil hydrolyzate by recombinant Escherichia coli cells expressing PpoC from Aspergillus nidulans. Appl Microbiol Biotechnol. doi:10.1007/s00253-00016-07508-00256
Hou CT (2000) Biotransformation of unsaturated fatty acids to industrial products. Adv Appl Microbiol 47:201–220
Kim KR, Oh DK (2013) Production of hydroxy fatty acids by microbial fatty acid-hydroxylation enzymes. Biotechnol Adv 31:1473–1485
Kuo TM, Huang JK, Labeda D, Wen L, Knothe G (2008) Production of 10-hydroxy-8(E)-octadecenoic acid from oleic acid conversion by strains of Pseudomonas aeruginosa. Curr Microbiol 57:437–441
Liberek K, Lewandowska A, Zietkiewicz S (2008) Chaperones in control of protein disaggregation. EMBO J 27:328–335
Marasco EK, Vay K, Schmidt-Dannert C (2006) Identification of carotenoid cleavage dioxygenases from Nostoc sp. PCC 7120 with different cleavage activities. J Biol Chem 281:31583–31593
Martin-Arjol I, Busquets M, Manresa A (2013) Production of 10(S)-hydroxy-8(E)-octadecenoic acid mono-estolides by lipases in non-aqueous media. Proc Biochem 48:224–230
Nishihara K, Kanemori M, Kitagawa M, Yanagi H, Yura T (1998) Chaperone co-expression plasmids: differential and synergistic roles of DnaK-DnaJ-GrpE and GroEL-GroES in assisting folding of an allergen of Japanese cedar pollen, Cryj2, in Escherichia coli. Appl Environ Microbiol 64:1694–1699
Oliw EH, Su C, Skogström T, Benthin G (1998) Analysis of novel hydroperoxides and other metabolites of oleic, linoleic, and linolenic acids by liquid chromatography-mass spectrometry with ion trap MSn. Lipids 33:843–852
Seo MJ, Shin KC, Oh DK (2014) Production of 5,8-dihydroxy-9,12(Z, Z)-octadecadienoic acid from linoleic acid by whole recombinant Escherichia coli cells expressing diol synthase from Aspergillus nidulans. Appl Microbiol Biotechnol 98:7447–7456
Sim DH, Shin KC, Oh DK (2015) 13-Hydroxy-9Z,11E-octadecadienoic acid production by recombinant cells expressing Burkholderia thailandensis 13-lipoxygenase. J Am Oil Chem Soc 92:1259–1266
Zheng CJ, Yoo JS, Lee TG, Cho HY, Kim YH, Kim WG (2005) Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Lett 579:5157–5162
Acknowledgments
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health & Welfare, Republic of Korea (No. 2012-009).
Supporting information
Supplementary Fig. 1—LC–MS/MS spectra for the products obtained from the conversion of unsaturated fatty acids to 10S-hydroxy fatty acids by recombinant cells expressing 10S-dioxygenase from N. punctiforme PCC 73102.
Supplementary Fig. 2—Effects of pH and temperature on the conversion of oleic acid to 10S-hydroxy-8(E)-octadecenoic acid by recombinant cells co-expressing the 10S-dioxygenase gene of N. punctiforme with pGro7.
Supplementary Fig. 3—Effect of dimethyl sulfoxide concentration on the conversion of oleic acid to 10S-hydroxy-8(E)-octadecenoic acid by recombinant cells co-expressing the 10S-dioxygenase gene of N. punctiforme with pGro7.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, MJ., Seo, MJ., Shin, KC. et al. Production of 10S-hydroxy-8(E)-octadecenoic acid from oleic acid by whole recombinant Escherichia coli cells expressing 10S-dioxygenase from Nostoc punctiforme PCC 73102 with the aid of a chaperone. Biotechnol Lett 39, 133–139 (2017). https://doi.org/10.1007/s10529-016-2225-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-016-2225-x