Abstract
Due to negative environmental influence and limited availability, petroleum-derived fuels need to be replaced by renewable biofuels. Biodiesel has attracted intensive attention as an important biofuel. Microalgae have numerous advantages for biodiesel production over many terrestrial plants. There are a series of consecutive processes for biodiesel production with microalgae as feedstock, including selection of adequate microalgal strains, mass culture, cell harvesting, oil extraction and transesterification. To reduce the overall production cost, technology development and process optimization are necessary. Genetic engineering also plays an important role in manipulating lipid biosynthesis in microalgae. Many approaches, such as sequestering carbon dioxide from industrial plants for the carbon source, using wastewater for the nutrient supply, and maximizing the values of by-products, have shown a potential for cost reduction. This review provides a brief overview of the process of biodiesel production with microalgae as feedstock. The methods associated with this process (e.g. lipid determination, mass culture, oil extraction) are also compared and discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biofuels are renewable liquid fuels produced predominantly from crop-derived feedstock. Currently, biofuels include biodiesel from oil crops such as soy and palm, animal fat, and waste cooking oil (Spolaore et al. 2006), bioethanol and other alcohols from sugarcane and corn starch, H2, long-chain hydrocarbons, and biogas (Scott et al. 2010). Although oil crops are renewable resources, biodiesel production from oil crops in large quantities has been deemed unsustainable (Chisti 2008). Production of crop-derived biodiesel will require large amounts of arable land, which has to compete with the cultivation of food crops. This has led to the controversy of “food versus fuel” (Searchinger et al. 2008; Singh et al. 2011). The increasing criticism of the sustainability of many first-generation biofuels has stimulated the interest in developing second-generation biofuels produced from non-food feedstocks, such as lignocellulose. Recently, microalgae as feedstock for biofuels have received considerable attention due to their advantages over higher plants and other organisms. Although long-term research and development in this field have been carried out, commercial implementation of microalgal biodiesel is still in its infancy. Many key technologies need to be developed and optimized at almost all stages of the microalgal biodiesel pipeline, from screening of suitable microalgal strains to downstream processing. Moreover, fundamental mechanisms underlying lipid metabolism in microalgae remain unknown.
In this paper, we provide an overview of advances on these aspects, with emphasis on the choice of ideal microalgal strains, mass cultivation, biomass harvesting, lipid extraction and transesterification. Methodologies involving determination of microalgal lipid content and lipid extraction are also discussed. It should also be noted that production of other forms of biofuels and useful by-products from microalgae must be integrated into biodiesel production, so that when large-scale biodiesel production projects using microalgae as feedstock are initiated, an integrated comprehensive biodiesel system can be developed and optimized to reduce overall costs and energy requirements (Wijffels and Barbosa 2010).
Advantages of microalgae as biodiesel feedstock
Microalgae appear to be the only promising alternative to biofuel crop plants because of the following facts:
(i) Microalgae grow rapidly and many contain high content of lipids (Metting 1996; Chisti 2007) which can provide sufficient feedstock for large-scale biodiesel production; (ii) Non-requirement of arable land for microalgal culture makes their growth without conflict with food production. According to a calculation by Chisti (2007), meeting only half the existing U.S. transport fuel needs by biodiesel would require 24% of the total cropland to grow oil palm with the highest oil productivity (5,950 l ha−1). On the other hand, only 2.5% of existing cropping area would be required for cultivation of microalgae with 30% oil in biomass, which can also produce equivalent biodiesel. The percent of cropping area required can be still lower, because 30% oil content in the biomass can be achieved easily for many oleaginous microalgae; (iii) Microalgal cells have photosynthetic mechanism similar to those of higher plants to fix CO2 in air and convert the C to carbohydrates and lipids, with some species accumulating large amounts of triacylglycerides (TAGs), which are suitable for biodiesel production (Scott et al. 2010). The photosynthetic mechanism of microalgae is cost-effective compared with oil-producing heterotrophic microorganisms that utilize glucose and other organic carbon sources (Lee et al. 2008); (iv) Microalgae can remove large amounts of CO2 emitted by power plants and other industrial sources, contributing to GHG mitigation (Packer 2009); (v) From an environmental standpoint, some microalgae can efficiently treat highly polluted municipal and agricultural wastewater that contain excess nitrogen and phosphorus nutrients (Mulbry et al. 2008; de Godos et al. 2009); (vi) As an attractive bioreactor system, microalgae can produce useful by-products including long-chain polyunsaturated fatty acids, carotenoids for foodstuffs (Walker et al. 2005), and other compounds used in the cosmetic and pharmaceutical industries (Olaizola 2003). Integrated utilization of these by-products will make an important contribution to the reduction of overall production costs. Microalgae can also produce other fuels such as alkanes, ethanol, butanol and hydrogen by genetic engineering (Radakovits et al. 2010); (vii) The use of biodiesel from microalgae results in minimal release of sulphur dioxide, nitrous oxide and other contaminants when compared to petroleum-derived diesel (Li et al. 2008; Mutanda et al. 2011).
Selection of microalgal strains
Biodiversity of microalgae lipid properties
Microalgae comprise several groups of unicellular, colonial or filamentous, photosynthetic or heterotrophic micro-organisms containing chlorophyll and other pigments. Microalgae can grow autotrophically or heterotrophically, with a wide range of tolerance to different temperature, salinity, pH and nutrient availabilities (Hu et al. 2008; Brennan and Owende 2010). More than 40,000 microalgal species have been classified as prokaryotes (cyanobacteria) and several eukaryotes including green algae, diatoms, yellow–green algae, golden algae, red algae, brown algae, dinoflagellates and others (Hu et al. 2008; Packer 2009).
Many different classes of lipids can be produced in microalgal cells. Based on chemical structures and polarity, these lipids are divided into polar and neutral lipids. In most cases, polar lipids function as membrane structure components, which commonly include phospholipids (e.g. phosphatidylinositol, phosphatidylcholine and phosphatidylethanolamine) and glycolipids (e.g. monogalactosyldiacylglycerol and digalactosyldiacylglycerol). Neutral lipids include tri-, di- and mono-acylglycerols, waxes and isoprenoid-type lipids (e.g. carotenoids), among which triacylglycerols (TAGs) are frequently found to be accumulated as energy storage under various stress conditions (Roessler 1988; Bigogno et al. 2002; Mansour et al. 2003; Basova 2005; Khozin-Goldberg and Cohen 2006). Although almost all types of microalgal lipids can be extracted, only TAGs are easily transesterified into biodiesel by traditional methods. Analysis of thousands of microalgal species have shown tremendous difference in lipid content among different strains, ranging from 1% to approximately 85% of dry cell weight (DCW) (Spolaore et al. 2006; Chisti 2007; Li et al. 2008). Microalgae produce a wide variety of fatty acids with chain length from C10 to C24 (Hu et al. 2008), depending on species or strains. For example, the filamentous cyanobacterium Trichodesmium erythraeum can synthesize C10 fatty acid accounting for almost 50% of total fatty acids (Parker et al. 1967); whereas the dinoflagellate Crypthecodinium cohnii can produce docosahexaenoic acid (C22:6ω3, DHA) as high as 30–50% of total fatty acids (De Swaaf et al. 1999). Moreover, for any one microalgal strain, the lipid content, lipid class and fatty acid composition fluctuate under different culture conditions (Emdadi and Berland 1989; Peeler et al. 1989; Reitan et al. 1994; Khozin-Goldberg and Cohen 2006).
Screening of oleaginous microalgae
Due to the variation and diversity of microalgal lipids, selection of oleaginous microalgal strains suitable for biodiesel production will require screening large number of microalgal strains. The first large-scale collection and screening of oleaginous algae dates back to 1978, when the Aquatic Species Program (ASP) was launched by U.S. National Renewable Energy Laboratory (NREL) for production of biodiesel from high lipid-content algae. With 8 years of effort, over 3,000 strains were collected and eventually around 300 species were identified as oil-rich algae (Sheehan et al. 1998). Recently, some studies on screening of oleaginous microalgae were reported, focusing on optimizing culture conditions to increase lipid productivity and evaluation of the potential for biodiesel production (Gouveia et al. 2009; Rodolfi et al. 2009; Li et al. 2010a, b). The routine procedures for screening of microalgae involve sampling from the field or an algae collection library, isolation and purification, microalgal identification and maintenance, and evaluation of potential for lipid production, all of which have been well reviewed (Mutanda et al. 2011).
The main indexes determining the potential of microalgal strains as biodiesel feedstock are growth rate, lipid content, and lipid productivity. Table 1 shows both lipid content and productivity of some microalgal species, indicating that suitable oleaginous strains have the potential with no less than 20% (w/w) lipid content and 40 mg l−1 day−1 lipid productivity. Further adaptation to local environment, genetic improvement and alteration of cultivation method might enhance lipid productivities of microalgae. For example, Chlorella protothecoides grown heterotrophically had a 55% lipid content compared with 14.5% of autotrophically-cells (Miao and Wu 2006). For the present, however, genetic improvement remains a distant goal because methods formulation, selection, and recombination are not developed for most strains.
Free fatty acid content and composition influence the quantity and quality of synthesized biodiesel, especially when some microalgal strains are capable of producing polyunsaturated fatty acids. Free fatty acids cause the occurrence of saponification during the esterification of lipids, while excess unsaturated fatty acids may lead to tar formation induced by cross linking of fatty acid chains (Bruton et al. 2009), and low cetane numbers (Ramos et al. 2009). Moreover, increased content of polyunsaturated methyl esters causes decreased oxidation stability of biodiesel (Ramos et al. 2009). Other performances factors need to be taken into account when one strain is chosen for further evaluation, including the ability of nutrient removal of wastewater effluent, CO2 capture from industrial sources (Gonzales et al. 1997; Iwasaki et al. 1998; Lee and Lee 2001), environmental tolerance of high salinity, extreme pH, temperatures and high light intensity, production of by-products with commercial values, and rapid growth in photoreactors. The best microalgal species or strains for biodiesel production will satisfy all these requirements.
Determination of microalgal lipid content
Screening of oleaginous microalgal strains requires frequent measurement of lipid content, a crucial parameter of microalgal lipid properties. The conventional method used for determination of lipid content involves a complicated lipid extraction with solvent, separation, concentration and gravimetric determination (Bligh and Dyer 1959). Although this method has high accuracy, its major limitations are time-consuming, labor intensive and requirement of large amounts of microalgal biomass (no less than 10–15 mg wet cell weight), thus not suitable for large-scale screening of microalgal strains. Chromatographic methods with internal standards can provide information on both fatty acid quantity and profile in a single analysis, and was a commonly employed method in several fields (Carvalho and Malcata 2005). Odd chain fatty acids have often been considered as useful internal standard since they are structurally similar to the fatty acids being analyzed and do not interfere with the chromatographic analysis. The fatty acid content determined by internal standard method can be used for quantitative analysis of neutral lipids that are suitable for biodiesel production because in oil-accumulating microalgae TAGs comprise a major proportion of the total lipids. Although internal standard method requires the extraction and derivatizing of fatty acids, it may be applicable to quantitative analysis of lipid content of microalgae when it is performed in batch mode.
Some in situ measurement methods have been evaluated and used in determination of microalgal lipid content, including Nile Red (NR) staining, time-domain nuclear magnetic resonance (TD-NMR), colorimetric quantification and others. NR is a red phenoxazine dye that can effectively detect neutral and polar lipids within microalgae from certain classes (Lee et al. 1998; Elsey et al. 2007). Selecting adequate excitation and emission wavelengths can yield the greatest sensitivity of this method for different species (Elsey et al. 2007). Improved NR staining can also be used as a high throughput technique for screening of green algal species, many of which have thick and rigid cell wall (Chen et al. 2009). Although some factors may have impact on its accuracy, NR staining is still a rapid and efficient method in preliminary screening of microalgal strains with high content of neutral lipid. Another lipophilic fluorescent dye BODIPY 505/515 has recently been used for detecting lipids within viable microalgal cells (Cooper et al. 2010). Screening of oil-rich microalgal strains can be operated with a micromanipulator system and flow cytometry. TD-NMR is a fast and convenient method to quantify lipid of C. protothecoides (Gao et al. 2008), and its application for other microalgal species needs further investigation. Wawrik and Harriman (2010) developed a simple colorimetric method for quantification of algal lipid from small cultures. Algal lipids are saponified into fatty acids and reacted with a copper reagent. The resulting copper soaps are then colorimetrically measured by addition of substrate to produce a colored product. However, fatty acids of chain length with less than 12 carbons can not be detected, leading to underestimation of total lipid content in some algal species. The advantages and limitations of these methods are summarized in Table 2. Based on the analysis of these methods, measurements of neutral lipid contents using fluorescent dyes (e.g., Nile Red or BODIPY) have been most effective at this time. It is advisable that in situ measurement methods and chromatographic method can be considered for high-throughput preliminary screening of naturally occurred or genetically modified microalgal strains, while traditional gravimetric method can be used for final examination of resulting candidate oleaginous strains. The maximum accuracy of this method will depend on effective cell disruption and extraction of microalgal lipids.
Mechanisms of lipid accumulation in microalgae
Microalgal strains differ tremendously in the lipid content and productivity because of the vast diversity of microalgae. In addition, the levels of lipid accumulation are also affected by nutrients especially nitrogen deprivation and other environmental stress conditions. Understanding biochemistry and molecular mechanisms of lipid accumulation of microalgae needs to answer two important questions, that are why do some strains accumulate more lipids than others, and what triggers lipid accumulation under stress conditions.
In contrast to the large numbers of studies on the effects of environmental stress on TAG biosynthesis and the change of fatty acid profile (Guschina and Harwood 2006), there are few reports on the enzymes and pathways that trigger and control accumulation of storage TAGs in microalgal cells. However, important progress has been achieved in filamentous fungi. There is a strong correlation between the activity of malic enzyme and the extent of lipid accumulation, indicating that malic enzyme plays a determining role in controlling the lipid accumulation in fungi (Wynn et al. 1999; Zhang et al. 2007). While overexpressing genes involved in fatty acid synthesis has achieved small successes and malic enzyme is an NADPH-generating enzyme, the overall regulation of fatty acid biosynthesis may not lie in the flux of carbon into lipid but in the provision of reducing equivalents (Zhang et al. 2007). These findings have explained to a large extent why different fungal cells have different capacities for storage lipid accumulation. However, whether this conclusion can be extended from the fungi to microalgae remains to be determined. Recent studies in Chlamydomonas reinhardtii have revealed the possibility of identifying key genes involving lipid accumulation. C. reinhardtii is capable of accumulating large amounts of TAGs in lipid droplets upon nitrogen deprivation. Sixteen proteins potentially involved in lipid biosynthesis were identified from a lipid droplet-enriched fraction, and a novel protein, designated major lipid droplet protein (MLDP) was found associated with droplet formation (Moellering and Benning 2010). Expression of the gene encoding MLDP was found to correlate with the pattern of TAG accumulation upon nitrogen deprivation. Although inhibition of MLDP gene expression by RNA interference only affected lipid droplet size, but not TAGs content or metabolism, MLDP is still a potential molecular marker for lipid droplet and TAG accumulation.
In green microalgae, such as C. reinhardtii, lipid synthesis shares common carbon precursors with starch synthesis. Blocking starch synthesis by inactivation of ADP-glucose pyrophosphorylase had led to remarkable increase of TAG content and lipid droplet formation (Li et al. 2010a, b), suggesting that shift of carbon precursors from starch to TAG biosynthesis was an efficient strategy for enhancing oil content. Some algal species accumulate large amounts of TAG under nutrient limitation, which also led to a decrease in cell division. It was presumed that decrease or cessation of cell division triggered shunting of excess fixed carbons into storage lipids. Alternatively, inhibition of cell division caused the decreased utilization of storage lipids while synthesis of new lipids continued (Sheehan et al. 1998). It was also possible that TAG turnover was associated with the assembly of membrane lipids (Thompson 1996).
Genetic engineering of microalgae
Microalgae are unicellular photosynthetic organisms, making it possible in principle to manipulate metabolic pathways of microalgal cells by genetic engineering. Several reviews deal with the modest advances that have been achieved in the past decades on genetic engineering for improving the performance of lipid accumulation in microalgae (Rosenberg et al. 2008; Costa and de Morais 2011; Radakovits et al. 2010). In the present review, some topics on genetic engineering for biodiesel production from microalgae are discussed. The earliest work on the molecular biology and genetics of microalgae was isolation and overexpression of Acetyl CoA Carboxylase (ACCase) from Cyclotella cryptica. This enzyme catalyzes a key metabolic step in the synthesis of fatty acid in algae. Although the full-length ACCase gene was overexpressed in yeast and C. cryptica, no increased lipid production was observed (Sheehan et al. 1998). Many attempts to up-regulate the ACCase encoding gene and other genes in the pathway of fatty acid synthesis failed to achieve anticipated results, showing that direct manipulation of the fatty acid synthesis pathway is not a hopeful strategy. However, up-regulation of TAG assembly genes, such as glycerol-3-phosphate acyltransferase or diacylglycerol acyltransferase had enhanced oil content in many plant seeds (Radakovits et al. 2010), suggesting that enzymes in TAG assembly pathway are interesting candidates for genetic manipulation to enhance lipid biosynthesis in microalgae.
Current strategies for enhancing lipid biosynthesis or modifying fatty acid profile include: (i) elimination of fatty acid β-oxidation; (ii) increasing the supply of reducing power; (iii) introduction of plant-derived thioesterases to optimize the chain length of fatty acids; (iv) overexpression of thioesterase to decrease feedback inhibition resulting from increased acyl-ACP concentration (Lu et al. 2008; Courchesne et al. 2009). Another avenue is in vivo direct synthesis of biodiesel, although this has been realized only in Escherichia coli. Fatty acid ethyl esters could be synthesized by co-expressions of the ethanolic enzymes pyruvate decarboxylase and alcohol dehydrogenase from Zymomonas mobilis, and the wax ester synthase/acyl-CoA-diacylglycerol acyltransferase (WS-DGAT) from Acinetobacter baylyi strain ADP1 in E. coli (Kalscheuer et al. 2006). It will be tempting to try direct biodiesel production with microalgal cells as green factories.
Microalgal cultivation and harvesting
The commercial production of biodiesel requires enormous biomass through mass culturing of microalgae. Microalgae can be grown on a large scale in enclosed photobioreactors or in open ponds, each of which has advantages and limitations throughout the production process. Many factors must be considered and compared between these two culture methods.
Open ponds
The infrastructures of open ponds or raceways are not expensive and easy to operate. To minimize the cost, microalgal cells in open ponds must utilize sunlight and CO2 in the atmosphere. However, the quality and quantity of natural sunlight are affected by daily and seasonal fluctuation (Grobbelaar et al. 1996; Chisti 2008). For most open ponds or raceways, shallow water depths of 0.2–0.3 m are generally used to provide sufficient sunlight for most microalgal cells. The mixing of nutrients and water flow is mechanically achieved by paddle wheels and guided by baffles in recirculation channels (Grobbelaar 1994; Sheehan et al. 1998; Chisti 2007), which avoid settlement of microalgal cells and boost gas exchange. The biomass productivities of open ponds or raceways are determined to a certain extent by the areas of the ponds due to the restricted water depth. This not only increases the costs of land use and harvesting of cells (Borowitzka 1999; Hase et al. 2000; Scott et al. 2010), but also makes it difficult to control temperature on extremely hot or cold days.
Microbial contamination is inevitable because open ponds or raceways cannot be sterilized or kept under axenic conditions (Packer 2009). Under highly selective conditions, open ponds are suitable for mass culture of some microalgal species such as native species or those that tolerate high salinity or pH. For example, Dunaliella salina can dominate under high salt conditions in an open pond system. An alternative is to provide large quantities of inoculum or starter cultures for open ponds by smaller photobioreactors, which guarantees the dominance of selected microalgal strains (Singh et al. 2011). Although open ponds or raceways have some advantages compared to photobioreactors, the main drawback is their relatively low productivity.
Enclosed photobioreactors
Enclosed photobioreactors have received much attention because of high biomass productivity and easier control of culture conditions. There are various types of photoreactors designed for different purposes (for reviews see Carvalho et al. 2006; Eriksen 2008; Ugwu et al. 2008). Flat-plate, tubular photobioreactors with large illumination surface area are suitable for outdoor mass cultures of microalgae (Ugwu et al. 2008), whereas column bioreactors have the advantages of efficient mixing, high volumetric gas transfer rate and flexible control of growth conditions (Eriksen 2008). Tubular photobioreactors are often considered the most suitable for commercial large-scale production of microalgal biomass, which partly relies on the multiplication of bioreactor unit (Janssen et al. 2003; Chisti 2008). In indoor closed photobioreactors, illumination can be from natural solar light or metal halide lamps; whereas outdoor photobioreactors use natural sunlight or solar collection devices (Acién Fernández et al. 1997; Greenwell et al. 2010). Sunlight is considered only for large-scale biodiesel production purpose. To ensure optimum photosynthetic efficiency for each of the cells in an outdoor photobioreactor, a mechanical pump or airlift pump is used to mix the cultures (Chisti 2007).
The productivities of photobioreactors are affected by the supplies of light and CO2, changes of temperature, pH, dissolved O2 levels of cultures (Molina Grima et al. 2001; Chisti 2008), and the performance of selected microalgal strains. Biomass measurements or growth rate evaluations are critical in assessing the potential of photobioreactors and the performance of the algal strains. Microalgal biomass productivity can be evaluated in photobioreactors based on areal productivity (productivity per unit of occupied land area or illuminated reactor surface per unit of time), volumetric productivity, photosynthetic efficiency or biomass yield. Table 3 summarizes the maximal biomass productivities measured in three types of bioreactors, in which most microalgal strains can reach productivities of 10–30 g m−2 day−1 or 0.2–3.0 g l−1 day−1. The maximal biomass concentration ranges from 1 to 7 g l−1. In some cases, the maximal biomass productivity was reached at relatively low biomass concentration (Cuaresma et al. 2009; Meiser et al. 2004). Microalgal growth rate can be affected by the average illumination, light saturation constant and biomass concentration. Maximal biomass productivity can be achieved under optimal culture conditions. To meet transport fuel needs, large scale production of biodiesel requires producing sufficient biomass that must be provided by appropriate culture systems. Biomass productivity data and the volume of mass cultures allow an estimate of the potential annual biomass yield of photobioreactors. Culture volume is limited by the configuration and scalability of photobioreactors. Tubular reactors can be scaled-up by increasing the length and diameter of the tubes, or the number of units. However, an optimal configuration of tubular reactors was found to be a length of 80 m and diameter of 6 cm (Molina Grima et al. 2001; Chisti 2007), and thus the culture volume can only be scaled to 315 m3 on one hectare of land. The largest flat panel reactor was composed of five individual units of 200 l, and had a total capacity of 1,000 l (Richmond and Zhang 2001). Maximizing the number of this type of reactor units allows an attainable scale of 900 m3 per hectare of land. There are limited examples of large-scale applications of vertical column reactors. Chini Zitelli et al. (2006) described an outdoor annular column reactor with a height of 2 m and diameter of 0.5 m, and an optimal arrangement allowed 0.81 columns per square meter. Extrapolation of these data indicates a maximum culture volume of 972 m3 on one hectare of land. Without considering the cost, at the maximum biomass productivity of 3.8 g l−1 day−1, and if average oil content is valued at 40% of the biomass by dry weight, the maximum annual oil yield in different photobioreactors can be estimated to 143–443 tons per hectare of land (300 days of productive growing season per year). Therefore, to replace the current total U.S. transportation fuel needs (about 0.76 billion m3 per year), production of microalgal biodiesel would require land of approximately 1.5–4.6 M hectares. Although these estimates are approximate, they illustrate the potential of long-term research and development to improve the prospects for microalgal biodiesel production. However, Rodolfi et al. (2009) estimated an annual yield of 20–30 tons of microalgal oil per hectare on the local areas, according to the biomass and lipid productivities of Nannochloropsis sp. based on a two-phase (a nutrient sufficient phase followed by a nitrogen deprived phase) cultivation process with 110 l Green Wall Panel photobioreactors. Such low estimated yield relative to the theoretical value would be mainly attributed to the low biomass productivity (on average 0.3 g l−1 day−1). Therefore, narrowing this gap of microalgal oil yields would require a major advance in engineering improvements of photobioreactors and choosing suitable strains to significantly enhance the biomass productivity. Notably, scaling-up of photobioreactors, especially column reactors in an economical way would also present great challenges. With present technologies, closed photobioreactors have been considered to produce starter cultures (inocula) or high-value products.
In contrast to open ponds, photobioreactors have the advantages of low contamination, high productivity, minimal evaporation, attainable high microalgae densities or biomass concentrations, reduced CO2 losses and better control over culture conditions (Merchuk et al. 2000; Richmond 2004). The major drawbacks of photobioreactors are the high costs of construction, operating and maintenance. Although these can be partially compensated by higher productivity, they still limit the cost-effective production of microalgal biomass on a scale required for biodiesel production. Hybrid algae production system comprising photobioreactors and open ponds may be a promising way. Sufficient contaminant-free inocula can be produced by photobioreactors, followed by transfer to open ponds or raceways to attain the biomass needed for biodiesel production (Greenwell et al. 2010).
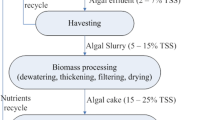
Harvesting of microalgal biomass
The small size (2–20 μm diam.) of microalgal cells and high water content of the broth make harvesting of microalgal biomass a costly process, accounting for approximately 20–30% of the total cost (Molina Grima et al. 2003). Microalgae can be harvested by sedimentation, flotation, filtration and centrifugation. Cost-effectiveness and high recovery efficiency are two ultimate purposes of selecting adequate harvesting methods, which depend greatly on the physical property of microalgal strain, culture method (open pond or bioreactor), the final products and downstream processing. The harvested biomass is mainly used to extract oils for biodiesel production and other by-products.
Flocculation is a low-cost pretreatment process used for aggregating microalgal cells carrying negative charges to form larger clumps, which can promote subsequent filtration, centrifugation, and sedimentation (Elmaleh et al. 1991; Jiang et al. 1993). Multivalent metal salts such as ferric chloride (FeCl3), aluminum sulfate (Al2(SO4)3) and ferric sulfate (Fe2(SO4)3) are the most frequently used flocculants or coagulants. Alum is also an effective flocculant for Scenedesmus and Chlorella (Golueke and Oswald 1965). Cationic polymers are an effective alternative to metal salts, which can aggregate particles through a bridging process (Tenney et al. 1969; Tilton et al. 1972). Polymeric flocculants are also extensively used to recover the biomass of some fresh water microalgae such as Chlorella (Tilton et al. 1972; Molina Grima et al. 2003). Flocculation alone is not sufficient for recovering microalgae cultures, and typically need to be combined with other processes. Sedimentation is only suitable for a few microalgal species with naturally high sedimentation rates, whereas some species with low specific gravity can naturally float at the surface of water. The flotation can be aided by dissolved air floatation (DAF), which uses tiny bubbles (less than 10 μm diameter) to attach the flocs and then float rapidly to the surface (Packer 2009). For recovery of most unicellular microalgae cultured in open ponds or raceways, flocculation is often a pre-treatment step to increase the particle size. Gravity sedimentation is a preferred method due to low capital costs even if large scale basins are needed.
Centrifugation and filtration are used to harvest microalgal biomass from photobioreactors, where microalgal cells attain higher density. Centrifugal recovery is rapid but energy intensive (McCausland et al. 1999). The settling characteristics of microalgal cells and residence time of slurry in the centrifuge can affect the recovery efficiency and cost of centrifugation (Heasman et al. 2000). Centrifugation is a method only for recovery of microalgal biomass with high value products (Molina Grima et al. 2003), but not applicable for large scale operations due to the large energy costs. There are many modes of filtration used for harvesting microalgae, and the filter productivities greatly depend on the size of algal cells. Conventional filtrations include rotary drum precoat filtration and press filters (Gudin and Therpenier 1986; Gudin and Chaumont 1991). Membrane microfiltration and ultrafiltration were proved feasible for recovering small quantities of algal biomass, but not applicable to large-scale use (Rossignol et al. 1999). The cost of membrane filtration is primarily dependent on membrane replacement and pumping. There is still considerable scope to increase productivity and lower cost in membrane filtration technology for harvesting microalgal biomass (Greenwell et al. 2010).
Extraction of microalgal lipid
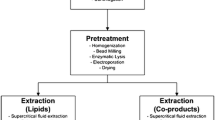
The wet microalgal biomass with high water content can be directly converted into liquid fuel by thermo-chemical liquefaction. The advantage of this technology is that no drying process is needed, and thus might be an alternative to many conventional extraction steps (Mutanda et al. 2011). However, biomass drying is another step that often needs to be taken into consideration for most methods of lipid extraction. Sun drying is the cheapest but time-consuming for dehydration of microalgal biomass (Prakash et al. 1997). Some efficient but costly drying technologies include drum drying, spray drying, fluidized bed drying, freeze drying, and refractance window dehydration technology (Li et al. 2008). There are several different methods for extracting lipids from dewatered microalgal biomass, including presses, conventional solvent extraction, enzymatic extraction, and other methods used only at laboratory scale, such as ultrasonic-assisted extraction, osmotic shock and supercritical carbon dioxide extraction.
Presses use mechanical force to rupture algal cells, and their design must be tailored to each microalgal strain. There are different presses available on the market, i.e. screw, expeller, and piston. Screw presses are used for extracting oils from a variety of oil seeds. Although applying this method to microalgal cells was not reported in the literature, it should be considered as a viable option for commercial scale extraction of microalgal oils. The use of solvents (such as hexane and chloroform) is a quick and efficient method for the extraction of lipids from the whole cells. A combination of press and solvent extraction is sometimes used. However, the disadvantages of using solvent method at a large scale are that the process requires additional energy input because the extracted lipids and solvent need to be separated by distillation, and the risk of fire and explosion should be considered (Greenwell et al. 2010). Nevertheless, solvent extraction is the most economical method currently available.
Osmotic shock is a sudden reduction in osmotic pressure, causing microalgal cells in solution to rupture and release cellular oil and other components. Osmotic shock is particularly suitable for some marine species of microalgae lacking a thick cell wall, such as Dunaliella sp. Supercritical CO2 extraction is an efficient method for complete extraction of oils. The low viscosity and high diffusivity of supercritical CO2 can result in higher extraction efficiency, and CO2 can be recycled during this process (Eller and King 2000). Enzymatic extraction uses enzymes to degrade cell walls with water acting as the solvent. This makes fractionation of oil much easier. The cost, efficiency, toxicity, and the ease of oil extraction process are the most important aspects to be considered when an appropriate oil extraction method is chosen. Supercritical carbon dioxide extraction and osmotic shock are used at laboratory scale, but not a suitable method for large-scale production of microalgal oils due to high operating costs at this time. Enzymatic extraction of microalgal oils is commercially feasible, but the effort is essential to reduce the cost of this process. Because there are limited methods that can be considered to apply for large-scale extraction of microalgal oils, other commercially viable approaches are still needed to be developed for microalgal biodiesel production to reduce the cost, minimize the co-extraction of non-lipid contaminants, and maximize the desirable lipid fractions (Fajardo et al. 2007; Halim et al. 2011).
Transesterification of microalgal oil to biodiesel
The extracted microalgal oil is needed for further conversion to produce biodiesel. Although there are several ways to make biodiesel, one of the most common processes for producing biodiesel from vegetable oils, animal fat and microalgal oils is transesterification (Li et al. 2007; Demirbas 2008). Transesterification is a multi-step, consecutive reaction, where TAGs are usually reacted with methanol (methanolysis) and converted to diglycerides, monoglycerides, finally yielding corresponding fatty acid methyl ester (FAME) and glycerol (by-product). The transesterification reaction is shown as Fig. 1, where different fatty acids are located in the carbon backbone of glycerol molecule. Other short chain alcohols such as ethanol, propanol, butanol, and amyl alcohol are also suitable for this process, but ethanol is most frequently used due to its low cost and physical and chemical advantages. The transesterification reaction can be achieved by homogenous catalysts (acid, base), and heterogeneous catalysts (solid) (Fukuda et al. 2001). Homogeneous alkali catalysts (e.g. using NaOH or KOH) are widely used in many industrial processes in a stirred reactor in batch mode, and NaOH is well accepted for use because of its low cost and high production yield (Demirbas 2003). If microalgal species contain high levels of free fatty acids (due to internal autolysis prior to processing) that exceed the threshold of 0.5%, a change in the partial reaction to saponification and formation of soap in alkaline catalyzed transesterification may occur (Fukuda et al. 2001). Soap will decrease the catalytic efficiency and introduce difficulty in separation of glycerol from products. Water in microalgal oil has a similar effect on catalytic effectiveness and downstream processing. Therefore, high levels of free fatty acid and water need to be removed from algal oils prior to alkaline catalyzed transesterification. A major drawback of homogeneously catalyzed reaction is the complicated purification process when the products and catalyst have to been separated (Demirbas 2008). Heterogeneous catalysis has less complicated post-processing than homogeneous catalysis. For example, compounds suitable as catalysts such as calcined layered double hydroxides (LDHs) or mixed metal oxides (MMOs) can be easily separated and rapidly recycled, lowering the production cost (Cantrell et al. 2005; Macala et al. 2008).
Enzymatic reaction with lipases as catalysts in transesterification is a promising alternative to produce biodiesel. Both extracellular and intracellular lipases can effectively convert TAGs to biodiesel in either aqueous or nonaqueous systems. Moreover, the by-product glycerol can be easily recovered and the catalytic effectiveness is not affected by free fatty acids and water in the reactors (Bisen et al. 2010). The main hurdle for commercialization of enzyme-catalyzed transesterification is high cost of enzyme, but immobilization of lipases in suitable support particle, recombinant DNA technology and protein engineering would dramatically lower the cost, increase the catalytic efficiency and solve the problems related to downstream processing (Ranganathan et al. 2008; Bisen et al. 2010), which make it attractive as a catalytic transesterification method.
Transesterification can also be conducted in the absence of catalysts by the supercritical methanol process, which requires high temperature in the range of 200–350°C and pressure around 20–50 MPa. The reaction can be finished within extremely short time, only 4 min (Saka and Kusdiana 2001). Supercritical methanol has the advantages of no catalyst cost, no saponification, easier post-treatment, less sensibility to water content and fewer waste products, when compared to a catalytic process, so the equipment cost and energy requirement for higher temperature and pressure may be compensated by these advantages. As non-catalytic biodiesel production method, supercritical methanol was studied only with vegetable and animal oils, but it might emerge as the most promising alternative to conventional catalytic transesterification.
Commercial attempts for algae-based biofuels production
There have been many attempts to commercialize microalgal biofuels in recent years. The U.S. Department of Energy (DOE) announced an investment of up to $24 million for three research groups aimed at commercializing biofuels derived from algae in June 2010. The Sustainable Algal Biofuels Consortium of Mesa, Arizona, led by Arizona State University, is getting $6 million to investigate biochemical conversion of algae to biofuels and products, as well as chemical properties of algal fuels and intermediates. Another team led by the University of California, San Diego, is receiving $9 million to concentrate on developing algae as a robust biofuels feedstock. The final $9 million is allocated to a group led by Cellana, a joint venture formed in 2007 between Royal Dutch Shell PLC and HR BioPetroleum Inc., and this group will investigate large-scale production of fuels and feed from microalgae grown in seawater. Several companies are also attempting to commercialize microalgal biodiesel. For example, in July 2009, Exxon Mobil Corporation announced an alliance with Synthetic Genomics Inc. to develop next generation biofuels from photosynthetic algae. One year later, they announced the opening of a greenhouse facility to test whether large-scale quantities of affordable fuel can be produced from algae. If their research and development proceeds smoothly, Exxon Mobil expects to invest more than $600 million in the algae biofuels program over the next decade. More recently, DOE released the final National Algal Biofuels Technology Roadmap developed from the National Algal Biofuels workshop in May 2010. This final roadmap is intended to guide future work and investments in algae-based biofuels. In U.K., Carbon Trust Company has invested millions of dollars in the commercialization and utilization of algae-based biofuel through Algae Biofuels Challenge project. The U.K. government announced it would contribute to the further funding of this project. Although the investments in biofuel production from algae are being increased world wide, several challenges must be tackled before commercial-scale production of biofuels from algae can be achieved.
Conclusions and future challenges
Microalgae are a diverse group of prokaryotic and eukaryotic microorganisms, and they are considered as the most promising biodiesel feedstock because of rapid growth, high lipid productivity, not competing with food supply, capacity of reducing CO2 emission, and potential of producing a large variety of high-value bioactive compounds with wide application in medicine, food, and cosmetic industries. Adequate oleaginous microalgal strains with wide tolerance to varying environmental conditions can be grown in photobioreactors or open ponds at large scale required for biodiesel production. There are many new technologies needed to be developed and improved, involving the harvesting of microalgal biomass, dewatering, extraction of microalgal oil, transesterification, and downstream processing. Genetic and metabolic engineering have demonstrated the feasibility of manipulating microalgae to enhance biomass and lipid productivities, which require ultimate understanding of biochemical mechanism of lipid accumulation in microalgae.
Biodiesel production with microalgae as feedstock is one of the most promising areas of research today. The main hurdle of microalgal biodiesel production is lowering the cost to make it competitive with petroleum derived fuels. Biodiesel production from microalgae requires integration of multidisciplinary knowledge including phycology, system biology, genetic engineering, biochemistry, bioprocess engineering, biorefining and others (Wijffels and Barbosa 2010). Figure 2 shows an integrated biodiesel production system, and it implies that there is much work to do from carbon dioxide to biodiesel. To lower the overall costs, several attempts are proposed and being investigated. These include: (i) sequestering CO2 from thermal power plants for enhancing microalgal biomass productivity; (ii) combination with treatment of nutrients enriched wastewater; (iii) based on the characteristics of microalgal strains, development of outdoor photobioreactors suitable for large-scale cultivation of microalgae with low cost; (iv) reasonable development and effective utilization of by-products from microalgal biomass; (v) optimization of biodiesel production by genetic and metabolic engineering. These require development of suitable methods before genetically modified microalgal strains can be applied to large-scale outdoor cultures in the future.
References
Acién Fernández FG, García Camacho F, Sánchez Pérez JA, Fernández Sevilla JM, Molina Grima E (1997) A model for light distribution and average solar irradiance inside outdoor tubular photobioreactors for the microalgal mass culture. Biotechnol Bioeng 55:701–714
Basova MM (2005) Fatty acid composition of lipids in microalgae. Int J Algae 7:33–57
Bigogno C, Khozin-Goldberg I, Boussiba S, Vonshak A, Cohen Z (2002) Lipid and fatty acid composition of the green oleaginous alga Parietochloris incisa, the richest plant source of arachidonic acid. Phytochemistry 60:497–503
Bisen PS, Sanodiya BS, Thakur GS, Baghel RK, Prasad GBKS (2010) Biodiesel production with special emphasis on lipase-catalyzed transesterification. Biotechnol Lett 32:1019–1030
Bligh EG, Dyer WJ (1959) A rapid method for total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Borowitzka MA (1999) Commercial production of microalgae: ponds, tanks, tubes and fermenters. J Biotechnol 70:313–321
Brennan L, Owende P (2010) Biofuels from microalgae—a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sustain Energy Rev 14:557–577
Bruton T, Lyons H, Lerat Y, Stanley M, BoRasmussen M (2009) A review of the potential of marine algae as a source of biofuel in Ireland. Sustain Energy Irel 30–31
Cantrell DG, Gillie LJ, Lee AF, Wilson K (2005) Structure-reactivity correlations in MgAl hydrotalcite catalysts for biodiesel synthesis. Appl Catal A Gen 287:183–190
Carlozzi P (2000) Hydrodynamic aspects and Arthrospira growth in two outdoor tubular undulating row photobioreactors. Appl Microbiol Biotechnol 54:14–22
Carvalho AP, Malcata FX (2005) Preparation of fatty acid methyl esters for gas-chromatographic analysis of marine lipids: insight studies. J Agric Food Chem 53:5049–5059
Carvalho AP, Meireles LA, Malcata FX (2006) Microalgal reactors: a review of enclosed system designs and performances. Biotechnol Prog 22:1490–1506
Chen W, Zhang CW, Song LR, Sommerfeld M, Hu Q (2009) A high throughput Nile Red method for quantitative measurement of neutral lipids in microalgae. J Microbiol Methods 77:41–47
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Chisti Y (2008) Biodiesel from microalgae beats bioethanol. Trends Biotechnol 26(3):126–131
Cooper MS, Hardin WR, Petersen TW, Cattolico RN (2010) Visualizing green oil in live algal cells. J Biosci Bioeng 109:198–201
Costa JAV, de Morais MG (2011) The role of biochemical engineering in the production of biofuels from microalgae. Bioresour Technol 102:2–9
Courchesne NM, Parisien A, Wang B, Lan CQ (2009) Enhancement of lipid production using biochemical, genetic and transcription factor engineering approaches. J Biotechnol 141:31–41
Cuaresma M, Janssen M, Vílchez C, Wijffels RH (2009) Productivity of Chlorella sorokiniana in a short light-path (SLP) panel photobioreactor under high irradiance. Biotechnol Bioeng 104:352–359
de Godos I, Blanco S, García-Encina PA, Becares E, Muñoz R (2009) Long-term operation of high rate algal ponds for the bioremediation of piggery wastewaters at high loading rates. Bioresour Technol 100(19):4332–4339
de Morais MG, Costa JA (2007) Biofixation of carbon dioxide by Spirulina sp. and Scenedesmus obliquus cultivated in a three-stage serial tubular photobioreactor. J Biotechnol 129:439–445
de Swaaf ME, de Rijk TC, Eggink G, Sijtsma L (1999) Optimisation of docosahexaenoic acid production in batch cultivation by Crypthecodinium cohnii. J Biotechnol 70:185–192
Demirbas A (2003) Biodiesel fuels from vegetable oils via catalytic and noncatalytic supercritical alcohol transesterifications and other methods: a survey. Energy Convers Manag 44:2093–2109
Demirbas A (2008) Comparison of transesterification methods for production of biodiesel from vegetable oils and fats. Energy Convers Manag 49:125–130
Eller FJ, King JW (2000) Supercritical carbon dioxide extraction of cedarwood oil: a study of extraction parameters and oil characteristics. Phytochem Anal 11:226–231
Elmaleh S, Coma J, Grasmick A, Bourgade L (1991) Magnesium induced algal flocculation in a fluidized bed. Water Sci Technol 23:1695–1702
Elsey D, Jameson D, Raleigh B, Cooney MJ (2007) Fluorescent measurement of microalgal neutral lipids. J Microbiol Methods 68:639–642
Emdadi D, Berland B (1989) Variation in lipid class composition during batch growth of Nannochloropsis salina and Pavlova lutheri. Mar Chem 26(3):215–225
Eriksen NT (2008) The technology of microalgal culturing. Biotechnol Lett 30:1525–1536
Fajardo AR, Cerdan LE, Medina AR, Fernandez FGA, Moreno PAG, Grima EM (2007) Lipid extraction from the microalga Phaedactylum tricornutum. Eur J Lipid Sci Technol 109:120–126
Fukuda H, Kondo A, Noda H (2001) Biodiesel fuel production by transesterification of oils. J Biosci Bioeng 92(5):405–416
Gao CF, Xiong W, Zhang YL, Yuan WQ, Wu QY (2008) Rapid quantitation of lipid in microalgae by time-domain nuclear magnetic resonance. J Microbiol Methods 75(3):437–440
García-González M, Moreno J, Carlos Manzano J, Javier Florencio F, Guerrero MG (2005) Production of Dunaliella salina biomass rich in 9-cis-β-carotene and lutein in a closed tubular photobioreactor. J Biotechnol 115:81–90
García-Malea López MC, Del Río Sánchez E, Casas López JL, Acién Fernández Sevilla JM, Rivas J, Guerrero MG, Molina Grima E (2006) Comparative analysis of the outdoor culture of Haematococcus pluvialis in tubular and bubble column photobioreactors. J Biotechnol 123:329–342
Golueke CG, Oswald WJ (1965) Harvesting and processing sewage grown planktonic algae. J Water Pollut Control Fed 37:471–498
Gonzales LE, Canizares RO, Baena S (1997) Efficiency of ammonia and phosphorus removal from a Colombian agroindustrial wastewater by the microalgae Chlorealla vulgaris and Scenedesmus dimorphus. Bioresour Technol 60:259–262
Gouveia L, Marques AE, Silva TL, Reis A (2009) Neochloris oleabundans UTEX #1185: a suitable renewable lipid source for biofuel production. J Ind Microbiol Biotechnol 36:821–826
Greenwell HC, Laurens LML, Shields RJ, Lovitt RW, Flynn KJ (2010) Placing microalgae on the biofuels priority list: a review of the technological challenges. J R Soc Interface 7:703–726
Grobbelaar JU (1994) Turbulence in algal mass cultures and the role of light/dark fluctuations. J Appl Phycol 6:331–335
Grobbelaar J, Nedbal L, Tichy V (1996) Influence of high frequency light/dark fluctuations on photosynthetic characteristics of microalgae photo acclimated to different light intensities and implications for mass algal cultivation. J Appl Phycol 8:335–343
Gudin C, Chaumont D (1991) Cell fragility, the key problem of microalgae mass production in closed photobioreactors. Bioresour Technol 38:141–151
Gudin C, Therpenier C (1986) Bioconversion of solar energy into organic chemicals by microalgae. Adv Biotechnol Process 6:73–110
Guschina IA, Harwood JL (2006) Lipids and lipid metabolism in eukaryotic algae. Prog Lipid Res 45:160–186
Halim R, Gladman B, Danquah MK, Webley PA (2011) Oil extraction from microalgae for biodiesel production. Bioresour Technol 102:178–185
Hase R, Oikawa H, Sasao C, Morita M, Watanabe Y (2000) Photosynthetic production of microalgal biomass in a raceway system under greenhouse conditions in Sendai City. J Biosci Bioeng 89:157–163
Heasman M, Diemar J, O’Connor W, Sushames T, Foulkes L, Nell JA (2000) Development of extended shelf-life microalgae concentrate diets harvested by centrifugation for bivalve mollusks—a summary. Aquacult Res 31(8–9):637–659
Hu Q, Milton Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54:621–639
Iwasaki I, Hu Q, Kurano N, Miyachi S (1998) Effect of extremely high-CO2 stress on energy distribution between photosystem I and photosystem II in a ‘high-CO2’ tolerant green alga. Chlorococcum littorale and the intolerant green alga Stichococcus bacillaris. J Photochem Photobiol B44:184–190
Janssen M, Tramper J, Mur LR, Wijffels RH (2003) Enclosed outdoor photobioreactors: light regime, photosynthetic efficiency, scale-up, and future prospects. Biotechnol Bioeng 81:193–210
Jiang JQ, Graham NJD, Harward C (1993) Comparison of polyferric sulphate with other coagulants for the removal of algae and algae-derived organic matter. Water Sci Technol 27:221–230
Kalscheuer R, Stolting T, Steinbuchel A (2006) Microdiesel: Escherichia coli engineered for fuel production. Microbiology 152:2529–2536
Khozin-Goldberg I, Cohen Z (2006) The effect of phosphate starvation on the lipid and fatty acid composition of the fresh water eustigmatophyte Monodus subterraneus. Phytochemistry 67:696–701
Lee K, Lee CG (2001) Effect of light/dark cycles on wastewater treatments by microalgae. Biotechnol Bioprocess Eng 6:194–199
Lee SJ, Yoon BD, Oh HM (1998) Rapid method for the determination of lipid from the green alga Botryococcus braunii. Biotechnol Technol 12:553–556
Lee SK, Chou H, Ham TS, Lee TS, Keasling JD (2008) Metabolic engineering of microorganisms for biofuels production: from bugs to synthetic biology to fuels. Curr Opin Biotechnol 19:556–563
Li XF, Xu H, Wu QY (2007) Large-scale biodiesel production from microalga Chlorella protothecoides through heterotrophic cultivation in bioreactors. Biotechnol Bioeng 98(4):764–771
Li Y, Horsman M, Wu N, Lan CQ, Dubois-Calero N (2008) Biofuels from microalgae. Biotechnol Prog 24(4):815–820
Li X, Hu HY, Yang J (2010) Lipid accumulation and nutrient removal properties of a newly isolated freshwater microalga, Scenedesmus sp. LX1, growing in secondary effluent. New Biotechnol 27(1):59–63
Li Y, Han D, Hu G, Dauvillee D, Sommerfeld M, Ball S, Hu Q (2010) Chlamydomonas starchless mutant defective in ADP-glucose pyrophosphorylase hyper-accumulates triacylglycerol. Metab Eng 12:387–391
Lu XF, Vora H, Khosla C (2008) Overproduction of free fatty acids in E. coli: implications for biodiesel production. Metab Eng 10:333–339
Macala GS, Robertson AW, Johnson CL, Day ZB, Lewis RS, White MG, Iretskii AV, Ford PC (2008) Transesterification catalysts from iron doped hydrotalcite-like precursors: solid bases for biodiesel production. Catal Lett 122:205–209
Mansour MP, Volkman JK, Blackburn SI (2003) The effect of growth phase on the lipid class, fatty acid and sterol composition in the marine dinoflagellate, Gymnodinium sp. in batch culture. Phytochemistry 63:145–153
Masojídek J, Papacek S, Sergejevova M, Jirka V, Cerveny J, Kunc J, Korecko J, Vervobikova O, Kopecky J, Stys D, Torzillo G (2003) A closed solar photobioreactor for cultivation of microalgae under supra-high irradiance: basic design and performance. J Appl Phycol 15:239–248
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review. Renew Sustain Energy Rev 14:217–232
McCausland MA, Brown MR, Barrett SM, Diemar JA, Heasman MP (1999) Evaluation of live microalgae and microbial pastes as supplementary food for juvenile Pacific oyster (Crassostrea gigas). Aquaculture 174:323–342
Meiser A, Schmid-Staiger U, Trösch W (2004) Optimization of eicosapentaenoic acid production by Phaeodactylum tricornutum in the flat panel airlift (FPA) reactor. J Appl Phycol 16:215–225
Merchuk JC, Gluz M, Mukmenev I (2000) Comparison of photobioreactors for cultivation of the microalga Porphyridium sp. J Chem Technol Biotechnol 75:1119–1126
Metting FB (1996) Biodiversity and application of microalgae. J Ind Microbiol 17:477–489
Miao XL, Wu QY (2006) Biodiesel production from heterotrophic microalgal oil. Bioresour Technol 97:841–846
Moellering ER, Benning C (2010) RNA interference silencing of a major lipid droplet protein affects lipid droplet size in Chlamydomonas reinhardtii. Eukaryot Cell 9:97–106
Molina Grima E, Fernández J, Acién FG, Chisti Y (2001) Tubular photobioreactor design for algal cultures. J Biotechnol 92:113–131
Molina Grima E, Belarbi EH, Fernandes FGA, Robles M, Christi Y (2003) Recovery of microalgal biomass and metabolites: process options and economics. Biotechnol Adv 20:491–515
Mulbry W, Kondrad S, Pizarro C, Kebede-Westhead E (2008) Treatment of dairy manure effluent using freshwater algae: algal productivity and recovery of manure nutrients using pilot-scale algal turf scrubbers. Bioresour Technol 99:8137–8142
Mutanda T, Ramesh D, Karthikeyan S, Kumari S, Anandraj A, Bux F (2011) Bioprospecting for hyper-lipid producing microalgal strains for sustainable biofuel production. Bioresour Technol 102:57–70
Olaizola M (2003) Commercial development of microalgal biotechnology: from the test tube to the marketplace. Biomol Eng 20:459–466
Packer M (2009) Algal capture of carbon dioxide; biomass generation as a tool for greenhouse gas mitigation with reference to New Zealand energy strategy and policy. Energy Policy 37:3428–3437
Parker PL, van Baalen C, Maurer L (1967) Fatty acids in eleven species of blue-green algae: geochemical significance. Science 155:707–708
Peeler TC, Stephenson MB, Einsphar KJ, Thompson GA (1989) Lipid characterization of enriched plasma membrane fraction of Dunaliella salina grown in media of varying salinity. Plant Physiol 89:970–976
Prakash J, Pushparaj B, Carlozzi P, Torzillo G, Montaini E, Materassi R (1997) Microalgal biomass drying by a simple solar device. Int J Solar Energy 18:303–311
Pruvost J, Vooren V, Cogne G, Legrand J (2009) Investigation of biomass and lipids production with Neochloris oleoabundans in photobioreactor. Bioresour Technol 100:5988–5995
Radakovits R, Jinkerson RE, Darzins A, Posewitz MC (2010) Genetic engineering of algae for enhanced biofuel production. Eukaryot Cell 9:486–501
Ramos MJ, Fernández CM, Casas A, Rodríguez L, Pérez Á (2009) Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour Technol 100:261–268
Ranganathan SV, Narasimhan SL, Muthukumar K (2008) An overview of enzymatic production of biodiesel. Bioresour Technol 99(10):3975–3981
Reitan KI, Rainuzzo JR, Olsen Y (1994) Effect of nutrient limitation on fatty acid and lipid content of marine microalgae. J Phycol 30(6):972–979
Richmond A (2004) Biological principals of mass cultivation. In: Richmond A (ed) Handbook of microalgal culture: biotechnology, applied phycology. Blackwell Science, London, pp 125–177
Richmond A, Zhang CW (2001) Optimization of a flat plate glass reactor for mass production of Nannochloropsis sp. outdoors. J Biotechnol 85:259–269
Rodolfi L, Zittelli GC, Bassi N, Padovani G, Biondi N, Bonini G, Tredici MR (2009) Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol Bioeng 102(1):100–112
Roessler PG (1988) Changes in the activities of various lipid and carbohydrate biosynthetic enzymes in the diatom Cyclotella cryptica in response to silicon deficiency. Arch Biochem Biophys 267:521–528
Rosenberg JN, Oyler GA, Wilkinson L, Betenbaugh MJ (2008) A green light for engineered algae: redirecting metabolism to fuel a biotechnology revolution. Curr Opin Biotechnol 19:430–436
Rossignol N, Vandanjon L, Jaouen P, Quemeneur F (1999) Membrane technology for the continuous separation microalgae/culture medium: compared performances of cross-flow microfiltration and ultrafiltration. Aquacult Eng 20:191–208
Saka S, Kusdiana D (2001) Biodiesel fuel from rapeseed oil as prepared in supercritical methanol. Fuel 80:225–231
Scott SA, Davey MP, Dennis JS et al (2010) Biodiesel from algae: challenges and prospects. Curr Opin Biotechnol 21:277–286
Searchinger T, Heimlich R, Houghton RA, Dong F, Elobeid A, Fabiosa J, Tokgoz S, Hayes D, Yu TH (2008) Use of US croplands for biofuels increases greenhouse gases through emissions from land use change. Science 319:1238–1240
Sheehan J, Dunahay T, Benemann J, Roessler P (1998) A look back at the U.S. Department of Energy’s Aquatic Species Program: biodiesel from algae. TP-580-24190. National Renewable Energy Laboratory, Golden
Singh A, Nigam PS, Murphy JD (2011) Mechanism and challenges in commercialisation of algal biofuels. Bioresour Technol 102:26–34
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101:87–96
Tenney MW, Echelberger WF, Schuessler RG, Pavoni JL (1969) Algal flocculation with synthetic organic polyelectrolytes. Appl Bacteriol 18:965–971
Thompson GA (1996) Lipids and membrane function in green algae. Biochim Biophys Acta 1302:17–45
Tilton RC, Murphy J, Dixon JK (1972) The flocculation of algae with synthetic polymeric flocculants. Water Res 6:155–164
Ugwu CU, Aoyagi H, Uchiyama H (2008) Photobioreactors for mass cultivation of algae. Bioresour Technol 99:4021–4028
Walker TL, Purton S, Becker DK, Collet C (2005) Microalgae as bioreactors. Plant Cell Rep 24:629–641
Wawrik B, Harriman B (2010) Rapid, colorimetric quantification of lipid from algal cultures. J Microbiol Methods 80(3):262–266
Wijffels RH, Barbosa MJ (2010) An outlook on microalgal biofuels. Science 329:796–799
Wiltshire KH, Boersma M, Möller A, Buhtz H (2000) Extraction of pigments and fatty acids from the green alga Scenedesmus obliquus (Chlorophyceae). Aquat Ecol 34:119–126
Wynn JP, Hamid ABA, Ratledge C (1999) The role of malic enzyme in the regulation of lipid accumulation in filamentous fungi. Microbiology 145:1911–1917
Zhang Y, Adams IP, Ratledge C (2007) Malic enzyme: the controlling activity for lipid production? Overexpression of malic enzyme in Mucor circinelloides leads to a 2.5-fold increase in lipid accumulation. Microbiology 153:2013–2025
Zitelli GC, Rodolfi L, Biondi N, Tredici MR (2006) Productivity and photosynthetic efficiency of outdoor cultures of Tetraselmis suecica in annular columns. Aquaculture 261:932–943
Acknowledgments
We acknowledge the financial support from the National Natural Science Foundation (no. 30970262) and the Major S&T Projects on the Cultivation of New Varieties of Genetically Modified Organisms (Grant 2009ZX08009-120B). We are grateful to Prof. Ratledge and Archer for valuable suggestions on the manuscript, and the referees who made a great effort to improve it. We are also grateful to Prof. Robert Haselkorn (University of Chicago) for his kind help in improving the manuscript. We apologize to colleagues whose original work has not been cited or replaced by reviews, due to space limitation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gong, Y., Jiang, M. Biodiesel production with microalgae as feedstock: from strains to biodiesel. Biotechnol Lett 33, 1269–1284 (2011). https://doi.org/10.1007/s10529-011-0574-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-011-0574-z