Abstract
Thianthrene (TA) was desulfurized by an isolated strain, Gordonia sp. IITR100. The reaction is accompanied with the formation of TA-sulfoxide, TA-sulfone and 2-phenylsulfanylphenol. The formed 2-phenylsulfanylphenol undergoes further oxidation to o-hydroxyphenyl phenylsulfone that accumulates as an end product. Metabolism of TA to TA-sulfone can also occur by E. coli-DszC i.e. E. coli cells that were harboring the gene coding for the enzyme dibenzothiophene desulfurase C. When presented to E. coli-DszC in a binary combination with dibenzothiophene, TA metabolism was completely inhibited. Metabolism of TA–TA-sulfone by E. coli-DszC, as well as the nature of metabolites formed by IITR100, suggests that the desulfurization pathway for TA is similar to that of the thiophenic compounds. This is first report on the desulfurization of thianthrene, and has implications on biodesulfurization when multiple sulfur compounds are present together.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sulfur compounds that are present in petroleum and its fractions need to be removed to prevent toxic emissions, generated after their combustion in different engines (Van Hamme et al. 2003; Xu et al. 2009). For this reason, bio-desulfurization of sulfur compounds has been studied extensively (Gray et al. 2003; Xu et al. 2009). Most of the studies have been done on the model thiophenic compounds, dibenzothiophene (DBT) and benzothiophene (Mohebali and Ball 2008). Briefly, the desulfurization is mediated by a ‘4S’ pathway (Piddington et al. 1995; Gray et al. 2003) that includes serial activity of dibenzothiophene desulfurases, DszC, DszA and DszB, leading to the release of sulfur as sulfite ions and formation of hydroxy-biphenyl as an end product. Intermediates formed in the reaction, DBT-sulfoxide, DBT-sulfone and DBT-sulfinate, were also characterized. Studies on the desulfurization of non-thiophenic sulfur compounds like 1,4-dithiane or thianthrene, however, are scarce (Kirkwood et al. 2005; 2007). While Rhodococcus erythropolis EPWF, Pseudomonas sp. K1oA and Rhodococcus sp. KIbD can use 1, 4-dithiane as sulfur source, no metabolites were detected in any of the culture extracts (Kirkwood et al. 2005). The desulfurization of 1, 4-dithiane by Rhodococcus sp. K1bD was inhibited by >90 % in the presence of DBT (Kirkwood et al. 2005), but results with other bacteria are not available. Similarly, growth of an isolated strain Rhodococcus sp. IGTS8 by using thianthrene (TA) as sulfur source has been demonstrated (Kayser et al. 1993) but, again, no information is available about the formed metabolites. Oxidation of TA–TA-monosulfoxide by the ligninase from Phanerochaete chrysosporium has also been reported (Schreiner et al. 1988). In the present study, we describe the desulfurization of TA by a strain of Gordona, when present alone or together with DBT, and have also characterized the formed metabolites.
Materials and methods
Chemicals
Thiantherene, dibenzothiophene and acetonitrile were from Sigma-Aldrich. All other chemicals were of analytical grade.
Bacterium
Gordonia sp. IITR100 (16S ribosomal RNA accession no; GU084407) was used. It was obtained by selective enrichment on dimethyl-DBT from an oil-contaminated soil, present around a refinery in Gujarat, India, (Singh et al. 2011). Nucleotide sequence of its dszABC genes (accession no; KC693733.1) is >99 % identical to the corresponding genes of Gordonia alkanivorans 1B (Alves et al. 2007).
Desulfurization by IITR100
For TA desulfurization, 15 flasks, each containing 20 ml medium (no.1) [Na2HPO4, 2 g; KH2PO4, 1 g; MgCl2.6H2O, 0.4 g; (NH4)2C2O4, 4.25 g; Al(OH)3, 0.1 g; SnCl2.2H2O, 0.5 g; KI, 0.05 g; LiCl, 0.01 g; MnCl2.4H2O, 0.8 g; H3BO3, 0.05 g; ZnCl2, 0.1 g; CoCl2.6H2O, 0.1 g; NiCl2.6H2O, 0.1 g; BaCl2, 0.05 g; (NH4)6Mo7O24.4H2O, 0.05 g, and sucrose, 17.1 g/l], along with 0.25 µM TA as sulfur source, were inoculated with IITR100. Three flasks for each time point were removed after 0, 2, 4, 6, and 8 days of incubation. After estimation of growth (OD600), the reaction was stopped by acidification to pH < 2 by HCl, and levels of residual TA and the formed metabolites were analyzed. Uninoculated flasks were run in parallel and were processed similarly. A similar experiment was set up for studying the desulfurization of DBT, except that 0.25 µM DBT was used as sulfur source. For experiments, where desulfurization of TA and DBT was to be evaluated, when these were present together, similar set up was used except that 0.25 µM each of TA and DBT was used as sulfur source.
Oxidation by recombinant E. coli-dszC cells
Recombinant E. coli (E. coli-DszC) that was expressing DszC (dibenzothiophene desulfurase C), was prepared by ligation of the amplified dszC gene, with pET28a vector, and was followed by their cloning in E. coli-BL21 as described earlier (Macwan et al. 2012). After induction by IPTG, the cells from one liter culture were harvested by centrifugation at 3,500×g, washed with 50 ml medium (no. 1) and suspended in 100 ml of the same medium. For evaluation of the activity of DszC with the substrates, 0.25 µM TA, or DBT, or mixture of TA and DBT (0.25 µM each) were incubated with 10 ml suspension of the E. coli-DszC. After incubation at 30 °C for different times, the reaction was stopped by acidification and residual substrate along with the formed metabolites was analyzed, as described below.
Analytical methods
The acidified culture medium was extracted three times with equal volume of ethyl acetate. The residue obtained after complete evaporation of the solvent was dissolved in 1 ml ethyl acetate. Suitable aliquots were analyzed by using Trace GC Ultra coupled with TSQ Quantum XLS mass spectrometer, equipped with a Triplus Auto Sampler (Thermo Scientific, USA) and a TG-5MS capillary column (30 m × 0.25 mm I.D. × 0.25 µm film thickness). GC conditions were oven temperature at 100 °C (1 min), then increased to 250 at 10 °C min−1 (15 min). The other conditions were: injector; 250 °C, CT split; 20:1, mass range; 50–600 amu, source; 220 °C and transfer line; 290 °C. Helium was used as carrier gas at 1 ml min−1.
All the experiments were carried out at least twice and three samples for each time point were analysed on each occasion.
Results and discussion
Desulfurization of thianthrene by IITR100 and E. coli-DszC
The isolated strain, Gordonia IITR100, inoculated in the liquid medium that contained either DBT or TA as sulfur source, exhibited growth after a lag-phase of 3 days. The growth was highest on the sixth day but leveled off thereafter (Fig. 1a). The growth with TA was higher, compared to DBT, possibly because TA and its subsequent metabolites are accepted more readily by enzymes of the degradative pathway. GC–MS analysis of the culture extract revealed that the growth on TA was accompanied with a progressive fall in the levels of TA and formation of two metabolites M1 (Rt 11.16 min) & M2 (13.44 min; Figs. 1b, 2).
Mass spectrum of M1 consisted of major ions m/z 201.9, 170.3, 95.87, & 76.84 and was tentatively identified as 2-phenylsulfanyl-phenol. Similarly, mass spectrum of M2 consisted of major ions m/z 234, 200, 169, 141, 113 and 76.94, which being similar to the spectrum of hydroxyphenyl phenylsulfone, was identified as o-hydroxyphenyl phenylsulfone (Fig. 2). Incubation of TA with recombinant E. coli-DszC led to the formation of another two metabolites M3 (16.05 min) and M4 (17.85 min), whose levels increased progressively up to 12 h of incubation (Fig. 3). The mass spectrum of M3 consisted of major ions (m/z) 231.9, 202.9, 183.9, 170.9, 138.9 and 107.8 (Fig. 3) which tentatively identified it as TA-sulfoxide. Similarly, major ions 247.9, 199.9, 183.9 and 138.9 in the mass spectrum of M4 identify it as TA-sulfone (Fig. 3).
Based on the metabolites formed, desulfurization of TA is proposed to undergo desulfurization to 2-phenylsulfanyl-phenol via the intermediary metabolites TA-sulfoxide and TA-sulfone (Fig. 4), which is similar to the ‘4S’ pathway for the metabolism of the thiophenic compounds. The formed 2-phenylsulfanyl-phenol undergoes further oxidation into o-hydroxyphenyl phenylsulfone, which accumulated as the end product.
While some of the DBT desulfurizing organisms can grow on thianthrene as a sulfur source (Kayser et al. 1993), others cannot (Izumi et al. 1994). Although the pathway for metabolism of TA is similar to ‘4S’ and the enzyme, DszC, can mediate the oxidation of TA (Fig. 3), the enzymes for downstream reactions might be different. It is possible that additional presence of the downstream enzymes might confer the ability to the DBT-desulfurizers to grow on thianthrene also.
Metabolism of thianthrene and DBT, when present together
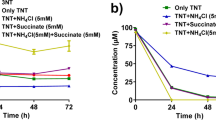
The desulfurization of TA and DBT by IITR100 was studied when the two chemicals were present separately, or together, in the reaction medium. When present separately, the desulfurization of TA was faster than DBT, and 10 % of TA & 60 % of DBT, remained in the medium after 6 days of incubation (Fig. 5a). However, when the two chemicals were present together, desulfurization of TA and DBT occurred at comparable rates, and ~60 % of each chemical remained in the medium after the same period of incubation (Fig. 5a). Similarly, the oxidation of TA by E. coli-DszC was faster than DBT, when the two chemicals were studied separately (Fig. 5b). But the oxidation of TA was almost completely inhibited when DBT was present along with it (Fig. 5b). There is a possibility that the DBT-sulfone, formed by the activity of DszC might be inhibitory to the oxidation of TA into TA-sulfone by this enzyme. With IITR100, on the other hand, the formed DBT-sulfone undergoes further metabolism by the activity of DszA and is, therefore, not available to inhibit the oxidation of TA. These results are similar to earlier study where desulfurization of 1,4-dithiane is inhibited in presence of DBT (Kirkwood et al. 2005). Competition between two substrates for their metabolism by E. coli DszC, however, has not been studied before. Overall, the results presented here underscore the importance of competition between various compounds and have an important bearing on the desulfurization of petroleum fractions, where several sulfur compounds are present together.
References
Alves L, Melo M, Mendonca D, Simoes F, Matos J, Tenreiro R, Girio FM (2007) Sequencing, cloning and expression of the dsz genes required for dibenzothiophene sulfone desulfurization from Gordonia alkanivorans strain 1B. Enz Microb Technol 40:1598–1603
Gray KA, Mrachko GT, Squires CH (2003) Biodesulfurization of fossil fuels. Curr Opin Microbiol 6:229–235
Izumi Y, Ohshiro T, Ogino H, Hine Y, Shimao M (1994) Selective desulfurization of dibenzothiophene by Rhodococcus erythropolis d-1. Appl Environ Microbiol 60:223–226
Kayser KJ, Bielaga-Jones BA, Jackowski K, Odusan O, Kilbane JJ (1993) Utilization of organosulfur compounds by axenic and mixed cultures of Rhodococcus rhodochrous IGTS8. J Gen Microbiol 139:3123–3129
Kirkwood KM, Ebert S, Foght JM, Fedorak PM, Gray MR (2005) Bacterial biodegradation of aliphatic sulfides under aerobic carbon-or sulfur-limited growth conditions. J Appl Microbiol 99:1444–1454
Kirkwood KM, Foght JM, Gray MR (2007) Selectivity among organic sulfur compounds in one- and two-liquid-phase cultures of Rhodococcus sp. strain JVH1. Biodegradation 18:473–480
Macwan AS, Kukshal V, Srivastava N, Javed S, Kumar A (2012) Crystal structure of the hexachlorocyclohexane dehydrochlorinase (LinA-Type2): mutational analysis, thermostability and enantioselectivity. PLoS ONE 7(11):e50373
Mohebali G, Ball AS (2008) Biocatalytic desulfurization (BDS) of petrodiesel fuels. Microbiology 154:2169–2183
Piddington CS, Kovacevich BR, Rambosek J (1995) Sequence and molecular characterization of a DNA region encoding the dibenzothiophene desulfurization operon of Rhodococcus sp. strain IGTS8. Appl Environ Microbiol 61:468–475
Schreiner BP, Stevens SE Jr, Sing T (1988) Oxidation of thianthrene by the ligninase of Phanerochaete chrysosporium. Appl Environ Microbiol 54:1858–1860
Singh P, Ahmad A, Faraz S, Macwan A, Kumar A, Srivastava P (2011) Desulfurization of recalcitrant organosulfur compound 4,6-DMDBT by the isolated strain IITR100 (Abstract). Proceedings of the 80th meeting of ‘Society of Biological Chemists (India)’ held at Lucknow, India
Van Hamme JD, Singh A, Ward OP (2003) Recent advances in petroleum microbiology. Microbiol Mol Biol Rev 67:503–549
Xu P, Feng J, Yu B, Li F, Ma C (2009) Recent developments in biodesulfurization of fossil fuels. Adv Biochem Eng Biotechnol 113:255–274
Acknowledgments
Financial assistance by a grant SIP-08 from Council of Scientific and Industrial Research (CSIR), India, is gratefully acknowledged. Authors AA and AKC sincerely thank CSIR and Indian Council of Medical Research (ICMR), India, respectively, for fellowship support. We are also thankful to our colleagues in the analytical facility of Indian Institute of Toxicology Research, Lucknow, for their help in HPLC and GC–MS analysis.
Conflict of interest
Authors do not have any financial relationship with the organization that sponsored the research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahmad, A., Chauhan, A.K., Javed, S. et al. Desulfurization of thianthrene by a Gordonia sp. IITR100. Biotechnol Lett 36, 2209–2214 (2014). https://doi.org/10.1007/s10529-014-1606-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-014-1606-2