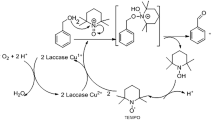
Abstract
Laccases play an important role in the biological break down of lignin and have great potential in the deconstruction of lignocellulosic feedstocks. We examined 16 laccases, both commercially prepared and crude extracts, for their ability to oxidize veratryl alcohol in the presence of various solvents and mediators. Screening revealed complete conversion of veratryl alcohol to veratraldehyde catalyzed by a crude preparation of the laccase from Trametes versicolor ATCC 11235 and the mediator TEMPO in 20 % (v/v) tert-butanol.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Laccases are polyphenol oxidases (EC 1.10.3.2) containing four copper atoms in their active sites and are the largest subclass of blue multicopper oxidases (MCO). They have been found in higher plants and fungi (Messerschmidt and Huber 1990), though fungal laccases have been the focus of most studies. Fungi produce several laccases, localized both intra- and extracellularly, to perform a large number of roles, including participating in host-pathogen interactions, sporulation and fungal morphogenesis, stress resistance, and lignin degradation (Gianfreda et al. 1999; Thurston 1994). Laccases have wide substrate specificity; they oxidize polyphenols, methoxy-substituted phenols, and a range of other compounds (Baldrian 2006). When combined with an additional small molecular weight compound to act as a mediator, laccases have increased reaction rates and broader specificity. Mediators are easily oxidized by the laccases and typically form radicals which are then capable of oxidizing other substrates (Bourbonnais and Paice 1990). In some instances highly active laccase-generated radicals can lead to the polymerization of substrates. Lignin degradation by laccases could lead to the generation of novel compounds with the potential to be converted into new polymers.
A typical laccase-mediated reaction results in the reduction of oxygen to water along with the oxidation of a substrate (Riva 2006). Selection of mediators appropriate for a given reaction is no small task. The first chemical used as a mediator in a laccase-mediated system was ABTS [2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)] (Bourbonnais and Paice 1990), and since 1990, more than 100 compounds have been tested for their ability to act as mediators in the laccase-mediator system. Different mediators can generate different products when coupled with the same laccase and substrate. This is due to the different structures of the mediator radicals generated by the reaction acting on different positions of the substrate (Baiocco et al. 2003; d’Acunzo et al. 2002). The most effective mediators have been N-heterocyclics bearing N–OH groups (Riva 2006), and there are thousands of compounds that have potential as laccase mediators.
In the current paper we study the activity of several commercial enzymes and several crude lysates from various fungi using a laccase-mediator system (LMS) in the oxidation of veratryl alcohol. We tested the enzymes in a combinatorial fashion with various mediators, as well as different solvents and at varying temperatures and pHs. To this point, the literature has contained many papers dealing with single laccase/mediator pairs. We have performed analysis on 30 mediators in order to create a more thorough resource for enzyme/mediator combinations.
Materials and methods
Chemicals
N-Hydroxyphthalamide (HPA), Cresol Red (CR1), Cresol Red sodium salt (CR2), phenolphthalein (PPT), humic acid (HA), 3,5-dimethoxy-4-hydroxyacetephenone (HAP), n-(2-hydroxyethyl)-phthalimide (nPA), 1-hydroxy-benzotriazole hydrate (HOBT), 4-oxo-TEMPO free radical (oxTEMPO), TEMPO free radical (TEMPO), 3,4-dimethoxybenzyl alcohol (veratryl alcohol), 3,4-dimethoxybenzaldehyde (veratraldehyde), dimethyl sulfoxide (DMSO), 1,4-dioxane, N,N-dimethylformamide (DMF) and 2,5-dimethylanline (xylidine) were obtained from Aldrich (Steinheim, Germany). Syringaldehyde (SYRald), 2,6-dichloroindophenol (DCIP), Chlorophenol Red sodium salt (CPR), aurintricarboxylic acid (ATCA), Phenol Red (PR), quercetin dihydrate (QUE), methyl 3,5-dimethoxy-4-hydroxybenzoate (HB), sodium 4-hydroxybenzenesulfonate dihydrate (HBS), 2-amino-4,6-dimethylpyrimidine (ADMP), n-hexane, and tert-butanol (tBuOH), were obtained from Alfa Aeser (Ward Hill, MA). Mordant Blue 29 (MB29), 4-hydroxybenzoic acid (HBA), anthraquinone (ATQ), 5-aminosalicylic acid (ASA), 3-hydroxyanthranilic acid (HAA), 2-methoxyphenothiazine (MPA), 4-hydroxy-3,5-dimethoxyacetophenone (DMOA), 1,2-dihydroxyanthraquinone (AZ), 1,3-dioxane, and tert-amyl alcohol (tAmOH) were obtained from TCI America (Portland, OR). Violuric acid (VIO) and 2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) were obtained from Fluka (Switzerland). Acetonitrile and tetrahydrofuran (THF) were from EMD Chemicals (Darmstadt, Germany). Syringamide (LccM) was received from AB Enzymes (Darmstadt, Germany); and syringaldazine (SYRaz) obtained from SAFC, St. Louis, MO).
Organisms and growth
Trametes versicolor (ATCC 20869 and ATCC 11235), Phlebia radiata (ATCC 64658), Pycnoporous cinnabarinus (ATCC 200478), Trichoderma atroviride (NRRL 25150), Fusarium proliferatum (NRRL 6413), F. verticillioides (NRRL 20956), and T. reesei (NRRL 1163) were maintained on PDA slants at 28 °C for 5 days. Mycelial plugs were used to inoculate starter cultures in malt extract broth that incubated 28 °C for 4 days. Mycelia generated from starter cultures were homogenized with PowerGen 700 homogenizer for 30 s and served to inoculate 5 % (v/v) in a basal liquid media containing phenylalanine, adenine, and CuSO4 (Fahraeus and Reinhammar 1967) at 30 °C, 130 rpm for enzyme production except for T. reesei, which was cultured 5 % (v/v) in a different liquid media containing peptone and also supplemented with CuSO4 (Chakroun et al. 2010). Liquid cultures were induced on the third day with 200 μM 2,5-xylidine (Fahraeus and Reinhammar 1967).
Laccases and crude enzyme preparations
Crude enzymes from supernatants of induced fungal cultures were harvested when maximum activity was observed with ABTS assay from culture supernatant. Cultures were centrifuged and supernatants filtered through a 0.45 μm membrane for a cell-free extract. Cell-free supernatants from T. versicolor and T. reesei were lyophilized and stored at −20 °C and those from P. radiata, P. cinnabarinus, T. atroviride and Fusarium spp. were concentrated using a Centricon Plus-70 polyethersulfone 5,000 MWCO centrifugal filter device and stored as a liquid at 4 °C.
Laccases from T. versicolor (Fluka 38429 and 53739) and Rhus vernificera (Sigma L2157) were purchased from Sigma-Aldrich; laccase C and laccase A were obtained from ASA Spezialenzyme GmbH (Wolfenbüttel, Germany); Laccase P was a sample from Americos Industries Inc. (Gujarat, India); Ecostone LCC10 was a sample from AB Enzymes (Darmstadt, Germany); and EcoFade LT100 was a sample from Genencor (Rochester, NY). Protein concentrations were determined by Bradford-based protein assay (Bio-Rad, Hercules, CA) for all crude enzyme preparations and commercial enzymes.
Assays for laccase optimum conditions
The optimum pH for each enzyme preparation was determined by oxidation of syringaldazine using a modified Sigma enzymatic assay of laccase procedure EC 1.10.3.2 (Ride 1980). Reactions were performed in triplicate in 100 μl using a 96-well polystyrene assay plate with 0.02 mM syringaldazine, 10 % (v/v) methanol, McIlvaine buffer at varying pH values, and a suitable amount of enzyme. Oxidation of syringaldazine was followed by the increase in absorbance at 530 nm for 5 min using a plate reader (Molecular Devices, Sunnyvale, CA). The enzyme activity in the presence of various solvents was performed in a similar manner using 200 μl in a glass 96-well assay plate with 0.05 mM syringaldazine, 1 % (v/v) methanol, and a range of 0–90 % (v/v) solvent in McIlvaine buffer at the enzyme’s pH optimum.
Laccase activity for both commercial laccases and crude enzyme preparations was also measured by oxidation of ABTS by following the increase in absorbance at 420 nm (ε420 = 3.6 × 104 M−1 cm−1 for the oxidation product) using a plate reader. The reaction mixtures contained 20 μl enzyme solution of measurable concentration and 180 μl ABTS (1 mM) in McIlvaine buffer (at optimum pH) at 30 °C. The enzyme specific activity was expressed in units (U = μmol min−1). Enzyme activities were also evaluated using ABTS under a range of temperatures (25–80 °C) in 1 ml with 1 mM ABTS and McIlvaine buffer at the enzyme’s optimum pH.
Veratryl alcohol oxidation using laccases and mediators
Oxidation reactions of veratryl alcohol to veratraldehyde were performed in duplicate in vials and initiated by the addition of laccase from commercial sources and fungal cell free crude extracts to 500 μl containing 5 mM veratryl alcohol and 4.5 mM mediator in McIlvaine buffer at appropriate pH containing 20 % (v/v) tert-butanol. Reactions were incubated in screw-cap vials at 30 °C, 240 rpm for 24 h and stopped by addition of an equal volume of DMSO. Control samples were incubated without the enzyme or a mediator. To compare oxidation in various solvents, reactions were set up in 20 % (v/v) solvent using laccase C with select mediators that showed activity in preliminary experiments with tBuOH.
A preparative oxidation of veratryl alcohol (12.6 mg) was performed using EcoFade LT100 laccase (30 mg) in a 15 ml reaction containing 20 % (v/v) tert-butanol in McIlvaine buffer (pH 5.4) containing 5 mM veratryl alcohol and 4.5 mM TEMPO. After 17 h the veratryl alcohol was completely converted to veratrylaldehyde. The reaction was extracted three times with 10 ml methylene chloride and the organic phase was removed in a separatory funnel, and the organic phases were combined and dried under N2. The dried organic extract was suspended in 50 % (v/v) ethyl acetate in hexane (5 ml), loaded on a silica column and eluted with the same solvent mixture: 11.8 mg of a beige powder (94 % yield) was recovered. Analysis by GC/MS, HPLC and NMR confirmed the product to be veratrylaldehyde.
HPLC analysis
Reaction products were analyzed by HPLC fitted with a photodiode array detector and using an Intertsil ODS-3 5 μm column (4.6 × 250 mm). Separation was obtained in gradient mode from 10 to 75 % (v/v) aqueous acetonitrile with 0.025 % trifluoroacetic acid at 1 ml/min. The eluate was monitored at 278 nm; and eluates absorbing from 200 to 800 nm were collected. Standard curves were prepared for veratryl alcohol, veratric acid, and veratraldehyde within the appropriate concentration range (five-point standard curve) and quantitative analysis performed using LC Solution (Shimadzu) and Microsoft Excel.
Results and discussion
Effect of reaction conditions on enzyme activity
The pH optima for laccases were determined by measuring the oxidation of syringaldazine in McIlvaine’s buffer at varying pH (pH 2.4–8.0). The pH optima of the enzymes were consistently in the range of pH 4.4–5.4 (Table 1). All of the laccases tested retained initial activity over a wide range of temperatures, consistent with other reported results (Hilden et al. 2009). However, enzyme activity decreased dramatically upon prolonged incubation at elevated temperatures. Indeed, T. versicolor ATCC 11235 enzyme had a temperature optimum at 55 °C (Fig. 1a), although the half-life of the enzyme increased from 1.3 to 8.2 h by lowering the reaction temperature from 50 to 30 °C (Fig. 1b). Decreasing the reaction temperature further to 23 °C resulted in slightly lower initial reaction rates (92 %) than at 30 °C, although there was another ca. two-fold increase in the half-life. The laccases from T. reesei NRRL 1163 and F. verticillioides NRRL 20956 had the lowest temperature optima, 30 and 35 °C, respectively, and the F. proliferatum NRRL 6413 laccase had the highest temperature optimum at 70 °C. Only one of the enzymes, T. reesei NRRL 1163, was inactivated at 55 °C; while the remaining enzymes were still active at 60 °C and seven remained active at 80 °C. Therefore, we selected 30 °C for all subsequent assays coupled with the optimum pH for each enzyme.
Evaluation of a temperature optimum and b thermostability of a T. versicolor ATCC 11235 laccase. Plot of a representative enzyme assayed for initial activity with ABTS from 25 to 80 °C (a) or activity after incubation for up to 4 h at both 30 and 50 °C (b). 100 % relative activity was 0.47 absorbency units/min monitored at 420 nm. Error bars represent one standard deviation for two independent measurements and are included for all data points
We tested a variety of organic solvents, including tBuOH, tAmOH, DMF, MeCN, 1,3-dioxane, THF, 1,4-dioxane, EtOAc, and diethyl ether, to assess their effect on the laccase C-TEMPO oxidation of veratryl alcohol. Reactions were carried out in the presence of 20 % (v/v) cosolvent in McIlvaine’s buffer. Solvent effects were varied, with some enzymes performing best with buffer alone, although many potential substrates for the LMS will not be soluble in a wholly aqueous system. Although there was not a clear relationship between solvent and enzyme activity, both THF and EtOAc were poor solvents for this reaction system. Generally, we found that 20 % (v/v) tBuOH was suitable for of the all enzymes tested and this allowed us to assess the activity of each enzyme in the same solvent/buffer combination. Activity in a range of solvent mixtures further demonstrates the robustness of the LMS and provides greater opportunity for developing commercial applications.
Comparison of commercially available laccases with crude preparations
Crude enzyme preparations were collected from cell free extracts of fungi grown in minimal media induced with 2,5-xylidine and compared to commercially available enzyme preparations. Relative molecular weights of the enzymes were compared through SDS-PAGE analysis by loading equal concentrations of total protein. Both sets of enzymes generally conformed to the 50–130 kDa range previously reported for laccases (Supplementary Fig. 1). Surprisingly, several of the crude extracts were composed of primarily single bands (P. cinnabarinus, T. reesei, T. versicolor), which was similar to that of the commercial enzyme preparations (Supplementary Fig. 1).
We had expected that several proteins of varying sizes would be observed in the extracellular fraction. The crude enzyme preparations were concentrated by lyophilization or ultrafiltration (some enzymes were inactivated by the lyophilization process), while the commercial preparations were used without further modification. Lyophilization was not responsible for the single bands observed in these induced cultures, as the same pattern was observed in the cell-free supernatants prior to concentration. The oxidation of veratryl alcohol was not observed in the absence of the mediator or enzyme nor was there ABTS-oxidation activity measured in reactions with supernatants from cultures that were not induced with 2,5-xylidine; thereby indicating that the primary oxidative activity was the result of a mediated laccase reaction.
Specific activities (U/mg) were determined for both our crude enzyme preparations and commercially available enzymes. The activity of the enzymes tested varied nearly 500-fold and the enzyme with the highest specific activity was T. versicolor (Fluka 53739) (Table 1). The crude enzyme preparations of T. versicolor (ATCC 11235) and P. cinnabarinus (ATCC 200478) showed high levels of specific activity similar to the commercial preparations. Overall, the crude enzyme preparations showed similar activities to the commercial enzymes.
Veratryl alcohol oxidation
Reactions were performed in a combinatorial fashion with 30 known mediators to assess the activities of 16 laccase enzyme preparations, including eight crude laccases and eight commercial laccases. Among the 30 chemicals screened, 15 were capable of acting as mediators in a quantifiable way for any of the laccases tested. It is clear that the choice of mediator is critical to the overall success of the reaction, as all of the tested chemicals have previously been shown to act as mediators. TEMPO was the most effective mediator, interacting with 7 of 8 crude enzyme preparations and 6 of 8 commercial enzymes (Supplementary Tables 1, 2), and d’Acunzo et al. 2002 previously showed that TEMPO as a mediator in buffer/solvent combinations improved access to substrates with limited solubility in buffer alone. We successfully applied one of the top reaction conditions identified in the screening to prepare veratrylaldehyde in 94 % isolated yield.
Generally, chemicals that can act as mediators with one laccase for veratryl alcohol oxidation are able to act as a mediator with other laccases. Additionally, the chemicals capable of acting as mediators were similar between both the commercial enzyme preparations and crude preparations generated for this study. The laccase from T. versicolor (Fluka 53739) had the least mediator specificity and utilized 11 mediators. Another T. versicolor ATCC 11235 laccase was the only enzyme that completely converted veratryl alcohol to veratraldehyde, although seven laccase-TEMPO combinations had >95 % conversion (Table 2). It is interesting to note that 11 enzyme-mediator pairs catalyzed >80 % conversion of veratryl alcohol, and seven of those instances involved TEMPO as the mediator; TEMPO has been previously reported to be an effective mediator of this reaction (Fabbrini et al. 2002). Furthermore, 14 of the molecules tested (MB29, HBA, AtQ, ASA, HAA, PPt, Que, MPA, HA, HBS, nPA, SYRaz, oxTEMPO, and ADMP) mediated this reaction to only trace levels with any of the enzymes tested, while none were completely incapable of mediating the reaction under the conditions that were employed (Supplementary Tables 1, 2).
In summary, the use of mediators in laccase-catalyzed oxidations has been well described (Riva 2006), and many of these reports have focused on the oxidation of veratryl alcohol to veratraldehyde. While many of the literature reports have described single enzyme-mediator pairs, we have described herein a combinatorial approach to the LMS where 16 enzymes were each paired with 30 different previously reported mediators. We determined the pH optimum (4.4–6.4) and operational temperature range (25–80 °C) for each enzyme. Although each of the mediators had been previously reported to serve as a laccase-mediator, it is clear that for a given substrate, it is necessary to examine a wide variety of enzyme-mediator combinations, and this combinatorial approach will enable the rapid identification of a suitable enzyme-mediator combination and optimization of reaction conditions.
References
Baiocco P, Barreca AN, Fabbrini M, Galli C, Gentili P (2003) Promoting laccase activity towards non-phenolic substrates: a mechanistic investigation with some laccase-mediator systems. Org Biomol Chem 1:191–197
Baldrian P (2006) Fungal laccases—occurrence and properties. FEMS Microbiol Rev 2:215–242
Bourbonnais R, Paice MG (1990) Oxidation of nonphenolic substrates—an expanded role for laccase in lignin biodegradation. FEBS Lett 267:99–102
Chakroun H, Mechichi T, Martinez MJ, Dhouib A, Sayadi S (2010) Purification and characterization of a novel laccase from the ascomycete Trichoderma atroviride: application on bioremediation of phenolic compounds. Proc Biochem 43:507–513
d’Acunzo F, Galli C, Masci B (2002) Oxidation of phenols by laccase and laccase-mediator systems: solubility and steric issues. Eur J Biochem 269:5330–5335
Fabbrini M, Galli C, Gentili P (2002) Comparing the catalytic efficiency of some mediators of laccase. J Mol Catal B Enzym 16:231–240
Fahraeus G, Reinhammar B (1967) Large-scale production and purification of laccase from cultures of the fungus Polyporus versicolor and some properties of laccase A. Acta Chem Scand 21:2367–2378
Gianfreda L, Xu F, Bollag JM (1999) Laccases: a useful group of oxidoreductase enzymes. Bioremediat J 3:1–26
Hilden K, Hakala TK, Lundell T (2009) Thermotolerant and thermostable laccases. Biotechnol Lett 31:1117–1128
Messerschmidt A, Huber R (1990) The blue oxidases, ascorbate oxidase, laccase and ceruloplasmin. Modeling and structural relationships. Eur J Biochem 2:341–352
Ride JP (1980) Physiol Plant Pathol 16:187–196
Riva S (2006) Laccases: blue enzymes for green chemistry. Trends Biotechnol 5:219–226
Thurston CF (1994) The structure and function of fungal laccases. Microbiology 140:19–26
Author information
Authors and Affiliations
Corresponding author
Additional information
Names are necessary to report factually on available data; however, the USDA neither guarantees nor warrants the standard of the product, and the use of the name by USDA implies no approval of the product to the exclusion of others that may also be suitable. USDA is an equal opportunity provider and employer.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Larson, T.M., Anderson, A.M. & Rich, J.O. Combinatorial evaluation of laccase-mediator system in the oxidation of veratryl alcohol. Biotechnol Lett 35, 225–231 (2013). https://doi.org/10.1007/s10529-012-1078-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-012-1078-1