Abstract
The growth rate and maximum biomass of Bacillus coagulans 2–6 were inhibited by lactate; inhibition by sodium lactate was stronger than by calcium lactate. The differences of protein expressions by B. coagulans 2–6 under the lactate stress were determined using two-dimensional electrophoresis coupled with mass spectrometric identification. Under the non-stress condition, calcium lactate stress and sodium lactate stress, the number of detected protein spots was 1,571 ± 117, 1,281 ± 231 and 904 ± 127, respectively. Four proteins with high expression under lactate stress were identified: lactate dehydrogenase, cysteine synthase A, aldo/keto reductase and ribosomal protein L7/L12. These proteins are thus potential targets for the reconstruction of B. coagulans to promote its resistance to lactate stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As one of the three major organic acids, lactic acid can serve as an acid, preservative, plant-growth regulator, a biodegradable material, drug and pesticide (Okano et al. 2010). A major industrial application of lactic acid is to produce polylactic acid, a biodegradable polymer, that can replace non-degradable petrochemical materials (Corma et al. 2007; John et al. 2007).
Microbial fermentation is the main method for industrial production of lactic acid (Datta and Henry 2006). Due to the inhibitory effect of products, a neutralizer needs to be added in the producing process to maintain the neutral or mildly acidic condition for fermentation liquid. The commonly-used neutralizing agents include CaCO3 and NaOH. Lactic acid in the fermentation liquid mainly exists as lactate. When the concentration of lactate reaches a limit lactic acid bacteria become stressed, resulting in a retardation of bacterial growth and the reduction of lactic acid yield. Maximum yields of lactic acid are higher when using calcium salts or alkali as neutralizer than that when using sodium salts or alkali as neutralizer (Bai et al. 2003; Ding and Tan 2006; Qin et al. 2010). Therefore, the response mechanisms of lactic acid bacteria to the calcium lactate and sodium lactate stress may be different.
Bacillus coagulans is newly applied to the fermentation and production of l-lactic acid. Compared with conventional strains, it has a number of advantages: high fermentation temperature, no need for sterilization of culture media, and high optical purity of products (Budhavaram and Fan 2009; Michelson et al. 2006). All these features conform to the production requirements for low energy consumption and high quality.
To our knowledge, little is known of the response of B. coagulans to lactate stress. The objective of this article was to determine the differences of B. coagulans 2–6 in growth and protein expression under sodium lactate stress, calcium lactate stress and non-stress conditions.
Materials and methods
Bacterial strain and culture media
Bacillus coagulans 2–6 (Qin et al. 2009) was used. It was cultured in three types of medium at 50 °C. Non-stress culture media: 40 g glucose l−1, 10 g yeast extract l−1, 1 g CaCl2 l−1, pH 6.5; for sodium lactate stress: 40 g glucose l−1, 10 g yeast extract l−1, 1 g CaCl2 l−1, 0.5 M l-sodium lactate, pH 6.5; for calcium lactate stress: 40 g glucose l−1, 10 g yeast extract l−1, 1 g CaCl2 l−1, 0.25 M l-calcium lactate, pH 6.5.
Culture conditions
Bacillus coagulans 2–6 was activated in non-stress culture media and inoculated into 100 ml culture media for sodium lactate stress, calcium lactate stress or non-stress. The three cultures were shaken at 150 rpm and 50 °C. Three repeated experiments were performed and the cell dry weight (CDW) was measured every few hours. For two-dimensional electrophoresis (2DE), samples were taken when the CDW reached 0.4 g l−1. The total protein was extracted from the samples to conduct 2DE.
Protein extraction and quantification
A bacterial sample, 200 μl, was added to 1 ml extraction buffer of 2DE (Sangon). The cells were oscillated at 4 °C for 2 h, and ultrasonically disrupted at 100 W for 3 min, then centrifugated at 12,000×g and 4 °C for 30 min. Then this procedure was repeated for the supernatant, and the final sediment was total protein.
Non-Interference Protein Assay Kit (Sangon) was employed to determine the protein concentration.
2DE
The loading amount of protein was 80 μg. IPG Ready Strip (pH 3–10 NL) was used to conduct first-dimension isoelectric focusing (IEF). The IPG strips were subjected to IEF with the following procedure: 30 V for 12 h, 500 V for 1 h, 1,000 V for 1 h, 8,000 V for 8 h, and a final phase of 500 V for 4 h.
The second-dimension electrophoresis was performed on 12.5 % SDS-PAGE gels. The parameters of electrophoresis were set as follows: 15 mA for 30 min; 30 mA until the end. The temperature for cooling circulation was 10 °C.
After electrophoresis, silver staining was performed according to the instruction of Mass Spectrometry-Compatible Rapid Silver Staining Kit (Sangon). The decolorized polyacrylamide gel was scanned at the resolution of 300 dpi. Image Master 2D Platinum was used for the analysis of 2DE patterns.
Mass spectrometric identification of protein spots
The differential protein spots on the gel were collected and digested with enzyme. Maldi-TOF-TOF-MS was employed for mass spectrometric identification and comparison.
Results
Growth of strains under lactate stress
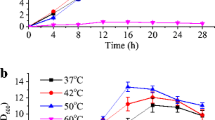
As shown in Fig. 1, under non-stress conditions, the CDW of B. coagulans 2–6 reached 0.83 g l−1 at 8 h, and a maximum of 1.22 g l−1 at 16 h. Under sodium lactate stress, the growth of B. coagulans 2–6 was the slowest; CDW was 0.24 g l−1 at 8 h and reached a maximum 0.5 g l−1 at 20 h. Under calcium lactate stress, CDW of B. coagulans 2–6 was 0.51 g l−1 at 8 h, and reached a maximum of 0.63 g l−1 at 16 h. Obviously, lactate stress had significant inhibitory effect on the growth of B. coagulans 2–6, and the inhibitory effect of sodium lactate was stronger than that of calcium lactate.
Influence of lactate stress on the protein expressions of B. coagulans 2–6
When the CDW of B. coagulans 2–6 cultured in sodium lactate stress, calcium lactate stress and non-stress conditions reached 0.4 g l−1, the strains were called NA, CA and GY, respectively. By total protein extraction, the protein concentrations of NA, CA and GY were 1.21, 1.78 and 2.01 g l−1, respectively. The two-dimensional electrophoresis (2DE) was conducted using 80 μg protein, with three replicates in each group. One of the electrophoregrams is shown in Fig. 2.
Two-dimensional electrophoretogram of total soluble protein of B. coagulans 2–6 cultured under different stress conditions. The two-dimensional electrophoresis was conducted using 80 μg of protein, with three replicates in each group. GY non-stress condition, CA calcium lactate stress, NA sodium lactate stress
The analysis indicated that GY sample contained 1,571 ± 117 protein spots, NA sample 904 ± 127 protein spots, and CA sample 1,281 ± 231 protein spots. Compared with the non-stress condition, the number of proteins expressed by B. coagulans 2–6 may be fewer under the lactate stress. Moreover, the number of expressed proteins under the sodium lactate stress was less than that under the calcium lactate stress.
The comparison between the 2D electrophoregrams of samples indicated that there were 73 protein spots with significant differences between NA and GY. The expression amount of all the differential proteins in GY samples was higher than that in NA sample. The high expression of protein spots was not found under the sodium lactate stress.
There were 44 protein spots with significant differences between CA and GY samples; 35 differentially expressed proteins showed higher expression in GY samples than in CA samples, and nine showed higher expression under the calcium lactate stress. The nine differential proteins were identified by mass spectroscopy, and four of them were successfully recognized as lactate dehydrogenase (GenBank accession no. ACR02673), cysteine synthase A (GenBank accession no. AEH52120), aldo/keto reductase (GenBank accession no. AEH52329) and ribosomal protein L7/L12 (GenBank accession no. AEH52167).
There were 46 protein spots with significant difference between CA and NA samples; 45 differential proteins showed higher expression in CA samples than in NA sample, and one showed a higher expression in NA samples. Unfortunately, this differential protein could not be identified by mass spectroscopy.
Discussion
Lactate stress is inevitable for lactic acid bacteria in the production of lactic acid. However, the existing domestic and foreign researches pay little attention to the response mechanism of B. coagulans to the stress of lactate. In this article, B. coagulans 2–6 with high yield of l-lactic acid screened at early stage (Qin et al. 2009) was used to determine the influence of two lactate stress conditions on the cell growth and protein expressions.
The results indicated that under the same concentration of lactates, sodium lactate had stronger stress on the B. coagulans 2–6 compared with calcium lactate (Fig. 1). In early stages of fermentation with this strain, NaOH and CaCO3 were used as neutralizer to produce l-lactic acid, respectively, and the yield was also lower in the former (Qin et al. 2009, 2010). It may be because the neutralization of 1 C3H6O3 requires 1 NaOH, but only 0.5 CaCO3. That is to say, if the concentrations of lactic acid are equal by using NaOH or CaCO3 as neutralizer, Na+ concentration should be twice that of Ca2+. Therefore, the high ion concentration of sodium lactate may be one reason for stronger stress.
Fewer proteins were detected by two-dimensional electrophoresis (2DE) under lactate stress conditions compared with non-stress condition (Fig. 2). It is speculated that part of the biological processes and metabolic pathways of B. coagulans 2–6 were inhibited under the lactate stress. After the comparison of differential protein spots, four proteins with high expressions under the calcium lactate stress were identified. As a key enzyme in lactic acid metabolism, lactate dehydrogenase can mediate the mutual conversion between pyruvic acid and lactic acid. When the lactic acid concentration is relatively high in the reaction system, the high expression of lactate dehydrogenase will facilitate the conversion of lactic acid to pyruvic acid, therefore reducing the stress of high-concentration lactic acid on the strains. Cysteine synthase A is a crucial enzyme in the synthetic pathway of glutathione. Glutathione can help Lactococcus lactis resist the acid stress and oxidative stress (Li et al. 2003; Zhang et al. 2007). The excess expression of aldo/keto reductase in Escherichia coli leads to increased resistance to methylglyoxal (Grant et al. 2003), and the excess expression of aldo/keto reductase in Clostridium beijerinckii could improve its resistance to furfural (Zhang and Ezeji 2013). Ribosomal protein L7/L12 plays an important role in the binding of RNA and ribosome and the process of accurate protein translation (Rosen et al. 2001). The reason for the excess expression of this protein may be that under the stress condition, the cells have to rapidly synthesize some specific protective proteins to resist the stress.
In summary: the influence of sodium lactate and calcium lactate on the growth and protein expression of B. coagulans 2–6 was studied. Four proteins related to anti-lactate stress were found. These proteins are potential targets for the reconstruction of B. coagulans 2–6 by metabolism engineering, so as to improve its resistance to lactate stress and its lactic acid production ability.
References
Bai DM, Wei Q, Yan ZH, Zhao XM, Li XG, Xu SM (2003) Fed-batch fermentation of Lactobacillus lactis for hyper-production of l-lactic acid. Biotechnol Lett 25:1833–1835
Budhavaram NK, Fan Z (2009) Production of lactic acid from paper sludge using acid-tolerant, thermophilic Bacillus coagulan strains. Bioresour Technol 100:5966–5972
Corma A, Iborra S, Velty A (2007) Chemical routes for the transformation of biomass into chemicals. Chem Rev 107:2411–2502
Datta R, Henry M (2006) Lactic acid: recent advances in products, processes and technologies—a review. J Chem Technol Biotechnol 81:1119–1129
Ding S, Tan T (2006) l-Lactic acid production by Lactobacillus casei fermentation using different fed-batch feeding strategies. Proc Biochem 41:1451–1454
Grant AW, Steel G, Waugh H, Ellis EM (2003) A novel aldo-keto reductase from Escherichia coli can increase resistance to methylglyoxal toxicity. FEMS Microbiol Lett 218:93–99
John RP, Nampoothiri KM, Pandey A (2007) Fermentative production of lactic acid from biomass: an overview on process developments and future perspectives. Appl Microbiol Biotechnol 74:524–534
Li Y, Hugenholtz J, Abee T, Molenaar D (2003) Glutathione protects Lactococcus lactis against oxidative stress. Appl Environ Microbiol 69:5739–5745
Michelson T, Kask K, Jõgi E, Talpsep E, Suitso I, Nurk A (2006) l(+)-Lactic acid producer Bacillus coagulans SIM-7 DSM 14043 and its comparison with Lactobacillus delbrueckii ssp. lactis DSM 20073. Enz Microb Technol 39:861–867
Okano K, Tanaka T, Ogino C, Fukuda H, Kondo A (2010) Biotechnological production of enantiomeric pure lactic acid from renewable resources—recent achievements, perspectives, and limits. Appl Microbiol Biotechnol 85:413–423
Qin J, Zhao B, Wang X, Wang L, Yu B, Ma Y, Ma C, Tang H, Sun J, Xu P (2009) Non-sterilized fermentative production of polymer-grade l-lactic acid by a newly isolated thermophilic strain Bacillus sp. 2–6. PLoS ONE 4:e4359
Qin J, Wang X, Zheng Z, Ma C, Tang H, Xu P (2010) Production of l-lactic acid by a thermophilic Bacillus mutant using sodium hydroxide as neutralizing agent. Bioresour Technol 101:7570–7576
Rosen R, Büttner K, Schmid R, Hecker M, Ron EZ (2001) Stress-induced proteins of Agrobacterium tumefaciens. FEMS Microbiol Ecol 35:277–285
Zhang Y, Ezeji TC (2013) Transcriptional analysis of Clostridium beijerinckii NCIMB 8052 to elucidate role of furfural stress during acetone butanol ethanol fermentation. Biotechnol Biofuel 6:66
Zhang J, Fu RY, Hugenholtz J, Li Y, Chen J (2007) Glutathione protects Lactococcus lactis against acid stress. Appl Environ Microbiol 73:5268–5275
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31200078), the Shandong Provincial Natural Science Foundation, China (ZR2001CQ025), and the CAS Key Laboratory of Microbial Physiological and Metabolic Engineering, Institute of Microbiology, Chinese Academy of Sciences (KLIM-201302).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, X., Qin, J., Wang, L. et al. A comparative proteomic analysis of Bacillus coagulans in response to lactate stress during the production of l-lactic acid. Biotechnol Lett 36, 2545–2549 (2014). https://doi.org/10.1007/s10529-014-1639-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-014-1639-6