Abstract
Mucor indicus can be used to produce ethanol from a variety of sugars, including pentose’s. An extract of it, produced by autolysis, could replace yeast extract in culture medium with improved production of ethanol. At 10 g l−1, the extract gave a higher ethanol yield (0.47 g g−1) and productivity (0.71 g l−1 h−1) compared to medium containing yeast extract (yield 0.45 g g−1; productivity 0.67 g l−1 h−1).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mucor indicus (formerly M. rouxii) can grow aerobically or anaerobically on various carbon sources, including hexoses and pentose’s, and produce ethanol with yield and productivity in the same order as Saccharomyces cerevisiae (Karimi et al. 2005; Lennartsson et al. 2009). Its high tolerance to sugar, ethanol and various possible inhibitors suggest it may have industrial applicability (Abtahi et al. 2010; Karimi et al. 2005). Furthermore, the biomass of this fungus can be used as a source of chitosan and also as a fish feed (Zamani 2010; Zamani et al. 2010).
The majority of studies on ethanol production by zygomycetes fungi have used media containing complex growth supplement, such as yeast extract, to a achieve high ethanol yield (Karimi et al. 2008; Sues et al. 2005; Millati et al. 2005). However, the cost of this supplement is not realistic for industrial ethanol production.
Fungal biomass can be autolysed by endogenous enzymes resulting in disintegration of the cells (Perez-Leblic et al. 1982). The resulting autolysate is a nutrient-rich solution of amino acids, peptides, phosphorus, and carbohydrates (Koutinas et al. 2005).
In the current work, M. indicus biomass was subjected to autolysis, the cell wall was separated for chitosan production, and the fungal autolysate was used as a nutrient for ethanol production by the same fungal strain in order to reduce the nutrient consumption in the fermentation.
Materials and methods
Microorganism and growth
Mucor indicus CCUG 22424 (Culture Collection University of Gothenburg, Sweden) was grown in Erlenmeyer flasks with 300 ml medium containing (per liter): 40 g glucose monohydrate, 5 g yeast extract, 7.5 g (NH4)2SO4, 3.5 g K2HPO4, 1.0 g CaCl2·2H2O, and 0.75 g MgSO4·7H2O at pH 5.5 ± 0.1 (Sues et al. 2005). The flasks were incubated at 32 ± 0.5°C and 180 rpm for 30 h.
Fungal autolysis
Fungal biomass was recovered by filtration, washed, and re-suspended in sterile distilled water to give 50 g biomass l−1. The pH was then adjusted to 5.2 using 1 M H2SO4. The autolyses were performed at 55 ± 1°C for 72 h by shaking at 120 rpm, with or without ultrasonication. The ultrasonication was carried out before autolysis for 20 min (38 kHz; Pulse 700, Italy).
After autolysis, the suspensions were centrifuged for 15 min (3,400×g). The solubilized cell constituents, present in the supernatant (autolysate), resulting from the autolysis process with or without ultrasonication were referred to as “ultrasonicated fungal extract” or “fungal extract”, respectively. The fungal biomass and the residual solids remained after the autolysis were dried at 55 ± 1°C and were referred to as “untreated fungal biomass” and “residual cellular materials”, respectively.
Ethanol production
Fermentations were carried out in 120 ml sealed anaerobic bottles with 50 ml medium containing 40 g glucose monohydrate l−1 and different supplements (Table 1). The media were autoclaved at 121°C for 20 min, and inoculated with 1 ml suspension containing 4.5 (±0.5) × 105 spores of M. indicus.
Analytical methods
The amount of the fungal extract in autolysates was measure by drying of 10 ml autolysate at 55 ± 1°C. Glucose, ethanol, glycerol, and other metabolites were determined by HPLC according to Karimi et al. (2008).
Results and discussion
In all previous studies on M. indicus, high yields of ethanol have been obtained only when a fully supplemented media, containing at least 5 g yeast extract l−1, was used (Sues et al. 2005; Karimi et al. 2008; Lennartsson et al. 2009). In the current work, the fungal biomass, an unavoidable byproduct of the ethanol production, was subjected to autolysis, and the autolysate was used as a supplementary nutrient which replaced yeast extract and other nutrients (mineral salts) in fermentation media. The nutritional effects of untreated fungal biomass and residual cellular materials were also investigated. Cultivation of the fungus with 5 g yeast extract l−1 and mineral salts supplementation as a fully supplemented medium (Sues et al. 2005) was used to establish a basis for the comparison in ethanol production (Fig. 1a, b).
Effect of the supplementation of untreated fungal biomass on a glucose assimilation and b ethanol production. The symbols = non-supplemented medium (40 g glucose l−1 with no nutrient supplementation) (filled square); yeast extract (5 g l−1) and mineral salts (filled triangle); untreated fungal biomass (5 g l−1) (filled circle); untreated fungal biomass (5 g l−1) and mineral salts (filled diamond). All fermentations were performed at 32 ± 0.5°C with 180 rpm for 72 h
In all cultivations, glycerol was the most abundant byproduct of the fermentation (Table 1). The other metabolites such as pyruvic, acetic, and succinic acids were also detected but at less than 10 mg g−1 in all experiments (data not shown).
Evaluation of untreated fungal biomass for media supplementation
Using a non-supplemented medium (40 g glucose l−1 with no nutrient supplementation), no ethanol was produced even after 72 h (Fig. 1a, b). Untreated fungal biomass as a supplement gave low ethanol yields with incomplete sugar consumption even after 72 h (Table 1). The low performance of the untreated fungal biomass may be attributed to M. indicus being unable to use unhydrolysed proteins or other polymeric materials of the fungal cell. Supplementation of the mineral salts together with the untreated fungal biomass improved ethanol yield and productivity; however, the results were low (Table 1; Fig. 1).
Evaluation of fungal autolysate for media supplementation
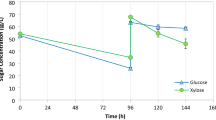
Supplementation of fungal autolysate, to give 2.5, 5, and 10 g fungal extract l−1 in fermentation media, was investigated (Table 1). Fungal extract at 5 g l−1 successfully replaced 5 g yeast extract l−1 with a better production of ethanol (Table 1; Fig. 2). The extract at 10 g l−1 gave complete sugar consumption in less than 24 h and an even higher ethanol yield and productivity, compared to the fully supplemented medium (Fig. 2a, b). This demonstrates that the fungal autolysate contains sufficient essential nutrients for ethanol fermentation. However, the rates of glucose consumption and ethanol production in media with 2.5 g fungal extract l−1 supplementation were low which indicated the existence of nutrient limitation.
Effect of supplementation of different fungal extract concentrations on (a and c) glucose assimilation and (b and d) ethanol production. The symbols = yeast extract (5 g l−1) (filled triangle); fungal extract (2.5 g l−1) (filled circle); fungal extract (5 g l−1) (filled diamond); fungal extract (10 g l−1) (filled square); fungal extract (2.5 g l−1) and mineral salts (white circle); fungal extract (5 g l−1) and mineral salts (lozenge); fungal extract (5 g l−1) and (Mg and Ca salts) (times symbol). All fermentations were performed at 32 ± 0.5°C with 180 rpm for 72 h
Effect of ultrasonic pretreatment on fungal autolysate for media supplementation
Ultrasonic treatment of fungal biomass before autolysis released more intracellular components and produced an autolysate with higher concentrations of fungal extract. Supplementation of the fungal autolysate containing ultrasonicated fungal extract gave higher ethanol yields with a higher productivity than those obtained from 5 g fungal extract l−1 supplementation (Table 1). Addition of either mineral salts or CaCl2 and MgSO4 to media containing untrasonicated fungal extract improved the results to the level which was achieved fermentation of the fully supplemented medium.
Evaluation of the residual cellular materials for media supplementation
Supplementation of the residual cellular materials in fermentation media resulted in incomplete sugar assimilation and low ethanol yield and productivity even after addition of mineral salts (Table 1). Therefore, the residual cellular materials, which mainly contain cell walls, have low nutritional values, and can probably be used for production of chitosan.
Chitosan extraction
The chitosan contents of residual cellular materials and untreated fungal biomass were extracted according to the method described by Zamani et al. (2010). The results showed that the residual cellular materials contain higher amounts of chitosan per dry alkali-insoluble material (AIM) compared with untreated fungal biomass (Fig. 3). Therefore, the residual cellular materials have a high potential for biological production of superabsorbent (Zamani 2010).
a Concentration of alkali-insoluble cell wall material (AIM) of M. indicus before and after autolysis process, measured as dry AIM per dry biomass (g g−1) and b concentration of chitosan in the AIM of materials, measured as chitosan per dry AIM (g g−1). The dried biomass was treated with 0.5 M NaOH at 120°C for 20 min to obtain AIM. The AIM was then subjected to two-steps treatment with dilute sulfuric acid, and the chitosan was subsequently recovered from the acid solution by precipitation at lowered temperature (Zamani et al. 2010)
Conclusions
A fermentation medium based on application of M. indicus biomass autolysate can be used for bioethanol production with a minimal or no additional nutrient requirement. The residual cellular materials as a byproduct of the autolysis process contain appreciable amounts of chitosan.
References
Abtahi Z, Millati R, Niklasson C, Taherzadeh MJ (2010) Ethanol production by Mucor indicus at high glucose and ethanol concentrations. Minerva Biotecnol 22:83–89
Edebo L (2002) Porous structure comprising fungi cell walls. US Patent 6423337
Karimi K, Brandberg T, Edebo L, Taherzadeh MJ (2005) Fed-batch cultivation of Mucor indicus in dilute-acid lignocellulosic hydrolyzate for ethanol production. Biotechnol Lett 27:1395–1400
Karimi K, Edebo L, Taherzadeh MJ (2008) Mucor indicus as a biofilter and fermenting organism in continuous ethanol production from lignocellulosic hydrolyzate. Biochem Eng J 39:383–388
Koutinas AA, Wang RH, Webb C (2005) Development of a process for the production of nutrient supplements for fermentations based on fungal autolysis. Enzyme Microb Tech 36:629–638
Lennartsson PR, Karimi K, Edebo L, Taherzadeh MJ (2009) Effects of different growth forms of Mucor indicus on cultivation on dilute-acid lignocellulosic hydrolyzate, inhibitor tolerance, and cell wall composition. J Biotechnol 143:255–261
Millati R, Edebo L, Taherzadeh M (2005) Performance of Rhizopus, Rhizomucor, and Mucor in ethanol production from glucose, xylose, and wood hydrolyzates. Enzyme Microb Tech 36:294–300
Perez-Leblic M, Reyes F, Martinez M, Lahoz R (1982) Cell wall degradation in the autolysis of filamentous fungi. Mycopathologia 80:147–155
Sues A, Millati R, Edebo L, Taherzadeh MJ (2005) Ethanol production from hexoses, pentoses, and dilute-acid hydrolyzate by Mucor indicus. Fems Yeast Res 5:669–676
Zamani A (2010) Superabsorbent polymers from the cell wall of zygomycetes fungi. Dissertation, Chalmers University of Technology
Zamani A, Edebo L, Niklasson C, Taherzadeh MJ (2010) Temperature shifts for extraction and purification of zygomycetes chitosan with dilute sulfuric acid. Int J Mol Sci 11:2976–2987
Acknowledgments
The authors are grateful to Professor Lars Edebo and Dr. Akram Zamani for their assistance and scientific supports.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Asachi, R., Karimi, K. & Taherzadeh, M.J. Fungal autolysate as a nutrient supplement for ethanol and chitosan production by Mucor indicus . Biotechnol Lett 33, 2405–2409 (2011). https://doi.org/10.1007/s10529-011-0725-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-011-0725-2