Abstract
Alternative biofuel sources, such as biodiesel, produced from nontoxic, biodegradable, and renewable materials are currently attracting great interest given the growing global energy demand. Microalgae are an exceptional biofuel source due to their potential for generating significant quantities of biomass and oil and for combining their production with environmental technologies such as wastewater treatment. Technologies for producing biodiesel from microalgae have been widely studied, especially increasing the lipid content in the cell, strain selection, lipid extraction, and transesterification methods and innovations that use wet biomass for extraction, which reduces the environmental impact and production cost. These aspects are explored in this chapter to identify the different production methods and technologies under development for expanding biodiesel production from microalgae.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Fossil fuels currently meet 27 % of the world energy demand. However, the scarce reserves, high prices, and environmental problems associated with using these fuels have driven the development of alternative energy sources to ensure economic and environmental sustainability of the energy supply (Stephens et al. 2010; Lam and Lee 2012; Hong et al. 2014).
Biodiesel is a fuel composed of alkyl esters obtained from long-chain fatty acid transformation. It can be produced by animal or plant triglyceride transesterification with short-chain alcohols, such as methanol and ethanol (Elshahed 2010; Velasquez-Orta et al. 2012; Likozar and Levec 2014).
Biodiesel is an exceptional renewable energy source because it releases less carbon dioxide, sulfur (SOx), and carbon monoxide (CO) into the atmosphere. Moreover, it does not contain aromatic compounds and other chemical substances that are harmful to the environment and human health; it is also more biodegradable than diesel (Kirrolia et al. 2013). The important properties of biodiesel are that it is a clean energy source with adequate viscosity and its inherent lubricity, high flash point, and elevated cetane number for use as a fuel (Knothe and Steidley 2011; Hong et al. 2014; Huang and Su 2014).
Multiple raw material sources are used for biodiesel production, including microalgae. These microorganisms contain lipids and fatty acids as cell membrane components, reserve substances, metabolites, and energy sources. Certain species are excessively rich in oils and may have an oil content greater than 80 %, which is efficiently converted into methyl esters (biodiesel) (Demirbas 2010).
Lipids from microalgae are mostly neutral lipids and typically include a high level of saturation, which renders the microalgae even more attractive for biofuel research (Rawat et al. 2013). In this context, using mixed cultures is also important because it may be advantageous to use biomass generated from wastewater treatment lagoons in biodiesel production (Wahlen et al. 2011).
In addition to using microalgae with a high oil content and improving the culture conditions to maximize biomass and oil production, the limiting step in producing biodiesel from microalgae is extracting the lipid content from the cells. This step can be performed separately or concurrently with transesterification (in situ) in the wet or dry biomass.
The aspects that should be explored to facilitate biodiesel production from microalgae include culture conditions that yield greater lipid accumulation in cells, oil extraction techniques, combination methods to avoid certain production stages, and optimizing the transesterification process.
2 An Overview of Microalgae Production for Obtaining Oil
Microalgae are among the most promising sources of biofuels due to their ability to rapidly multiply, even in low-quality water; absorb large quantities of CO2; and accumulate substantial quantities of reserve substances (Ashokkumar et al. 2014). Depending on the culture conditions and cultured species, large quantities of polysaccharides (sugars) and triglycerides (fats), which are raw materials for producing bioethanol and biodiesel, respectively, can be generated (Dębowski et al. 2013; Slade and Bauen 2013).
Maximizing lipid productivity is key for a successful biotechnological process using microalgae for biodiesel production, and one of the greatest challenges is scaling up from a laboratory to a commercial scale while maintaining high lipid productivity (Table 1) (Xu and Boeing 2014). Increased lipid extraction from microalgae depends on optimizing a series of nutritional conditions in the growth medium (Gupta et al. 2013; Huang and Su 2014).
Lipid accumulation in microalgae may result from a lack of nutrients, especially nitrogen, and excess organic carbon (Chandra et al. 2014). To abundantly produce valuable bioproducts from microalgae, particularly biofuels, it is important that the conditions offered to the microorganisms transition them toward a heterotrophic metabolism, where organic carbons, such as in sugars and organic acids, act as carbon and energy sources (Liang 2013).
Temperature, luminosity, pH, salinity, and salt minerals are other variables that, when controlled and applied as stress, can improve growth performance and energy reserve accumulation in microalgae (Venkata Mohan and Devi 2014).
Nitrogen, sulfur, and phosphorus are important components of algal cells and DNA and are essential for cell growth; these elements become limiting factors at low concentrations. Information on nitrogen deficiency in the algal metabolism is scarce, but certain reports indicate that a nitrogen deficit can lead to oxidative stress (Hockin et al. 2012). Zhang et al. (2013) observed substantial neutral lipid accumulation by Chlorella sorokiniana C3 in response to oxidative stress induced by a hydrogen peroxide treatment.
Nutrient limitation, particularly nitrogen, increases neutral lipid production and accumulation in microalgae. However, this limitation yields severe losses in biomass productivity. To reduce the negative effect of subjecting microalgae cultures to nutrient-limiting conditions, biomass production must first be maximized, and later, a neutral lipid accumulation-stimulating factor must be introduced (Bertozzini et al. 2014).
To maximize algal biomass production using Chlorella vulgaris and Scenedesmus sp., thereby increasing lipid accumulation , different concentrations of calcium, magnesium, and sodium chloride were examined. The results showed that a short-term reduction in the magnesium concentration induced lipid production (Chen et al. 2014).
Among the micronutrients, iron, potassium, and inorganic salts are the most important metabolic activators and are used in algal culture media formulations (Zeng et al. 2011; Cai et al. 2013).
Yang et al. (2014) applied a response surface method to define the optimal conditions for promoting greater lipid accumulation in Scenedesmus sp. The results showed that adding NaHCO3, i.e., a source of inorganic carbon, as well as NaH2PO4, 2H2O and NaNO3 significantly enhanced lipid accumulation in microalgae without influencing the fatty acid composition of Scenedesmus sp.
To synthesize cyanocobalamin (vitamin B12), algae require cobalt (Co), which should be used at low concentrations. According to Li et al. (2007), such care is necessary due to both the potential for toxicity to the algae and the researcher in addition to introducing this toxic element into the food chain.
Another important factor that merits study is supplementing the culture medium with glycerol, which is a by-product of biodiesel production that increases lipid production in microalgae. In studies conducted by Cerón-Garcıa et al. (2005), glycerol, fructose, glucose, mannose, and lactose were used as carbon sources to stimulate growth and fatty acid accumulation in Phaeodactylum tricornutum, which enabled effective gains in biomass yield and intracellular oil accumulation.
High glycerol concentration s in microalgal cultures subjected to mixotrophic growth conditions tend to change the thylakoids in chloroplasts that favor glycerol assimilation, which can be considered an adaptive response to stress imposed by mixotrophic conditions (Cerón-García et al. 2013).
Sun et al. (2014) studied the effects of culture media with different glycerol concentrations (1, 5, 10, 15, and 30 g L−1) and glucose (10 g L−1) as supplementary carbon sources on growth and lipid accumulation in Chlorella vulgaris. The presence of glycerol in the culture medium enhanced lipid production and accumulation, whereas the presence of glucose favored cell growth. The positive effect of glycerol supplementation on lipid accumulation in microalgae is limited over a given concentration (10 g L−1), after which inhibitory effects on biomass growth and lipid production were observed.
Biofuel production using current algal cultivation technology is not economical (Pittman et al. 2011). A more attractive option for reducing greenhouse gas emissions and the demand for freshwater resources and fertilizers is algal growth using wastewater. The high productivity of microalgal biomass grown in sewage and wastewater suggests that this culture method is a viable means for generating biofuels and will likely be one of the main technologies used for producing sustainable and renewable energy in the future.
Wastewaters contain organic carbon, nitrogen, phosphorus, and other compounds (Liang 2013; Sahu et al. 2013), which are used for microalgal growth and form a readily available substrate for biomass production that is easily assimilated and is inexpensive (Rawat et al. 2011; Ummalyma and Sukumaran 2014; Zhu et al. 2014).
Culturing microalgal using wastewater is influenced by multiple factors, and growth efficiency depends on controlling the variables considered critical, such as pH, temperature, light availability, CO2, O2, and especially nutrient concentrations, which can impede algal biomass development. Municipal wastewater typically contains approximately 350 mg L−1 chemical oxygen demand (COD), 50 mg L−1 of N—NH4+, and 10 mg L−1 of P—PO4 −3 as well as a considerable organic load and high nutrient concentrations (nitrogen and phosphorus) (Boelee et al. 2014).
Treated wastewater effluents also contain trace elements (K, Ca, Mg, Fe, Cu, and Mn) that are essential for microalgal metabolism and growth. Therefore, secondary and tertiary effluents may be broadly applied to microalgal culture media (Schneider et al. 2012; Aravantinou et al. 2013). However, the levels of cadmium, mercury, toxic organic compounds, and biotic contaminants, such as pathogenic bacteria and predators (zooplankton), in the culture medium should be considered because they can inhibit microalgal growth (Pittman et al. 2011; Van Den Hende et al. 2014).
Luminosity is a critical factor for microalgal growth when using urban wastewater as a culture medium due to the levels of suspended solids and turbidity in this type of effluent (Zhou et al. 2014). Microalgal cultures may overcome light limitations by consuming high levels of total organic carbon (TOC) as acetic acid, propionic acid, butyric acid, or ethanol from municipal wastewater for rapid growth under photoheterotrophic or mixotrophic conditions (Ji et al. 2014).
Likely one of the main requirements for large-scale biofuel production from microalgae is using microalgal strains adapted to local environmental conditions. Thus, there is a need for effective and rapid isolation of microalgal strains with a high potential for growth and biomass production as well as sufficiently high lipid and/or polysaccharide content for economic exploitation (Abdelaziz et al. 2014).
Microorganism communities overcome the limitations inherent to isolation through symbiosis. These interactions involve subtle signals and horizontal gene transfer, and they create competitive or cooperative scenarios where microorganisms can compete or provide resources. Cultures with multiple microbial species contain a broader range of genes and better metabolic capacity compared with monocultures (Hays et al. 2015).
The symbiotic relationship between microalgae and bacteria has been well characterized with regard to the oxygen supplied by microalgae for the aerobic bacteria to biodegrade organic pollutants and, in turn, consume the CO2 released during bacterial respiration. However, the interactions between these two microbial groups are not limited to CO2/O2 exchange. When properly characterized, the microalgae-bacteria consortium benefits both species; however, this association should be carefully evaluated because it can also produce antagonistic relationships (Oswald 2003; Schumacher et al. 2003).
Microbial consortia generally perform more complex tasks and can perform functions that are difficult or even impossible for individual species or strains (Brenner et al. 2008). Bacteria can increase the microalgal cell growth capacity by releasing certain growth factors, and microalgae assist bacteria by releasing biosurfactants and extracellular compounds in the medium, which improves bacteria-pollutant degradation activity (Tang et al. 2010).
Increased cell growth in the microalgae Chlorella minutissima was observed when it was cocultured with Escherichia coli under mixotrophic conditions, in which both light and organic compounds are used for energy and carbon production. This association demonstrates the potential for increasing microalgal biomass production with 1 % substrate consumption compared with isolated Chlorella minutissima cultures (Higgins et al. 2015).
Microalgae and bacteria co-selection in a consortium system is important when the objective is to increase biomass production, and the effects of these interactions may affect the system stability; thus, the system should be thoroughly investigated (Muñoz and Guieysse 2006).
Microalgal biomass can be converted into solid, liquid, or gaseous biofuels and may supply 30 % of the global fuel demand without affecting food production (López Barreiro et al. 2013) via various processes, including thermochemical (liquefaction, pyrolysis, and gasification) (Amin 2009) and biochemical processes (fermentation, transesterification, and anaerobic digestion) (Demirbas 2011).
When processed via chemical or biological reactions, in addition to biodiesel and bioethanol, microalgal biomass can provide different types of renewable biofuels, such as bio-hydrogen, bio-methane, and butanol, as well as light hydrocarbons, such as ethane (C2H6) and ethylene (C2H4), for direct use as energy sources or to generate electricity (Mussgnug et al. 2010; Efremenko et al. 2012; Nasir Uddin et al. 2013; Lakaniemi et al. 2013; Nayak et al. 2014). For microalgae-based fuels, the main focus is biodiesel production, whereas bioethanol and biomethane are considered a part of integrated processes (Oncel 2013) that generate residual biomass during oil extraction, which can be aerobically fermented into ethanol and anaerobically fermented into biomethane (Chinnasamy et al. 2010). The relevant factors for microalgal production and their potential applications in biofuel production are illustrated in Fig. 1.
3 Biodiesel Production
3.1 Oil Extraction
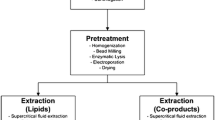
Several methods can be used to extract lipids from microalgae, including supercritical extraction, ultrasonic extraction, microwave extraction, high-pressure homogenizer extraction, hydrothermal liquefaction, and solvent extraction (Bligh and Dyer, 1959; Friedrich and Pryde 1984; Chao et al. 1993; Converti et al. 2009; Bucy et al. 2012; Iqbal and Theegala 2013; Toor et al. 2013; Reddy et al. 2014). However, high quantities of solvent are necessary, which may lead to environmental pollution and increase costs as well as energy consumption during the process (Xu et al. 2006).
Since the introduction of the Folch method, researchers have investigated various techniques to effectively extract lipids using different ratios of chloroform and methanol. These techniques include the Bligh-Dyer method, which is considered a conventional lipid extraction method that successfully uses a mixture of chloroform and methanol and is currently adjusted to the conditions of several laboratories (Bligh and Dyer 1959).
Halim et al. (2012) compared lipid extraction in different solvents. Although chloroform can dissolve the lipid content of cells, including acilglicerois e ácidos graxos livres as well as polar lipids, fosfolipídios e glicolipídios, researchers are reluctant to use chloroform in lipid extraction due to the toxicity of chlorinated solvents. Hexane is often suggested as a hydrophobic alternative because it selectively extracts neutral lipids and is less toxic. Another option is mixing hexane/isopropanol (3:2 v/v), which features good lipid extraction yield.
Ethanol is an inexpensive solvent and has a strong affinity for the lipid complex; thus, lipids can be efficiently extracted. Moreover, ethanol is a safe and eco-friendly solvent, which suggests that the residual biomass can be used in the food industry (Reddy et al. 2014). Several authors (Ibáñez González et al. 1998; Fajardo et al. 2007) have efficiently used ethanol to extract lipids from microalgae, such as Phaeodactylum tricornutum and P. tricornutum UTEX 640. However, these studies use dehydration or high temperatures for extraction.
Dimethyl carbonate (DMC)/methanol can also be used as an extraction solvent and generates a 38.9 % yield. In fact, the DMC can be subsequently used for transesterification (Lee et al. 2013). According to Su et al. (2009), short-chain dialkyl carbonates can be acyl group acceptors with carbon dioxide formation and favor methyl ester formation.
Because the microalgal biomass generated features a high water content, high levels of energy are consumed before extraction for drying and efficient extraction of the lipid content. Jiménez Callejón et al. (2014) studied extraction with wet biomass, and the results indicated that 69.1 % of the unsaponifiable lipids were extracted. The authors used the high-pressure homogenization technique and added hexane when using wet biomass for lipid extraction.
Therefore, lipids are typically extracted with solvents and dry biomass; other processes for using wet biomass will be discussed in the section on in situ transesterification.
3.2 Transesterification
Several processes can be used for microalgal lipid content transesterification, which are combined through transesterification of extracted oil or biomass directly using in situ methods. During optimization, researchers consider the necessary physical treatments associated with whether or not to use catalysts or biocatalysts. Certain biodiesel production initiatives are presented in Table 2 and are the most investigated methods for assessing potential biodiesel production from microalgae.
Catalysts should also be considered an important aspect for converting microalgal lipids into biodiesel. Two catalyst groups are considered, chemical and biocatalysts (enzyme catalysts), with their specific modes of action.
3.3 Chemical Catalysts
Methods that involve chemical catalysts during transesterification are the most attractive and widely used due to high yield and increased reaction speed. Because this reaction is reversible, excess alcohol is typically used to shift the equilibrium toward product formation (Arias-Peñaranda et al. 2013; Kiran et al. 2014).
Homogeneous alkaline catalysis has been the most widely used pathway for biodiesel production because the reaction is catalyzed at a low temperature and atmospheric pressure, and it yields high levels of conversion in less time. The most frequently used alkaline catalysts are homogeneous catalysts, such as potassium hydroxide, sodium hydroxide, sodium methoxide, and potassium methoxide (Puna et al. 2010; Macario and Giordano 2013).
Often, an alkaline catalyst is not effective for converting microalgal lipids through transesterification due to the high free fatty acid content. Soaps may form under this condition, and they will consume the catalyst and impede separation of the esters and glycerol formed (Gog et al. 2012).
More reports describe biodiesel production from microalgae via acid catalysis than alkali metals because acid catalysts esterify free fatty acids and transesterify triglycerides (Suwannakarn et al. 2009; Leung et al. 2010; Nigam and Singh 2011; Lee et al. 2014). Acid catalysts are typically employed for lipids with free fatty acid content greater than 2 % (Velasquez-Orta et al. 2012).
The most commonly used acids for homogeneous catalysts in biodiesel production are H2SO4 and sulfonic acid; HCl, BF3, and H3PO4 are also used (Bharathiraja et al. 2014). The use of this type of catalyst requires a greater response time and higher temperatures than alkaline catalysts (Hidalgo et al. 2013; Vonortas and Papayannakos 2014).
To overcome the problems with using homogeneous catalysts, certain authors report using both in the same transesterification process, which consists of two reaction steps. An acid catalyst may initially be used to convert free fatty acids to methyl esters by esterification such that the free fatty acid content in the oils is reduced to less than 1 %; thereafter, an alkaline catalyst is used (Park et al. 2015).
Reactions using homogeneous catalysts can generate many environmental and corrosion problems. Therefore, transesterification reactions with heterogeneous catalysts have emerged as an alternative for biodiesel production using different raw materials. Thus, researchers expect that heterogeneous catalysts will be easier to recover and reuse. Umdu et al. (2009) reported transesterification of oils from Nannochloropsis oculata using CaO and MgO supported on alumina with a 97.5 % yield. Using metal oxides composed of ZrO, TiO, and Al2O3, lipids from the microalgae Dunaliella tertiolecta and Nannochloropsis oculata were converted (85 %) into biodiesel with free fatty acid esterification and triglyceride transesterification simultaneously under supercritical conditions (Krohn et al. 2011).
3.4 Biocatalysts
Lipases (EC 3.1.1.3) are tools that catalyze various synthetic reactions, such as hydrolysis, esterification, transesterification, and aminolysis. Lipases are used as biocatalysts for transforming oil/lipids into biodiesel due to low operating costs and, particularly, high product purity under mild conditions (20–50 °C) (Gog et al. 2012; Tran et al. 2012; Christopher et al. 2014; Huang et al. 2015).
Furthermore, comparing the enzyme and chemical catalyst technologies, researchers have observed that enzymes catalyze free fatty acid (FFA) and triglyceride (TG) esterification in a reaction step without the need for a wash step and prevent by-product formation, which ensures an easily recovered product (Kulkarni and Dalai 2006; Robles-Medina et al. 2009).
Certain lipases synthesized by Mucor miehei, Rhizopus oryzae, Pseudomonas cepacia, Candida antarctica (Taher et al. 2011), Candida rugosa, Candida cylindracea, Pseudomonas fluorescens, Rhizomucor miehei (Teo et al. 2014), and Burkholderia sp. (Tran et al. 2012) are used as enzyme biocatalysts for biofuel production.
The lipases may exhibit regiospecificity, specificity for fatty acids or the nature of the alcohol, and stereospecificity, which includes sn-1,3 specific (hydrolyzable ester linkage at position sR1 or R3 of the triglyceride), sn-2 specific (hydrolyzable ester linkage in the R2 position of the TAG), and nonspecific (no distinction between the ester positions) (Kapoor and Gupta 2012).
In general, enzymatic transesterification for oils features certain disadvantages, including the reaction time and the potential enzyme inactivation by methanol (Persson et al. 2002; Adamczak and Bednarski 2004; Jung et al. 2006). These disadvantages limit the industrial applications of biodiesel production processes due to the high cost of enzymes associated with this process (Bajaj et al. 2010). Using the whole cell or immobilized enzyme yields higher conversion and less activity loss but allows for enzyme reuse (Teo et al. 2014; Huang et al. 2015).
The lipids are more viscous compared with the organic solvents, and dissolving lipids in this class of solvents may improve reactant diffusion in the liquid phase and hence transesterification performance (Lam and Lee 2013).
Studies on biocatalysis for transesterification are only performed in media with the alcohol used for the conversion or with cosolvents, especially hexane, which solubilizes oil and biodiesel well and is less aggressive toward enzymes (Tran et al. 2013a). Researchers have also examined using ionic liquids for biodiesel production using oil from three different microalgae (Botryococcus braunii, Chlorella vulgaris, and Chlorella pyrenoidosa). Researchers have observed high levels of methyl ester conversion using Penicillium expansum lipase (PEL) and Candida antarctica lipase B (Novozyme 435) (Lai et al. 2012).
According to Amoah et al. (2016), researchers must also consider the phospholipids in microalgae at concentrations reaching 30 % of the total lipid content, which negatively affect enzyme catalysis because they can form water-in-oil phospholipid-based reverse micelles. The water required for enzyme activity is trapped by the micelle.
To produce biodiesel from microalgae, Huang et al. (2015) used a recombinant Rhizomucor miehei lipase to convert oil from Chlorella vulgaris with more than 90 % conversion to methyl esters and ethyl esters.
Notably, enzymatic transesterification can be advantageous for in situ biodiesel production from alternative raw materials, such as microalgae.
3.5 In Situ Transesterification
In situ transesterification is an efficient means to directly convert lipids from biomass to biodiesel. Because it is a simple process, various studies have used in situ transesterification with different raw materials, including oilseed plants (Hincapié et al. 2011; Martínez Arias et al. 2012) and microalgae (Hidalgo et al. 2013). This method is an alternative to the conventional transesterification process that may reduce biodiesel production costs where the lipid/oil extraction step features a high environmental or economic impact.
In this process, fatty acids are converted into alkyl esters directly inside the biomass, which eliminates the extraction step and postharvest biomass drying (Amaro et al. 2011). Microalgae lipid content can be transformed to biodiesel by enzymatic transesterification in situ, which uses methanol as raw material for the process and as solvent to assist microalgae cell wall rupture (Steriti et al. 2014), as shown in Fig. 2.
Optical microscopy images of Desmodesmus sp. cells at different times of transesterification in situ: (a) intact cells at the beginning of the process (400x); (b) cell wall rupture after 48 h of transesterification (1000x); (c) the lipid content extracted from cells after transesterification in situ and evaporation of the solvent (400x)
This method may be particularly advantageous for microalgae because lipids are typically extracted using solvents, not through physical methods for oil extraction from conventional crops, as previously presented. Alcoholysis of the TG in the biomass produces better biodiesel yields compared with the conventional transesterification method with the additional advantage of reducing waste generation, power consumption, and consequently the environmental impact from these methods (Ehimen et al. 2010).
The reactions are simple and include adding alcohols, catalysts, biomass, and, occasionally, cosolvents (Rodrigues Da Silva Baumgartner et al. 2013; Atadashi et al. 2013).
For microalgae , this method has been applied using Nannochloropsis salina, Chlorella sp., Scenedesmus sp., Nannochloropsis gaditana, Chlorella pyrenoidosa, and Schizochytrium limacinum, among others (Carvalho et al. 2011; Cao et al. 2013; Jin et al. 2014; Sathish et al. 2014; Kim et al. 2015a, b; Ma et al. 2015). Tran et al. (2013b) used immobilized Burkholderia lipase for direct transesterification (in situ) to convert lipids into methyl esters after ultrasonically pretreating the biomass. The results showed that the ideal hexane/methanol ratio was 1.65, and different proportions of methanol relative to the lipid content yielded more than 90 % conversion to esters in the 48-h reaction period.
Biodiesel can also be produced from microalgae through in situ non-catalytic supercritical methanol transesterification, reaching 45.6 % biodiesel using the microalgae Chlorella sp. (native) (Jazzar et al. 2015) or Schizochytrium limacinum with supercritical CO2 and adding methanol, which generates a 37.5 % yield (Bi et al. 2015). Notably, supercritical fluids are promising for in situ transesterification and should be further explored in the near future (Zeng et al. 2014).
3.6 Prospects for Industrial Production
The viability of biodiesel production using microalgae at the industrial level faces several challenges involving reduction of energy loss. The process includes a pretreatment step for free fatty acid esterification with methanol and acid catalysis followed by alkaline-catalyzed transesterification, and the cost of purifying biodiesel and glycerol through distillation was recently calculated as $0.592/L of biodiesel. This value does not consider lipid extraction from the biomass, which also impacts the production cost and may be investigated to save energy (Song et al. 2015).
In the first phase of biodiesel production from microalgae, volatile solvents are used to separate the lipids and for subsequent transesterification, which are ineffective for microalgae with high water content according to Cooney et al. (2009). Thus, the microalgae must be dried. Next, the organic solvents must be removed through reducing the pressure. The energy cost associated with the drying, distillation, and solvent recovery processes must also be considered. Modifications to the process that decrease energy consumption and optimize the use of solvents and other inputs may be the factors responsible that render biodiesel production from microalgae feasible. Peralta-Ruiz et al. (2013) performed an energy analysis for the microalgal lipid extraction pathways, and hexane was the most suitable substance for large-scale production with a 51 % maximum energy efficiency.
Lipid extraction technologies that generate a high yield are developed on a laboratory scale and are not easily converted to larger scales, such as the industrial scale. Successful industrial production is directly linked to using innovative technologies, such as in situ transesterification.
Countries such as Japan, Israel, India, and the United States have successfully ventured into this area, but the potential for using microalgae on an industrial level may be much greater when it is associated with, for example, wastewater phytoremediation. The potential for biofuel production from microalgae has driven studies and the transfer of technology to industry (http://www.algaeindustrymagazine.com).
The environmental and economic impacts of biomass production, lipid extraction, and biodiesel production have been studied in recent years (Monari et al. 2016; Delrue et al. 2012; Dassey and Theegala 2013; Collet et al. 2014; Mata et al. 2014; Sawaengsak et al. 2014; Yu et al. 2015). However, different production systems must be studied with a focus on environmental issues and the real potential of microalgae in biodiesel production to determine the actual environmental and economic gains from industrial biodiesel production using microalgae, as shown by Yu et al. (2015) in Singapore.
4 Conclusion
Biodiesel production from microalgae depends on the species, biomass production conditions, and technologies used for lipid separation and transesterification, and it is becoming feasible or is already a reality in certain countries. Several initiatives in the field have sought to use techniques that consume less energy and produce a better final biodiesel yield. Among these technologies, recent microalgal culture studies have sought greater lipid accumulation in the cells through adding different sources of carbon, metals, and vitamins and have sought to avoid using extraction solvents, such as through in situ transesterification. Technological advances from research have facilitated efficient biodiesel production from microalgae, but large-scale feasibility has not been analyzed to maximize production potential. The environmental gains from these processes should also be highlighted to render biodiesel production from microalgal biomass even more relevant in the medium and long term.
References
Abdelaziz AEM, Leite GB, Belhaj MA, Hallenbeck PC (2014) Screening microalgae native to Quebec for wastewater treatment and biodiesel production. Bioresour Technol 157:140–148. doi:10.1016/j.biortech.2014.01.114
Adamczak M, Bednarski W (2004) Enhanced activity of intracellular lipases from Rhizomucor miehei and Yarrowia lipolytica by immobilization on biomass support particles. Process Biochem 39:1347–1361. doi:10.1016/S0032-9592(03)00266-8
Ahmad AL, Yasin NHM, Derek CJC, Lim JK (2014) Comparison of harvesting methods for microalgae Chlorella sp. and its potential use as a biodiesel feedstock. Environ Technol 35:2244–2253. doi:10.1080/09593330.2014.900117
Amaro HM, Guedes AC, Malcata FX (2011) Advances and perspectives in using microalgae to produce biodiesel. Appl Energy 88:3402–3410. doi:10.1016/j.apenergy.2010.12.014
Amin S (2009) Review on biofuel oil and gas production processes from microalgae. Energy Covers Manage 50:1834–1840. doi:10.1016/j.enconman.2009.03.001
Amoah J, Ho SH, Hama S, Yoshida A, Nakanishi A, Hasunuma T, Ogino C, Kondo A (2016) Converting oils high in phospholipids to biodiesel using immobilized Aspergillus oryzae whole-cell biocatalysts expressing Fusarium heterosporum lipase. Biochem Eng J 105:10–15. doi:10.1016/j.bej.2015.08.007
Aravantinou AF, Theodorakopoulos MA, Manariotis ID (2013) Selection of microalgae for wastewater treatment and potential lipids production. Bioresour Technol 147:130–134. doi:10.1016/j.biortech.2013.08.024
Arias-Peñaranda MT, Cristiani-Urbina E, Montes-Horcasitas C, Esparza-Garcı AF, Torzillo G, Cañizares-Villanueva RO (2013) Scenedesmus incrassatulus CLHE-Si01: a potential source of renewable lipid for high quality biodiesel production. Bioresour Technol 140:158–164. doi:10.1016/j.biortech.2013.04.080
Ashokkumar V, Rengasamy R, Deepalakshmi S, Sivalingam A, Sivakumar P (2014) Mass cultivation of microalgae and extraction of total hydrocarbons: a kinetic and thermodynamic study. Fuel 119:308–312. doi:10.1016/j.fuel.2013.11.062
Atadashi IM, Aroua MK, Abdul Aziz AR, Sulaiman NMN (2013) The effects of catalysts in biodiesel production: a review. J Ind Eng Chem 19:14–26. doi:10.1016/j.jiec.2012.07.009
Bajaj A, Lohan P, Jha PN, Mehrotra R (2010) Biodiesel production through lipase catalyzed trans-esterification: an overview. J Mol Catal B Enzym 62:9–14. doi:10.1016/j.molcatb.2009.09.018
Bertozzini E, Galluzzi L, Penna A, Magnani M (2014) Enhancing neutral lipid content in Skeletonema marinoi through multiple phase growth in a bench photobioreactor. Algal Res 5:32–36. doi:10.1016/j.algal.2014.05.004
Bharathiraja B, Chakravarthy M, Kumar RR, Yuvaraj D, Jayamuthunagai J, Kumar RP, Palani S (2014) Biodiesel production using chemical and biological methods—a review of process, catalyst, acyl acceptor, source and process variables. Renew Sustain Energy Rev 38:368–382. doi:10.1016/j.rser.2014.05.084
Bi Z, He BB, Mcdonald AG (2015) Biodiesel production from green microalgae schizochytrium limacinum via in situ trans-esterification. Energy Fuels 29:5018–5027. doi:10.1021/acs.energyfuels.5b00559
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917. doi:10.1139/o59-099
Boelee NC, Temmink H, Janssen M, Buisman CJN, Wijffels RH (2014) Balancing the organic load and light supply in symbiotic microalgal-bacterial biofilm reactors treating synthetic municipal wastewater. Ecol Eng 64:213–221. doi:10.1016/j.ecoleng.2013.12.035
Brenner K, You L, Arnold FH (2008) Engineering microbial consortia: a new frontier in synthetic biology. Trends Biotechnol 26:483–489. doi:10.1016/j.tibtech.2008.05.004
Bucy HB, Baumgardner ME, Marchese AJ (2012) Chemical and physical properties of algal methyl ester biodiesel containing varying levels of methyl eicosapentaenoate and methyl docosahexaenoate. Algal Res 1:57–69. doi:10.1016/j.algal.2012.02.001
Cai T, Park SY, Li Y (2013) Nutrient recovery from wastewater streams by microalgae: status and prospects. Renew Sustain Energy Rev 19:360–369. doi:10.1016/j.rser.2012.11.030
Cao H, Zhang Z, Wu X, Miao X (2013) Direct biodiesel production from wet microalgae biomass of chlorella pyrenoidosa through in situ trans-esterification. BioMed Res Int 2013:1–6. doi:10.1155/2013/930686
Carvalho RM Jr, Vargas JVC, Ramos LP, Marino CEB, Torres JCL (2011) Microalgae biodiesel via in situ methanolysis. J Chem Technol Biotechnol 86:1418–1427. doi:10.1002/jctb.2652
Cerón Garcı AMC, Sánchez Mirón A, Fernández Sevilla JM, Molina Grima E, Garcıa Camacho F (2005) Mixotrophic growth of the microalga Phaeodactylum tricornutum: influence of different nitrogen and organic carbon sources on productivity and biomass composition. Process Biochem 40:297–305. doi:10.1016/j.procbio.2004.01.016
Cerón-García MC, Fernández-Sevilla JM, Sánchez-Mirón A, García-Camacho F, Contreras-Gómez A, Molina-Grima E (2013) Mixotrophic growth of Phaeodactylum tricornutum on fructose and glycerol in fed-batch and semi-continuous modes. Bioresour Technol 147:569–576. doi:10.1016/j.biortech.2013.08.092
Chandra R, Rohit MV, Swamy YV, Venkata Mohan S (2014) Regulatory function of organic carbon supplementation on biodiesel production during growth and nutrient stress phases of mixotrophic microalgae cultivation. Bioresour Technol 165:279–287. doi:10.1016/j.biortech.2014.02.102
Chao R, Mulvaney S, Huang H (1993) Effects of extraction and fractionation pressures on supercritical extraction of cholesterol from beef tallow. J Am Oil Chem Soc 70:139–143. doi:10.1007/BF02542616
Chen X, Liu T, Wang Q (2014) The growth of Scenedesmus sp. attachment on different materials surface. Microb Cell Fact 13:142. doi:10.1186/s12934-014-0142-z
Chen CL, Huang CC, Ho KC, Hsiao PX, Wu MS, Chang JS (2015) Biodiesel production from wet microalgae feedstock using sequential wet extraction/trans-esterification and direct trans-esterification processes. Bioresour Technol 194:179–186. doi:10.1016/j.biortech.2015.07.021
Cheng J, Yu T, Li T, Zhou J, Cen K (2013) Using wet microalgae for direct biodiesel production via microwave irradiation. Bioresour Technol 131:531–535. doi:10.1016/j.biortech.2013.01.045
Chinnasamy S, Bhatnagar A, Claxton R, Das KC (2010) Biomass and bioenergy production potential of microalgae consortium in open and closed bioreactors using untreated carpet industry effluent as growth medium. Bioresour Technol 101:6751–6760. doi:10.1016/j.biortech.2010.03.094
Christopher LP, Hemanathan K, Zambare VP (2014) Enzymatic biodiesel: challenges and opportunities. Appl Energy 119:497–520. doi:10.1016/j.apenergy.2014.01.017
Collet P, Lardon L, Hélias A, Bricout S, Lombaert-Valot I, Perrier B, Lépine O, Steyer J-P, Bernard O (2014) Biodiesel from microalgae—life cycle assessment and recommendations for potential improvements. Renew Energy 71:525–533. doi:10.1016/j.renene.2014.06.009
Converti A, Casazza AA, Ortiz EY, Perego P, Del Borghi M (2009) Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem Eng Proces Process Intens 48:1146–1151. doi:10.1016/j.cep.2009.03.006
Cooney M, Young G, Nagle N (2009) Extraction of bio-oils from microalgae. Sep Purif Rev 38:291–325. doi:10.1080/15422110903327919
Dassey AJ, Theegala CS (2013) Harvesting economics and strategies using centrifugation for cost effective separation of microalgae cells for biodiesel applications. Bioresour Technol 128:241–245. doi:10.1016/j.biortech.2012.10.061
Dębowski M, Zieliński M, Grala A, Dudek M (2013) Algae biomass as an alternative substrate in biogas production technologies—review. Renew Sustain Energy Rev 27:596–604. doi:10.1016/j.rser.2013.07.029
Delrue F, Setier PA, Sahut C, Cournac L, Roubaud A, Peltier G, Froment AK (2012) An economic, sustainability, and energetic model of biodiesel production from microalgae. Bioresour Technol 111:191–200. doi:10.1016/j.biortech.2012.02.020
Demirbas A (2010) Use of algae as biofuel sources. Energy Covers Manage 51:2738–2749. doi:10.1016/j.enconman.2010.06.010
Demirbas MF (2011) Biofuels from algae for sustainable development. Appl Energy 88:3473–3480. doi:10.1016/j.apenergy.2011.01.059
Dragone G, Fernandes BD, Vicente AA, Teixeira JA (2010) Third generation biofuels from microalgae. In: Méndez-Vilas A (ed) Current research, technology and education topics in applied microbiology and microbial biotechnology, pp 1355–1366. Formatex Spain. ISBN-13:978–84–614-6195-0
Efremenko EN, Nikolskaya AB, Lyagin IV, Senko OV, Makhlis TA, Stepanov NA, Maslova OV, Mamedova F, Varfolomeev SD (2012) Production of biofuels from pretreated microalgae biomass by anaerobic fermentation with immobilized Clostridium acetobutylicum cells. Bioresour Technol 114:342–348. doi:10.1016/j.biortech.2012.03.049
Ehimen EA, Sun ZF, Carrington CG (2010) Variables affecting the in situ trans-esterification of microalgae lipids. Fuel 89:677–684. doi:10.1016/j.fuel.2009.10.011
Elshahed MS (2010) Microbiological aspects of biofuel production: current status and future directions. J Adv Res 1:103–111. doi:10.1016/j.jare.2010.03.001
Fajardo AR, Cerdán LE, Medina AR, Fernández FGA, Moreno PG, Grima EM (2007) Lipid extraction from the microalga Phaeodactylum tricornutum. Eur J Lipid Sci Tech 109:120–126. doi:10.1002/ejlt.200600216
Friedrich JP, Pryde EH (1984) Supercritical CO2 extraction of lipid-bearing materials and characterization of the products. J Am Oil Chem Soc 61:223–228. doi:10.1007/BF02678773
Gog A, Roman M, Toşa M, Paizs C, Irimie FD (2012) Biodiesel production using enzymatic trans-esterification—current state and perspectives. Renew Energy 39:10–16. doi:10.1016/j.renene.2011.08.007
Guldhe A, Singh P, Kumari S, Rawat I, Permaul K, Bux F (2016) Biodiesel synthesis from microalgae using immobilized Aspergillus niger whole cell lipase biocatalyst. Renew Energy 85:1002–1010. doi:10.1016/j.renene.2015.07.059
Gupta A, Singh D, Barrow CJ, Puri M (2013) Exploring potential use of Australian thraustochytrids for the bioconversion of glycerol to omega-3 and carotenoids production. BiochemEng J 78:11–17. doi:10.1016/j.bej.2013.04.028
Halim R, Danquah MK, Webley PA (2012) Extraction of oil from microalgae for biodiesel production: a review. Biotechnol Adv 30:709–732. doi:10.1016/j.biotechadv.2012.01.001
Hays SG, Patrick WG, Ziesack M, Oxman N, Silver PA (2015) Better together: engineering and application of microbial symbioses. Curr Opin Biotechnol 36:40–49. doi:10.1016/j.copbio.2015.08.008
Heredia-Arroyo T, Wei W, Ruan R, Hu B (2011) Mixotrophic cultivation of Chlorella vulgaris and its potential application for the oil accumulation from non-sugar materials. Biomass Bioenergy 35:2245–2253. doi:10.1016/j.biombioe.2011.02.036
Hidalgo P, Toro C, Ciudad G, Navia R (2013) Advances in direct trans-esterification of microalgal biomass for biodiesel production. Rev Environ Sci Bio 12:179–199. doi:10.1007/s11157-013-9308-0
Higgins BT, Labavitch JM, Vandergheynst JS (2015) Co-culturing Chlorella minutissima with Escherichia coli can increase neutral lipid production and improve biodiesel quality. Biotechnol Bioeng 112:1801–1809. doi:10.1002/bit.25609
Hincapié G, Mondragón F, López D (2011) Conventional and in situ trans-esterification of castor seed oil for biodiesel production. Fuel 90:1618–1623. doi:10.1016/j.fuel.2011.01.027
Hockin NL, Mock T, Mulholland F, Kopriva S, Malin G (2012) The response of diatom central carbon metabolism to nitrogen starvation is different from that of green algae and higher plants. Plant Physiol 158:299–312. doi:10.1104/pp.111.184333
Hong IK, Jeon GS, Lee SB (2014) Prediction of biodiesel fuel properties from fatty acid alkyl ester. J Ind Eng Chem 20:2348–2353. doi:10.1016/j.jiec.2013.10.011
Huang YT, Su CP (2014) High lipid content and productivity of microalgae cultivating under elevated carbon dioxide. Int J Environl Sci Tec 11:703–710. doi:10.1007/s13762-013-0251-y
Huang J, Xia J, Jiang W, Li Y, Li J (2015) Biodiesel production from microalgae oil catalyzed by a recombinant lipase. Bioresour Technol 180:47–53. doi:10.1016/j.biortech.2014.12.072
Ibáñez González MJ, Robles Medina A, Grima EM, Giménez AG, Carstens M, Cerdán LE (1998) Optimization of fatty acid extraction from Phaeodactylum tricornutum UTEX 640 biomass. J Am Oil Chem Soc 75:1735–1740. doi:10.1007/s11746-998-0325-z
Im H, Lee H, Park MS, Yang J-W, Lee JW (2014) Concurrent extraction and reaction for the production of biodiesel from wet microalgae. Bioresour Technol 152:534–537. doi:10.1016/j.biortech.2013.11.023
Iqbal J, Theegala C (2013) Microwave assisted lipid extraction from microalgae using biodiesel as co-solvent. Algal Res 2:34–42. doi:10.1016/j.algal.2012.10.001
Jazzar S, Quesada-Medina J, Olivares-Carrillo P, Marzouki MN, Acién-Fernández FG, Fernández-Sevilla JM, Molina-Grima E, Smaali I (2015) A whole biodiesel conversion process combining isolation, cultivation and in situ supercritical methanol trans-esterification of native microalgae. Bioresour Technol 190:281–288. doi:10.1016/j.biortech.2015.04.097
Ji M-K, Kabra AN, Salama E-S, Roh H-S, Kim JR, Lee DS, Jeon B-H (2014) Effect of mine wastewater on nutrient removal and lipid production by a green microalga Micratinium reisseri from concentrated municipal wastewater. Bioresour Technol 157:84–90. doi:10.1016/j.biortech.2014.01.087
Jiménez Callejón MJ, Robles Medina A, Macías Sánchez MD, Hita Peña E, Esteban Cerdán L, González Moreno PA, Molina Grima E (2014) Extraction of saponifiable lipids from wet microalgal biomass for biodiesel production. Bioresour Technol 169:198–205. doi:10.1016/j.biortech.2014.06.106
Jin B, Duan P, Xu Y, Wang B, Wang F, Zhang L (2014) Lewis acid-catalyzed in situ trans-esterification/esterification of microalgae in supercritical ethanol. Bioresour Technol 162:341–349. doi:10.1016/j.biortech.2014.03.157
Jung H-C, Kwon S-J, Pan J-G (2006) Display of a thermostable lipase on the surface of a solvent-resistant bacterium, Pseudomonas putida GM730, and its applications in whole-cell biocatalysis. BMC Biotechnol 6:23–23. doi:10.1186/1472-6750-6-23
Kaiwan-Arporn P, Hai PD, Thu NT, Annachhatre AP (2012) Cultivation of cyanobacteria for extraction of lipids. Biomass Bioenergy 44:142–149. doi:10.1016/j.biombioe.2012.04.017
Kapoor M, Gupta MN (2012) Lipase promiscuity and its biochemical applications. Process Biochem 47:555–569. doi:10.1016/j.procbio.2012.01.011
Kim B, Im H, Lee JW (2015a) In situ trans-esterification of highly wet microalgae using hydrochloric acid. Bioresour Technol 185:421–425. doi:10.1016/j.biortech.2015.02.092
Kim TH, Suh WI, Yoo G, Mishra SK, Farooq W, Moon M, Shrivastav A, Park MS, Yang JW (2015b) Development of direct conversion method for microalgal biodiesel production using wet biomass of Nannochloropsis salina. Bioresour Technol 191:438–444. doi:10.1016/j.biortech.2015.03.033
Kiran B, Kumar R, Deshmukh D (2014) Perspectives of microalgal biofuels as a renewable source of energy. Energy Covers Manage 88:1228–1244. doi:10.1016/j.enconman.2014.06.022
Kirrolia A, Bishnoi NR, Singh R (2013) Microalgae as a boon for sustainable energy production and its future research & development aspects. Renew Sustain Energy Rev 20:642–656. doi:10.1016/j.rser.2012.12.003
Knothe G, Steidley KR (2011) Kinematic viscosity of fatty acid methyl esters: prediction, calculated viscosity contribution of esters with unavailable data, and carbon-oxygen equivalents. Fuel 90:3217–3224. doi:10.1016/j.fuel.2011.06.016
Krohn BJ, Mcneff CV, Yan B, Nowlan D (2011) Production of algae-based biodiesel using the continuous catalytic Mcgyan® process. Bioresour Technol 102:94–100. doi:10.1016/j.biortech.2010.05.035
Kulkarni MG, Dalai AK (2006) Waste cooking oil an economical source for biodiesel: a review. Ind Eng Chem Res 45:2901–2913. doi:10.1021/ie0510526
Lai JQ, Hu ZL, Wang PW, Yang Z (2012) Enzymatic production of microalgal biodiesel in ionic liquid [BMIm][PF6]. Fuel 95:329–333. doi:10.1016/j.fuel.2011.11.001
Lakaniemi A-M, Tuovinen OH, Puhakka JA (2013) Anaerobic conversion of microalgal biomass to sustainable energy carriers—a review. Bioresour Technol 135:222–231. doi:10.1016/j.biortech.2012.08.096
Lam MK, Lee KT (2012) Microalgae biofuels: a critical review of issues, problems and the way forward. Biotechnol Adv 30:673–690. doi:10.1016/j.biotechadv.2011.11.008
Lam MK, Lee KT (2013) Catalytic trans-esterification of high viscosity crude microalgae lipid to biodiesel: effect of co-solvent. Fuel Process Technol 110:242–248. doi:10.1016/j.fuproc.2012.12.021
Lee OK, Kim YH, Na JG, Oh YK, Lee EY (2013) Highly efficient extraction and lipase-catalyzed trans-esterification of triglycerides from Chlorella sp. KR-1 for production of biodiesel. Bioresour Technol 147:240–245. doi:10.1016/j.biortech.2013.08.037
Lee AF, Bennett JA, Manayil JC, Wilson K (2014) Heterogeneous catalysis for sustainable biodiesel production via esterification and trans-esterification. Chem Soc Rev 43:7887–7916. doi:10.1039/C4CS00189C
Leung DYC, Wu X, Leung MKH (2010) A review on biodiesel production using catalyzed trans-esterification. Appl Energy 87:1083–1095. doi:10.1016/j.apenergy.2009.10.006
Li M, Zhu Q, Hu CW, Chen L, Liu ZL, Kong ZM (2007) Cobalt and manganese stress in the microalga Pavlova viridis (Prymnesiophyceae): effects on lipid peroxidation and antioxidant enzymes. J Environ Sci 19:1330–1335. doi:10.1016/S1001-0742(07)60217-4
Liang Y (2013) Producing liquid transportation fuels from heterotrophic microalgae. Appl Energy 104:860–868. doi:10.1016/j.apenergy.2012.10.067
Likozar B, Levec J (2014) Effect of process conditions on equilibrium, reaction kinetics and mass transfer for triglyceride trans-esterification to biodiesel: experimental and modeling based on fatty acid composition. Fuel Process Technol 122:30–41. doi:10.1016/j.fuproc.2014.01.017
López Barreiro D, Prins W, Ronsse F, Brilman W (2013) Hydrothermal liquefaction (HTL) of microalgae for biofuel production: state of the art review and future prospects. Biomass Bioenergy 53:113–127. doi:10.1016/j.biombioe.2012.12.029
Ma G, Hu W, Pei H, Jiang L, Song M, Mu R (2015) In situ heterogeneous trans-esterification of microalgae using combined ultrasound and microwave irradiation. Energy Covers Manage 90:41–46. doi:10.1016/j.enconman.2014.10.061
Macario A, Giordano G (2013) Catalytic conversion of renewable sources for biodiesel production: a comparison between biocatalysts and inorganic catalysts. Catal Lett 143:159–168. doi:10.1007/s10562-012-0949-3
Maity JP, Bundschuh J, Chen CY, Bhattacharya P (2014) Microalgae for third generation biofuel production, mitigation of greenhouse gas emissions and wastewater treatment: present and future perspectives—a mini review. Energy 78:104–113. doi:10.1016/j.energy.2014.04.003
Martínez Arias EL, Fazzio Martins P, Jardini Munhoz AL, Gutierrez-Rivera L, Maciel Filho R (2012) Continuous synthesis and in situ monitoring of biodiesel production in different microfluidic devices. Ind Eng Chem Res 51:10755–10767. doi:10.1021/ie300486v
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review. Renew Sustain Energy Rev 14:217–232. doi:10.1016/j.rser.2009.07.020
Mata TM, Mendes AM, Caetano NS, Martins AA (2014) Sustainability and economic evaluation of microalgae grown in brewery wastewater. Bioresour Technol 168:151–158. doi:10.1016/j.biortech.2014.04.091
Monari C, Righi S, Olsen SI (2016) Greenhouse gas emissions and energy balance of biodiesel production from microalgae cultivated in photobioreactors in Denmark: a life-cycle modeling. J Clean Prod 112:4084–4092. doi:10.1016/j.jclepro.2015.08.112
Muñoz R, Guieysse B (2006) Algal–bacterial processes for the treatment of hazardous contaminants: a review. Water Res 40:2799–2815. doi:10.1016/j.watres.2006.06.011
Mussgnug JH, Klassen V, Schlüter A, Kruse O (2010) Microalgae as substrates for fermentative biogas production in a combined biorefinery concept. J Biotechnol 150:51–56. doi:10.1016/j.jbiotec.2010.07.030
Nasir Uddin M, Daud WMAW, Abbas HF (2013) Potential hydrogen and non-condensable gases production from biomass pyrolysis: insights into the process variables. Renew Sustain Energy Rev 27:204–224. doi:10.1016/j.rser.2013.06.031
Nayak BK, Roy S, Das D (2014) Biohydrogen production from algal biomass (Anabaena sp. PCC 7120) cultivated in airlift photobioreactor. Int J Hydrogen Energy 39:7553–7560. doi:10.1016/j.ijhydene.2013.07.120
Nigam PS, Singh A (2011) Production of liquid biofuels from renewable resources. Prog Energy Combust Sci 37:52–68. doi:10.1016/j.pecs.2010.01.003
Oncel SS (2013) Microalgae for a macroenergy world. Renew Sustain Energy Rev 26:241–264. doi:10.1016/j.rser.2013.05.059
Oswald WJ (2003) My sixty years in applied algology. J Appl Phycol 15:99–106. doi:10.1023/a:1023871903434
Park J-Y, Park MS, Lee Y-C, Yang J-W (2015) Advances in direct trans-esterification of algal oils from wet biomass. Bioresour Technol 184:267–275. doi:10.1016/j.biortech.2014.10.089
Patil PD, Gude VG, Mannarswamy A, Deng S, Cooke P, Munson-Mcgee S, Rhodes I, Lammers P, Nirmalakhandan N (2011) Optimization of direct conversion of wet algae to biodiesel under supercritical methanol conditions. Bioresour Technol 102:118–122. doi:10.1016/j.biortech.2010.06.031
Peralta-Ruiz Y, González-Delgado AD, Kafarov V (2013) Evaluation of alternatives for microalgae oil extraction based on exergy analysis. Appl Energy 101:226–236. doi:10.1016/j.apenergy.2012.06.065
Persson M, Mladenoska I, Wehtje E, Adlercreutz P (2002) Preparation of lipases for use in organic solvents. Enzyme Microb Technol 31:833–841. doi:10.1016/S0141-0229(02)00184-9
Pittman JK, Dean AP, Osundeko O (2011) The potential of sustainable algal biofuel production using wastewater resources. Bioresour Technol 102:17–25. doi:10.1016/j.biortech.2010.06.035
Puna JF, Gomes JF, Correia MJN, Soares Dias AP, Bordado JC (2010) Advances on the development of novel heterogeneous catalysts for trans-esterification of triglycerides in biodiesel. Fuel 89:3602–3606. doi:10.1016/j.fuel.2010.05.035
Rawat I, Ranjith Kumar R, Mutanda T, Bux F (2011) Dual role of microalgae: phycoremediation of domestic wastewater and biomass production for sustainable biofuels production. Appl Energy 88:3411–3424. doi:10.1016/j.apenergy.2010.11.025
Rawat I, Ranjith Kumar R, Mutanda T, Bux F (2013) Biodiesel from microalgae: a critical evaluation from laboratory to large scale production. Appl Energy 103:444–467. doi:10.1016/j.apenergy.2012.10.004
Reddy HK, Muppaneni T, Patil PD, Ponnusamy S, Cooke P, Schaub T, Deng S (2014) Direct conversion of wet algae to crude biodiesel under supercritical ethanol conditions. Fuel 115:720–726. doi:10.1016/j.fuel.2013.07.090
Robles-Medina A, González-Moreno PA, Esteban-Cerdán L, Molina-Grima E (2009) Biocatalysis: towards ever greener biodiesel production. Biotechnol Adv 27:398–408. doi:10.1016/j.biotechadv.2008.10.008
Rodrigues Da Silva Baumgartner T, Mendes Burak JA, Baumgartner D, Zanin GM, Arroyo PA (2013) Biomass production and ester synthesis by in situ trans-esterification/esterification using the microalga spirulina platensis. Int J Chem Eng 2013:1–7. doi:10.1155/2013/425604
Sahu AK, Siljudalen J, Trydal T, Rusten B (2013) Utilisation of wastewater nutrients for microalgae growth for anaerobic co-digestion. J Environ Manage 122:113–120. doi:10.1016/j.jenvman.2013.02.038
Sathish A, Smith BR, Sims RC (2014) Effect of moisture on in situ trans-esterification of microalgae for biodiesel production. J Chem Technol Biotechnol 89:137–142. doi:10.1002/jctb.4125
Sawaengsak W, Silalertruksa T, Bangviwat A, Gheewala SH (2014) Life cycle cost of biodiesel production from microalgae in Thailand. Energy Sustain Dev 18:67–74. doi:10.1016/j.esd.2013.12.003
Schneider RCS, Bjerk TR, Gressler PD, Souza MP, Corbellini VA, Lobo EA (2012) Potential production of biofuel from microalgae biomass produced in wastewater. In: Fang Z (ed) Biodiesel—feedstocks, production and applications, pp 3–24. InTech. ISBN:978–953–51-0910-5 doi:10.5772/52439
Schumacher G, Blume T, Sekoulov I (2003) Bacteria reduction and nutrient removal in small wastewater treatment plants by an algal biofilm. Water Sci Technol 47:195–202
Slade R, Bauen A (2013) Micro-algae cultivation for biofuels: cost, energy balance, environmental impacts and future prospects. Biomass Bioenergy 53:29–38. doi:10.1016/j.biombioe.2012.12.019
Song C, Chen G, Ji N, Liu Q, Kansha Y, Tsutsumi A (2015) Biodiesel production process from microalgae oil by waste heat recovery and process integration. Bioresour Technol 193:192–199. doi:10.1016/j.biortech.2015.06.116
Soydemir G, Keris-Sen UD, Sen U, Gurol MD (2015) Biodiesel production potential of mixed microalgal culture grown in domestic wastewater. Bioprocess Biosyst Eng 1–7. doi:10.1007/s00449–015–1487-3
Stephens E, Ross IL, Mussgnug JH, Wagner LD, Borowitzka MA, Posten C, Kruse O, Hankamer B (2010) Future prospects of microalgal biofuel production systems. Trends Plant Sci 15:554–564. doi:10.1016/j.tplants.2010.06.003
Steriti A, Rossi R, Concas A, Cao G (2014) A novel cell disruption technique to enhance lipid extraction from microalgae. Bioresour Technol 164:70–77. doi:10.1016/j.biortech.2014.04.056
Su E, You P, Wei D (2009) In situ lipase-catalyzed reactive extraction of oilseeds with short-chained dialkyl carbonates for biodiesel production. Bioresour Technol 100:5813–5817. doi:10.1016/j.biortech.2009.06.077
Sun Y, Liu J, Xie T, Xiong X, Liu W, Liang B, Zhang Y (2014) Enhanced lipid accumulation by Chlorella vulgaris in a two-stage fed-batch culture with glycerol. Energy Fuels 28:3172–3177. doi:10.1021/ef5000326
Suwannakarn K, Lotero E, Ngaosuwan K, Goodwin JG (2009) Simultaneous free fatty acid esterification and triglyceride trans-esterification using a solid acid catalyst with in situ removal of water and unreacted methanol. Ind Eng Chem Res 48:2810–2818. doi:10.1021/ie800889w
Taher H, Al-Zuhair S, Al-Marzouqi AH, Haik Y, Farid MM (2011) A review of enzymatic trans-esterification of microalgal oil-based biodiesel using supercritical technology. Enzyme Res 2011:1–25. doi:10.4061/2011/468292
Tang X, He LY, Tao XQ, Dang Z, Guo CL, Lu GN, Yi XY (2010) Construction of an artificial microalgal-bacterial consortium that efficiently degrades crude oil. J Hazard Mater 181:1158–1162. doi:10.1016/j.jhazmat.2010.05.033
Teo CL, Jamaluddin H, Zain NM, Idris A (2014) Biodiesel production via lipase catalysed trans-esterification of microalgae lipids from Tetraselmis sp. Renew Energy 68:1–5. doi:10.1016/j.renene.2014.01.027
Toor SS, Reddy H, Deng S, Hoffmann J, Spangsmark D, Madsen LB, Holm-Nielsen JB, Rosendahl LA (2013) Hydrothermal liquefaction of Spirulina and Nannochloropsis salina under subcritical and supercritical water conditions. Bioresour Technol 131:413–419. doi:10.1016/j.biortech.2012.12.144
Tran D-T, Yeh K-L, Chen C-L, Chang J-S (2012) Enzymatic trans-esterification of microalgal oil from Chlorella vulgaris ESP-31 for biodiesel synthesis using immobilized Burkholderia lipase. Bioresour Technol 108:119–127. doi:10.1016/j.biortech.2011.12.145
Tran D-T, Chen C-L, Chang J-S (2013a) Effect of solvents and oil content on direct trans-esterification of wet oil-bearing microalgal biomass of Chlorella vulgaris ESP-31 for biodiesel synthesis using immobilized lipase as the biocatalyst. Bioresour Technol 135:213–221. doi:10.1016/j.biortech.2012.09.101
Tran DT, Chen CL, Chang JS (2013b) Effect of solvents and oil content on direct trans-esterification of wet oil-bearing microalgal biomass of Chlorella vulgaris ESP-31 for biodiesel synthesis using immobilized lipase as the biocatalyst. Bioresour Technol 135:213–221. doi:10.1016/j.biortech.2012.09.101
Umdu ES, Tuncer M, Seker E (2009) Trans-esterification of Nannochloropsis oculata microalga’s lipid to biodiesel on Al2O3 supported CaO and MgO catalysts. Bioresour Technol 100:2828–2831. doi:10.1016/j.biortech.2008.12.027
Ummalyma SB, Sukumaran RK (2014) Cultivation of microalgae in dairy effluent for oil production and removal of organic pollution load. Bioresour Technol 165:295–301. doi:10.1016/j.biortech.2014.03.028
Van Den Hende S, Carré E, Cocaud E, Beelen V, Boon N, Vervaeren H (2014) Treatment of industrial wastewaters by microalgal bacterial flocs in sequencing batch reactors. Bioresour Technol 161:245–254. doi:10.1016/j.biortech.2014.03.057
Velasquez-Orta SB, Lee JGM, Harvey A (2012) Alkaline in situ trans-esterification of Chlorella vulgaris. Fuel 94:544–550. doi:10.1016/j.fuel.2011.11.045
Venkata Mohan S, Devi MP (2014) Salinity stress induced lipid synthesis to harness biodiesel during dual mode cultivation of mixotrophic microalgae. Bioresour Technol 165:288–294. doi:10.1016/j.biortech.2014.02.103
Vonortas A, Papayannakos N (2014) Comparative analysis of biodiesel versus green diesel. Wiley Interdiscip Rev Energy Environ 3:3–23. doi:10.1002/wene.78
Wahlen BD, Willis RM, Seefeldt LC (2011) Biodiesel production by simultaneous extraction and conversion of total lipids from microalgae, cyanobacteria, and wild mixed-cultures. Bioresour Technol 102:2724–2730. doi:10.1016/j.biortech.2010.11.026
Wu LF, Chen PC, Huang AP, Lee CM (2012) The feasibility of biodiesel production by microalgae using industrial wastewater. Bioresour Technol 113:14–18. doi:10.1016/j.biortech.2011.12.128
Wu YH, Yu Y, Hu HY (2013) Potential biomass yield per phosphorus and lipid accumulation property of seven microalgal species. Bioresour Technol 130:599–602. doi:10.1016/j.biortech.2012.12.116
Xu Y, Boeing WJ (2014) Modeling maximum lipid productivity of microalgae: review and next step. Renew Sustain Energy Rev 32:29–39. doi:10.1016/j.rser.2014.01.002
Xu H, Miao X, Wu Q (2006) High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. J Biotechnol 126:499–507. doi:10.1016/j.jbiotec.2006.05.002
Yang F, Long L, Sun X, Wu H, Li T, Xiang W (2014) Optimization of medium using response surface methodology for lipid production by Scenedesmus sp. Marine Drugs 12:1245–1257. doi:10.3390/md12031245
Yu N, Dieu LTJ, Harvey S, Lee D-Y (2015) Optimization of process configuration and strain selection for microalgae-based biodiesel production. Bioresour Technol 193:25–34. doi:10.1016/j.biortech.2015.05.101
Zeng X, Danquah MK, Chen XD, Lu Y (2011) Microalgae bioengineering: From CO2 fixation to biofuel production. Renew Sustain Energy Rev 15:3252–3260. doi:10.1016/j.rser.2011.04.014
Zeng D, Li R, Yan T, Fang T (2014) Perspectives and advances of microalgal biodiesel production with supercritical fluid technology. RSC Adv 4:39771–39781. doi:10.1039/C4RA05766J
Zhang YM, Chen H, He CL, Wang Q (2013) Nitrogen starvation induced oxidative stress in an oil-producing green alga Chlorella sorokiniana C3. PLoS ONE 8:10. doi:1371/journal.pone.0069225
Zhou W, Chen P, Min M, Ma X, Wang J, Griffith R, Hussain F, Peng P, Xie Q, Li Y, Shi J, Meng J, Ruan R (2014) Environment-enhancing algal biofuel production using wastewaters. Renew Sustain Energy Rev 36:256–269. doi:10.1016/j.rser.2014.04.073
Zhu L, Hiltunen E, Shu Q, Zhou W, Li Z, Wang Z (2014) Biodiesel production from algae cultivated in winter with artificial wastewater through pH regulation by acetic acid. Appl Energy 128:103–110. doi:10.1016/j.apenergy.2014.04.039
Acknowledgments
The authors wish to thank FAP-UNISC, CNPq (306178/2012-5), Fapergs/Capes (DOCFIX n° 09/2012), and the Brazilian government through the Ministry of Science, Technology, and Innovation for providing necessary funds and facilities.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Pacheco, M.M., Hoeltz, M., de Souza, D., Benitez, L.B., Schneider, R.C.S., Müller, M.V.G. (2017). Current Approaches in Producing Oil and Biodiesel from Microalgal Biomass. In: Singh, L., Kalia, V. (eds) Waste Biomass Management – A Holistic Approach. Springer, Cham. https://doi.org/10.1007/978-3-319-49595-8_13
Download citation
DOI: https://doi.org/10.1007/978-3-319-49595-8_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-49594-1
Online ISBN: 978-3-319-49595-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)