Abstract
Pyruvate was produced from glucose by Escherichia coli BW25113 that contained formate dehydrogenase (FDH) from Mycobacterium vaccae. In aerobic shake-flask culture (K L a = 4.9 min−1), the recombinant strain produced 6.7 g pyruvate l−1 after 24 h with 4 g sodium formate l−1 and a yield of 0.34 g pyruvate g glucose−1. These values were higher than those of the original strain (0.2 g l−1 pyruvate and 0.02 g pyruvate g glucose−1). Based on the reaction mechanism of FDH, the introduction of FDH into E. coli enhances the accumulation of pyruvate by the regeneration of NADH from NAD+ since NAD+ is a shared cosubstrate with the pyruvate dehydrogenase complex, which decarboxylates pyruvate to acetyl-CoA and CO2. The oxygenation level was enough high to inactivate lactate dehydrogenase, which was of benefit to pyruvate accumulation without lactate as a by-product.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The manufacturing of pyruvate has received much attention for use as a food additive, or a weight-control supplement, as well as a starting material for synthesis of amino acids (Causey et al. 2004). In previous studies, microbial pyruvate production was achieved by using Escherichia coli mutants with the deletion of pyruvate-converting enzymes, such as the pyruvate dehydrogenase complex (PDC), alcohol dehydrogenase (ADH), lactate dehydrogenase (LDH) and those involved in acetate-producing pathways (Tomar et al. 2003; Causey et al. 2004). In particular, inactivation of LDH was primarily examined to accumulate pyruvate since lactate production via LDH was a main route of pyruvate metabolism under anaerobic or microaerobic conditions (Mat-Jan et al. 1989). To avoid the generation of this by-product, highly aerobic culture conditions are thought to be effective.
The metabolic fate of pyruvate depends on the redox state in microbial cells (Clark 1989). In the aerobic metabolism of glucose, pyruvate is decarboxylated by PDC to produce acetyl-CoA and CO2. O2 or other external compounds serve as a terminal electron acceptor, and NADH concentrations remain relatively low (Causey et al. 2004). In addition, a lipoic acid auxotroph of E. coli with a mutation in the F1ATPase component of (F1F0) H+-ATP synthase achieved a high yield of pyruvate due to the higher intracellular levels of NADH (Yokota et al. 1994; 1997; Noda et al. 2006). In these studies, higher NADH levels were caused by the cessation of NAD+ regeneration from NADH because the energy production through oxidative phosphorylation was inactivated by the mutation. Thus, the change in intracellular NADH/NAD+ ratio exerts a profound effect on the pyruvate metabolism of E. coli.
As an alternative strategy for enhancing the intracellular NADH/NAD+ ratio in E. coli the introduction of formate dehydrogenase (FDH) from Mycobacterium vaccae can be effective (Galkin et al. 1997) because the reaction of FDH regenerates NADH from NAD+ with the degradation of formate into CO2. It is thus expected that the regulation of the redox status by FDH introduction increases pyruvate production of E. coli from glucose. Figure 1 shows the conceptual drawing of the proposed strategy to accumulate pyruvate in E. coli. In the present study, pyruvate accumulation is examined under the condition of suppressing the activity of inherent LDH by sufficient oxygen supply to E. coli cells. Furthermore, FDH is introduced to decrease the decarboxylation of pyruvate by PDC since FDH is a direct competitor with PDC in using NAD+ as a cosubstrate.
Conceptual drawing of proposed strategy for accumulation of pyruvate in E. coli. In the present study, the pyruvate accumulation is examined under the conditions of suppressing the functions of LDH (broken line A) by aerobiosis and pyruvate the dehydrogenase complex (broken line B) by unbalancing NADH generation. LDH lactate dehydrogenase, PDC the pyruvate dehydrogenase complex, FDH formate dehydrogenase, POX pyruvate oxidase, PTA phosphotransacetylase, ACK acetate kinase
Materials and methods
Bacterial strains and plasmids
Escherichia coli BW25113 stain, and its poxB-deficient mutant JW0855 (named BW25113∆poxB) and pta-deficient mutant JW2294 (named BW25113∆pta) were obtained from the National BioResource Project (National Institute of Genetic (NIG), Mishima, Japan). The fdh gene of Mycobacterium vaccae obtained from pFDHAlaDH (Galkin et al. 1997) was used by digesting the plasmid with HindIII and SphI at both end sites of the fdh insert. Then the fdh gene was ligated into the pHSG399 vector which contains a pMB1 replicon (Takashita et al. 1987). This plasmid, named pHfdh, was introduced into BW25113, BW25113∆poxB and BW25113∆pta strains, which were denoted as BW25113/pHfdh, BW25113∆poxB/pHfdh and BW25113∆pta/pHfdh, respectively.
Cultures of Escherichia coli strains
The respective cells pre-cultured in liquid LB medium for 12 h at 37°C were inoculated into 40 ml test medium in a 200 ml baffled conical flask to give an initial OD660 ≅ 0.05. The test medium contained 20 g glucose, 4 g sodium formate, 4 g Polypepton (Wako Pure Chemical Industries, Ltd.), 2 g NaCl, 10 g (NH4)2SO4, 1 g KH2PO4, 0.5 g MgSO4·7H2O and 14.7 mg CaCl2·2H2O per litre of deionized water. When necessary, chloramphenicol (34 mg l−1) was added together with 0.5 mM IPTG for inducing the recombinant protein. The initial pH of the medium was adjusted to 8.0 using NaOH, and CaCO3 (20 g l−1) was included in the medium to avoid pH drop in the course of culturing. The cultures were grown at 37°C with shaking at 100 or 200 rpm with 4 cm amplitude to adjust the amount of O2 supplied at a given O2 mass transfer coefficient (K L a) level. The value of K L a under each condition was determined by the sodium sulfite method as described elsewhere (Cooper et al. 1944). Cell growth was recorded by measuring OD660 after dissolving solid CaCO3 with dilute HCl, and represented as dry cell weight (DCW) by calculating from DCW = 0.36 × OD660.
Analyses
Glucose was determined by an enzyme kit. Organic acids were analyzed by HPLC with an Aminex HPX-87H column at 55°C using 20 mM H2SO4 as solvent at 0.6 ml min−1. Detection was at 210 nm. The data were recorded as averages with standard deviations, obtained from three or four independent culture runs under the respective conditions.
Results and discussion
Effect of overexpression of fdh gene on culture property of E. coli in the presence of formate
Figure 2 compares the time profiles of cell growth, glucose consumption and organic acid production of E. coli BW25113 and BW25113/pHfdh strains. In the culture of the original strain BW25113 (Fig. 2a), DCW reached 1.12 g l−1 after 6 h, showing no appreciable growth afterwards (DCW = 1.33 g l−1 at t = 24 h). Glucose consumption rate was relatively slow and 13.4 g l−1 glucose remained at t = 24 h. Furthermore, formate concentration did not change throughout the culture period, which suggested that inherent FDH of E.coli did not function for formate utilization under the examined culture conditions. The dull cell growth and glucose consumption were attributable to cytotoxicity of residual formate (Östling and Lindgren 1993). Acetate was a dominant product detected in the culture and ultimately reached 4.06 g l−1. The production of other organic acids was not detected except the small amount of pyruvate (0.52 g l−1 at t = 24 h).
In the case of BW25113/pHfdh strain (Fig. 2b), gradual proliferation of cells lasted and attained a prevailing cell growth (DCW = 2 g l−1) over the BW25113 strain at the end of culture. BW25113/pHfdh cells continuously utilized glucose, followed by complete consumption at the end of the culture. In addition, formate was consumed rapidly during the growth phase, which indicated that the recombinant FDH functioned effectively in the cells. The consumption of formate released the cells from its cytotoxicity. Interestingly, the introduction of recombinant FDH resulted in the significant enhancement of pyruvate production during the later growth phase, achieving 5.51 g pyruvate l−1 at the end of culture. This value was ten times higher than that of the culture of BW25113 strain. Furthermore, lactate was not detected throughout the culture though this strain originally has LDH, suggesting that the DOT rate was high enough to inactivate the enzyme. This phenomenon offers a new insight into the production of pyruvate by changing the redox balance in the cells instead of using mutants with the deletion of genes involved in the pyruvate-dissimilating pathways.
Consideration of metabolic changes of pyruvate accumulation
Although pyruvate accumulation was achieved by the FDH reaction, the mechanism of this phenomenon is still unclear. To clarify the metabolic flow in the central metabolism, oxygen was chosen as a parameter for the following reasons. It was reported that the NADH/NAD+ ratio responses to changes in oxygen availability since oxygen is a terminal electron acceptor during NADH oxidation in oxidative phosphorylation (Wimpenny and Firth 1972). In addition, pyruvate oxidase (POX) catalyzes the oxidative decarboxylation of pyruvate to acetate and CO2. This reaction is accompanied by the transfer of electrons from the reduced flavoprotein to O2 (Cunningham and Hager 1975). These reports suggest that an elevated O2 supply may decrease pyruvate production in association with an increase in acetate generation by enhancing POX activity.
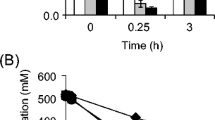
Figure 3 shows the time courses of DCW, glucose, and organic acids in the culture of BW25113/pHfdh at K L a = 4.9 min−1. Contrary to expectation, pyruvate production was 6.69 g l−1 at t = 18 h, which was higher compared with that at K L a = 1.5 min−1 (5.51 g l−1 at t = 24 h). Table 1 summarizes the results obtained from the culture runs at K L a = 4.9 min−1. Concerning BW25113/pHfdh strain, the yield of pyruvate was 0.34 g pyruvate g glucose−1, which was much higher than those obtained from the two control cultures (with formate and without recombinant FDH, and without formate and with recombinant FDH). These results demonstrate that the impact of FDH reaction on the accumulation of pyruvate is also effective even under a highly aerobic culture condition.
The acetate concentration did not change significantly between different oxygenation levels (Figs. 2, 3). It can therefore be inferred that acetate production by these strains is not through direct oxidation of pyruvate to acetate by POX, but from the conversion of acetyl-CoA to acetate by phosphotransacetylase (PTA) and acetate kinase (ACK). To confirm our hypothesis, mutant strains lacking poxB or pta gene were also examined focusing on pyruvate and acetate production with the introduction of FDH (Table 1). As expected, in the case of POX-deficient mutant (BW25113ΔpoxB/pHfdh), acetate at t = 18 h was 3.15 g l−1, a value comparable to that of BW25113/pHfdh.
This result revealed that POX is not the main contribution to acetate production under the examined culture conditions. On the other hand, deletion of the pta gene significantly suppressed acetate production (1.35 g l−1 at t = 18 h), suggesting that the conversion of acetyl-CoA to acetate was a dominant route for the production of acetate. Though acetate production was suppressed, pyruvate production was not significantly different between BW25113/pHfdh and BW25113Δpta/pHfdh strains. This can be explained by the increased DCW in the culture of BW25113Δpta/pHfdh strain, compared with BW25113/pHfdh strain. In other words, the pta deletion caused an increased flow of acetyl-CoA towards the TCA cycle, followed by enhancing of cell mass synthesis.
Based on the findings in the present study, we propose a possible mechanism for the accumulation of pyruvate in E. coli cells with recombinant FDH. In the case of BW25113/pHfdh strain, the NADH/NAD+ ratio is forced to be kept at a high level as a result of formate decomposition by the FDH reaction, which negatively affects the decarboxylation of pyruvate by PDC. This is because the oxidation of pyruvate to acetyl-CoA through lipoamide dehydrogenase, a component of PDC, needs NAD+ as a cofactor for the reaction. In addition, a high NADH level suppresses the activity of the TCA cycle through allosteric regulation of key enzymes such as a citrate synthase (Weitzman 1966). We also clarified that the impact of FDH reaction on the accumulation of pyruvate was effective even under a highly aerobic culture condition.
In conclusion, the introduction of FDH into E. coli enhanced the accumulation of pyruvate by increasing the regeneration of NADH from NAD+. This may be supported by the thought FDH become a direct competitor with PDC in using NAD+ as a cosubstrate at a lower level of intracellular NAD+. Our strategy offers a new insight into the production of pyruvate by changing the redox balance in the cells instead of using mutants with the deletion of genes involved in the pyruvate-dissimilating pathways.
References
Causey TB, Shanmugam KT, Yomano LP, Ingram LO (2004) Engineering Escherichia coli for efficient conversion of glucose to pyruvate. Proc Natl Acad Sci USA 101:2235–2240
Clark DP (1989) The fermentation pathways of Escherichia coli. FEMS Microbiol Rev 63:223–234
Cooper CM, Fernstrom GA, Miller SA (1944) Performance of agitated gas–liquid contactors. Ind Eng Chem 36:504–509
Cunningham CC, Hager LP (1975) Reactivation of the lipid-depleted pyruvate oxidase system from Escherichia coli with cell envelope neutral lipid. J Biol Chem 250:7139–7146
Galkin A, Kulakova L, Yoshimura T, Soda K, Esaki N (1997) Synthesis of optically active amino acids from α-keto acids with Escherichia coli cells expressing heterologous genes. Appl Environ Microbiol 63:4651–4656
Mat-Jan F, Alam KY, Clark DP (1989) Mutations of Escherichia coli deficient in the fermentative lactate dehydrogenase. J Bateriol 171:342–348
Noda S, Takezawa Y, Mizutani T, Asakura T, Nishiumi E, Onoe K, Wada M, Tomita F, Matsushita K, Yokota A (2006) Alterations of cellular physiology in Escherichia coli in response to oxidative phosphorylation impaired by defective F1-ATPase. J Bacteriol 188:6869–6876
Östling CE, Lindgren SE (1993) Inhibition of enterobacteria and Listeria growth by lactic, acetic and formic acids. J Appl Bacteriol 75:18–24
Takashita S, Sato M, Toba M, Masahashi W, Hashimoto-Gotoh T (1987) High-copy-number and low-copy-number plasmid vectors for lacZ-complementation and chloramphenicol- or kanamycin-resistance selection. Gene 61:63–74
Tomar A, Eiteman MA, Altman E (2003) The effect of acetate pathway mutations on the production of pyruvate in Escherichia coli. Appl Microbiol Biotechnol 62:76–82
Weitzman PDJ (1966) Regulation of citrate synthase activity in Escherichia coli. Biochim Biophys Acta 128:213–215
Wimpenny JWT, Firth A (1972) Levels of nicotinamide adenine dinucleotide and reduced nicotinamide adenine dinucleotide in facultative bacteria and the effect of oxygen. J Bacteriol 111:24–32
Yokota A, Terasawa Y, Takaoka N, Shimizu H, Tomita F (1994) Pyruvic acid production by an F1-ATPase-defective mutant of Escherichia coli W1485lip2. Biosci Biotechnol Biochem 58:2164–2167
Yokota A, Henmi M, Takaoka N, Hayashi C, Terasawa Y, Fukumori Y, Tomita F (1997) Enhancement of glucose metabolism in a pyruvic acid-hyperproducing Escherichia coli mutant defective in F1-ATPase activity. J Ferment Bioeng 83:132–138
Acknowledgment
We thank Dr. Kurihara of Kyoto University for kindly providing the pFDHADH plasmid.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ojima, Y., Suryadarma, P., Tsuchida, K. et al. Accumulation of pyruvate by changing the redox status in Escherichia coli . Biotechnol Lett 34, 889–893 (2012). https://doi.org/10.1007/s10529-011-0842-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-011-0842-y