Abstract
SnRK2 protein kinase family plays an important role in plant response to abiotic stress and has been identified in various plants. This study aimed to identify SnRK2 genes in tobacco and systematically analyze their expression under abscisic acid treatment and abiotic stress. We identified 22 NtSnRK2 members, which were divided into three groups and located on 13 chromosomes, mainly at both ends of the chromosomes; additionally, 11 duplicated NtSnRK2 gene pairs were observed. Phylogenetic analysis showed that these SnRK2 members were divided into three groups in tobacco. The motifs of NtSnRK2 proteins in the same group were highly similar. Subcellular localization indicated that NtSnRK2s in Group3 were present in the nucleus, cytomembrane, and cytoplasm. Gene expression pattern analysis revealed that NtSnRK2 genes played a role in the responses to several abiotic stresses (salt, drought, and low-temperature stress), indicating that they are widely involved in the adaptation of tobacco to adverse environmental conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The complex and changeable natural environment results in the generation of abiotic stressors that inevitably affect plants during their growth. Abnormal environmental factors, such as drought and flooding, hinder crop growth and decrease yield (Bailey-Serres and Voesenek 2008; Chaves et al. 2003; Wang et al. 2004). To adapt to environmental changes, plants have evolved a complex set of defense mechanisms to survive via natural selection (Blum 2017; Krasensky and Jonak 2012), and exploring these mechanisms is extremely important to improve crop yield and plant resistance.
SnRK2 is a plant protein kinase family, which is split into subgroups I to III (Umezawa et al. 2009, 2010) based on their sequence homology and respective responses to osmotic pressure and abscisic acid (ABA). The cDNA of PKABA1, a member of this gene family, was first identified from the cDNA library of Triticum aestivum L. endosperm treated with ABA (Anderberg and Walker-Simmons 1992); PKABA1 can phosphorylate the substrate Triticum aestivum ABA response element-binding factor (TaABF) and be induced by ABA, low temperature, high salinity, and drought conditions (Johnson et al. 2008). Subsequently, AAPK (abscisic acid-activated protein kinase) of the SnRK2 family was identified in fava bean and found to play a role in Ca2+-independent ABA signal transduction in guard cells (Li and Assmann 1996). In Arabidopsis thaliana, SnRK2 is composed of 10 family members, among which nine are induced by hypertonic stress, five by ABA, and none by low temperatures (Boudsocq et al. 2004). There are 11 SnRK2 family members in Zea mays L., among which five are induced by ABA, two are strongly induced by NaCl, two are induced by low temperatures, and three are inhibited by high temperatures. In addition, most members exhibit weak responses to salt stress (Huai et al. 2008). Ten SnRK2 members have been identified in Oryza sativa L., all of which are induced by hypertonic stress; however, three of these could be induced by ABA (Kobayashi et al. 2004).
Arabidopsis overexpressing maize SAPK8 and rice overexpressing SAPK4 show higher salt tolerance than the wild-type plants (Diédhiou et al. 2008; Ying et al. 2011). After NaCl solution treatment, the SAPK4 gene in rice decreases the accumulation of Na+ and Cl– in cells via reducing oxidative damage and regulating ion balance, thereby improving the salt tolerance of transgenic plants (Diédhiou et al. 2008). Overexpression of the TaSnRK2.4 gene in wheat induces the expression of downstream stress-related genes. This leads to a series of physiological changes, such as decreased tissue osmotic potential, increased relative water content, and enhanced cell membrane stability, thus improving the salt tolerance and cold and drought resistance of transgenic plants (Mao et al. 2010).
On the whole, members of the SnRK2 family provide support for plants adapting to abiotic stress. Tobacco is not only a special industrial crop but also a model crop (Chen et al. 2017). Therefore, clarifying the molecular mechanism underlying the action of SnRK2 family in tobacco under adverse conditions can provide an important theoretical basis for improving tobacco stress resistance.
In this study, sequences of the SnRK2 protein in Arabidopsis were used to screen NtSnRK2 family members. Thereafter, we analyzed the physical properties, chromosome and subcellular localization, evolutionary relationship, and conserved motifs of NtSnRK2 family members. To clarify the function of NtSnRK2 genes, we assessed the expression levels of NtSnRK2 genes under abiotic stresses (salt, drought, and low temperature) and ABA treatment. Our study could provide theoretical guidance for the stress resistance breeding of tobacco.
Materials and Methods
Identification of NtSnRK2 Genes
We obtained SnRK2 gene sequences of Oryza sativa, Zea mays, Nicotiana tabacum, and Arabidopsis thaliana from the Rice Genome Annotation Project database (http://rice.plantbiology.msu.edu/), Maize Genetics and Genomics Database (https://www.maizegenetics.net/), National Center for Biotechnology Information (NCBI) database (http://www.ncbi.nlm.nih.gov/mapview/index.html), and the Arabidopsis Information Resource (TAIR) database (http://www.arabidopsis.org/), respectively. Based on the identified protein sequences of the AtSnRK2 gene, the candidate sequences of tobacco SnRK2 were obtained using the Basic Local Alignment Search Tool (BLAST; E-value = 1e − 10). The hidden Markov model (HMM) obtained from the Pfam database (http://pfam.xfam.org, PF00069) (Finn et al. 2016) was used to predict the NtSnRK2 protein, and the HMMER software (HMM) was used to search for SnRK2 proteins from tobacco sequences. The predicted SnRK2 domains were detected using the SMART online tool (https://smart.embl-heidelberg.de/) to confirm the final protein sequences of NtSnRK2s. The ExPASy ProtParam online software (https://www.expasy.org/) was used to analyze the biophysical properties of the encoded NtSnRK2s. The subcellular localization and trans-membrane spanning region of NtSnRK2s were separately predicted using the WoLF PSORT online tool (https://www.genscript.com/wolf-psort.html) and TMHMM Server (http://www.cbs.dtu.dk/services/TMHMM/), respectively.
Chromosome Localization and Collinearity Analyses of NtSnRK2 Genes
We retrieved the chromosomal distribution information regarding NtSnRK2 genes from the Sol Genomics Network (https://solgenomics.net/). The MCScanX program was used to identify the Synteny blocks of tobacco genome containing the SnRK2 genes (Wang et al. 2012). For gene duplication analysis, the chromosomal distribution of NtSnRK2s and the collinearity relationships between NtSnRK2 homologs were verified and visualized using Circos (http://circos.ca/).
Phylogenetic Analysis of SnRK2 Proteins
The Clustal X program with default parameters was used to perform the multiple sequence alignment of SnRK2 genes in four species (Z. mays, A. thaliana, O. sativarice, and tobacco). MEGA 7.0 (Molecular Evolutionary Genetics Analysis) software with the neighbor-joining method was used to construct the unrooted phylogenetic tree, and set the Bootstrap value to 1000.
Gene Structure and Conserved Motifs Analysis of NtSnRK2s
We employed the Gene Structure Display Server 2.0 (GSDS 2.0) (http://gsds.cbi.pku.edu.cn/) to predict NtSnRK2s exon–intron structures using cDNAs and their corresponding genome sequence alignment. The conserved motifs in the NtSnRK2 protein sequences were discovered using MEME online software (https://meme-suite.org/meme/tools/meme), and the number of motif searches was set at 15.
Subcellular Localization of NtSnRK2 Proteins in Group 3
The open reading frame (ORF) of NtSnRK2 without a termination codon was fused upstream of green fluorescent protein (GFP) in the pJIT163-GFP vector under the control of the CaMV 35S promoter. The fusion construct was transformed into tobacco protoplasts by PEG-mediated transformation (Abel and Theologis 1994). Tobacco protoplasts were enzymically isolated from tobacco leaves of 3-week-old plants (Mahdieh and Mostajeran 2009). Incubated at 25 °C for 15 h after transformation, fluorescence signals were observed in the range of 500–570 nm wavelengths with a laser scanning confocal microscope (Leica TCSNT, Germany).
Gene Expression Pattern Analysis
K326 (Nicotiana tabacum L., cv. Kentucky 326), a widely cultivated flue-cured tobacco variety, was used to analyze the expression of NtSnRK2 genes. The tobacco seeds were planted in mixed soil (humus: vermiculite = 1:1) with water and germinated in a sieve plate (25 ℃, 16 h light and 8 h dark). Seedlings cultured for 4 weeks in treatment groups were exposed to drought (PEG 6000 solution, − 0.5 MPa water potential), salt (300 mM NaCl solution), cold (4 °C), or ABA (50 µM, spray), respectively. Leaves were collected at 0, 1, 3, 6, 12, 24, 48, and 72 h post-treatment, frozen using liquid nitrogen, and stored at − 80 °C for RNA isolation. Total RNA was isolated using TRIzol reagent (Applygen, Beijing, China), then treated with RNase-free DNase to remove residual DNA. Complementary DNA (cDNA) was then synthesized using a reverse transcription (RT) kit (Takara, Dalian, China).
The gene expression patterns were detected using qRT-PCR, which was performed in an ABI PRISM 7000 system (Applied Biosystems, Waltham, MA, USA) using SYBR Green RT-PCR kits (Takara, Dalian, China) according to the manufacturer’s instructions; all reactions were performed in triplicate. The L25 gene was used as an internal control to quantify the relative transcript levels (Gregor and Delaney 2010). Results were calculated using the 2−ΔΔCt method (Schmittgen 2001). The specific gene primers are listed in Table S1.
Results
Identification and Chromosomal Localization of the SnRK2 Gene in Tobacco
Arabidopsis SnRK2 protein sequences were used as the query sequences, and a local BLASTP search was used to identify the SnRK2 members in the tobacco genome. The candidate genes with default E-values were searched via the SnRK2 domain (PF00069) using the HMMER3.0 program to verify the screening results. Finally, 22 NtSnRK2 proteins were identified. Table 1 lists the characteristics of NtSnRK2, including the chromosomal localization, gene length, instability index, molecular mass, theoretical isoelectric point, number of amino acids, and subcellular localization. The results indicated a slight discrepancy among these NtSnRK2 genes: the lengths of NtSnRK2s were 2488–6043 bp, the protein sequences of NtSnRK2s comprised 282–363 amino acids, the molecular weights of NtSnRK2s were 32.77–41.57 kDa, and the isoelectric points of NtSnRK2 proteins ranged from 4.59 to 6.36, suggesting that all members of the NtSnRK2 gene family encode acidic proteins. The hydrophobicity index of proteins encoded by NtSnRK2s was less than zero, which indicated that NtSnRK2 proteins were hydrophobic. Subcellular localization was predicted using the WoLF PSORT online tool and revealed that members of the NtSnRK2 family were distributed in the cytoplasm, cytoskeleton, and mitochondria, respectively. Among the 22 NtSnRK2 members, 13 members exhibited an instability index greater than 40, which indicates that these members are unstable.
According to the chromosomal distribution information regarding 22 NtSnRK2 genes (Fig. 1), 16 NtSnRK2 genes were located on 13 chromosomes, and the other six were located on the scaffolds. Two NtSnRK2 genes were observed on chromosomes 6, 10, and 13, while one was observed on 10 other chromosomes (1, 3, 4, 5, 9, 14, 15, 17, 22, and 24). In addition, the location information and gene sequence homology analysis results showed that the 16 NtSnRK2 genes successfully located on the ends of the chromosome, and there were 11 duplicated gene pairs in 22 NtSnRK2 genes.
Phylogenetic Analysis of SnRK2 Proteins
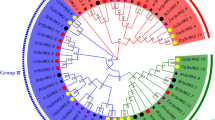
We used MEGA 7.0 with the neighbor-joining method to construct an unrooted phylogenetic tree and examine the evolutionary relationship among SnRK2 members. The phylogenetic tree contained 52 SnRK2 members from four species: 10 SnRK2s from Arabidopsis, 10 from rice, 10 from maize, and 22 from tobacco. The 52 SnRK2s were divided into three subgroups (Group 1–3, Fig. 2): LOC107766520, LOC107810314, LOC107817827, LOC107791496, LOC107798612, and LOC107826957 were clustered in Group 1; LOC107766134, LOC107765115, LOC107807198, LOC107819672, LOC107817951, and LOC107829367 belonged to Group 2; and the other tobacco SnRK2 proteins were assigned to the same branch (Group 3). It was found that each group contained SnRK2 members from tobacco, maize, rice, and Arabidopsis. Compared to maize or rice, SnRK2 proteins from tobacco and Arabidopsis were highly homologous. Furthermore, homologous SnRK2 proteins from maize and rice showed a close homology.
Phylogenetic analysis of SnRK2 proteins from Oryza sativa, Zea mays, Arabidopsis thaliana, and Nicotiana tabacum. The MEGA7.0 program was used to generate the unrooted phylogenetic tree using the neighbor-joining method with 1000 bootstrap replicates. The areas covered by the green, blue, and red arcs represent Group 1, Group 2, and Group 3, respectively. The red square represents Nicotiana tabacum, the green diamond represents Arabidopsis thaliana, the purple circle represents Zea mays, and the blue triangle represents Oryza sativa (Color figure online)
It is worth considering that in Group 1, LOC107798612 and LOC107826957 showed high similarity with AtSnRK2.1 and AtSnRK2.7, while AtSnRK2.7 could respond to osmotic stress and had a weak induction response to ABA. Similarly, LOC107766520, LOC107810314, LOC107817827, and LOC107791496 were closely related to AtSnRK2.4 and AtSnRK2.10, whereas AtSnRK2.4 and AtSnRK2.10 were induced by osmotic stress but not by ABA. In Group 2, LOC107777197, LOC107780983, LOC107802713, and LOC107770300 had high homology with ZmSnRK2.3 and OsSAPK3, while OsSAPK3 was induced by ABA, and ZmSnRK2.3 was induced by low temperature and salt stress. In Group 3, LOC107792070 and LOC107827494 were similar to AtSnRK2.2 and AtSnRK2.3; LOC107769218, LOC107790910, LOC107807681, and LOC107824451 were highly homologous with AtSnRK2.6; and AtSnRK2.2, AtSnRK2.3, and AtSnRK2.6 were strongly induced by ABA (Huai et al. 2008; Kobayashi et al. 2004).
Gene Structure and Motif Analyses of NtSnRK2s
To further understand the function of the NtSnRK2 gene family, a neighbor-joining phylogenetic tree, including 22 NtSnRK2 protein sequences, was constructed, and motifs were analyzed using the MEME online software. The neighbor-joining phylogenetic tree showed that the 22 NtSnRK2s were split into 11 varieties (NtSnRK2.1–NtSnRK2-11) based on differences in their genetic sequences (Fig. 3a). The results of gene structure analysis (Fig. 3b) showed that there was a little discrepancy (6–9 exons) in the number of introns among different members of the NtSnRK2 family. Group 1 had the most exons, ranging from eight to nine, while 15 of the NtSnRK2 gene family members had nine, four had eight, two had seven, and one had six.
Phylogenetic tree, exon–intron structure, and motif analysis of NtSnRK2s. a Phylogenetic analysis of NtSnRK2s using the neighbor-joining method. The boxes of different colors represent NtSnRK2.1 to NtSnRK2.11 from top to bottom. b Gene structure analysis of NtSnRK2 genes. Exons and introns are represented by blue rectangles and black lines, respectively. c NtSnRK2 protein motifs were analyzed using MEME tools. The order of the motifs corresponds to their position in the protein sequence. Conserved motifs are shown in different colored boxes (Color figure online)
As revealed by the motif analysis (Fig. 3c), NtSnRK2 members contained 15 conserved motifs. Furthermore, the protein motifs of Group 1 and Group 3 were relatively conserved. Motifs 1, 3, 7, 8, 9, and 11, which can be found in each NtSnRK2 protein, may be vital for their common functions, and more than 86.4% of the NtSnRK2 members contained motifs 2, 4, 5, and 6. It is worth mentioning that motifs 12 and 15 were specific to Groups 1 and 2, respectively, while motifs 10 and 14 were specific to Group 3. These motifs may explain the functional differences among the three groups.
Subcellular Localization of NtSnRK2 Proteins in Group 3
The NtSnRK2.9-11 proteins in Group 3 contained a trans-membrane spanning region (DVWSCGVTLYVMLVGAYPF) and a potential N-myristoylation site (SGVSY/FCH), indicating that NtSnRK2.9-11 might mediate the association between the nuclear and cell membrane systems (Fig. S1). To determine the subcellular localization of the three NtSnRK2 proteins, their coding regions were separately fused in-frame with the GFP gene, and the fusion constructs were then individually transfected into tobacco protoplasts. The GFP-labeled NtSnRK2 protein in tobacco protoplasts was used to assess the cellular distribution of green fluorescence. As shown in Fig. 4, the NtSnRK2.9-11 proteins in Group 3 were separately targeted to the cell outlines, likely the nucleus, cytomembrane, and cytoplasm.
Subcellular localization of NtSnRK2 proteins in Group 3. The ORF of NtSnRK2 without a termination codon was fused upstream of GFP in the pJIT163-GFP vector under the control of 35S promoter. NtSnRK2–GFP fusion proteins were transiently expressed in tobacco protoplasts by PEG-mediated transformation. Fluorescence signals were observed in the range of 500–570 nm wavelengths with a laser scanning confocal microscope. 1, GFP fluorescence; 2, bright field images; 3, Chl autofluorescence; 4, merged images. Bar = 100 µm
Expression Patterns of NtSnRK2s Under ABA Treatment and Abiotic Stress
To assess the potential functions of NtSnRK2s during abiotic stress, qRT-PCR was performed to determine the expression pattern of NtSnRK2s after ABA, drought, high salinity, and cold treatments. As shown in Fig. 5, although NtSnRK2 transcription was induced with a different expression profile, the expression patterns of NtSnRK2s in the same group were similar across the treatment groups. Distinct patterns were observed under ABA treatment among the three SnRK2 groups: three Group 1 NtSnRK2 members (NtSnRK2.6-8) showed no response to ABA; Group 2 NtSnRK2s (NtSnRK2.1-5) were weakly induced; and Group 3 NtSnRK2s (NtSnRK2.9-11) were strongly activated by ABA. We observed the rapid transcription induction of all NtSnRK2s under NaCl stress. The transcription of NtSnRK2s in Group 1 gradually increased and peaked at 48 h. Group 2 and 3 NtSnRK2s responded rapidly, reaching a peak at 3 or 6 h, and then their expression decreased. Under cold stress, the transcription of NtSnRK2s in Group 2 peaked at 1 h, declined gradually, and reached a maximum at 24 h. The expression of Group 1 and 3 NtSnRK2s increased gradually, reached a maximum at 24 h, and then decreased. All NtSnRK2s showed a similar expression pattern in response to PEG treatment. Transcription was induced gradually, peaked at 24 (Group 1 and 3) or 48 h (Group 2), and then decreased. These results suggest that NtSnRK2s may participate in abiotic stress responses in different manners.
Heat map of NtSnRK2s gene expression under ABA, NaCl, low temperature, and PEG treatments. The data from quantitative RT-PCR was used for hierarchical cluster analysis with MeV 4.9.0. The expression values of each NtSnRK2 were log2 transformed. Yellow indicates low level expression and red indicates high level expression (Color figure online)
Discussion
SnRK2s are a class of Ser/Thr protein kinases unique to plants, which play significant roles in abiotic stress and various signaling pathways. Numerous studies have demonstrated that each SnRK2 gene is differently involved in the response to multi-environmental stress. In the present study, we identified 11 NtSnRK2 gene members from the Nicotiana tabacum genome database using a homology search of the protein sequences of SnRK2 in Arabidopsis. A total of 15 motifs were present in the NtSnRK2 proteins, and the motif distribution patterns were similar within subgroups (Fig. 3c). This finding indicated that tobacco SnRK2 genes were relatively conserved during evolution.
Gene replication is an important mechanism in gene family evolution. As a general biological phenomenon, gene repetition can provide the most primitive genetic material basis for biological evolution, produce new genes or subfunctional genes, and promote species differentiation and diversity (Hu et al. 2009; Leitch et al. 2008; Yang et al. 2008). Thus, more gene members are commonly present in polyploid species. Compared with other plants (10 SnRK2 genes have been identified in maize, rice, and Arabidopsis), the Nicotiana tabacum SnRK2 family displays more members (Table 1). This may be because Nicotiana tabacum is a tetraploid plant, and replication of new genes occurred in the process of tetraploidization.
To better analyze the evolutionary relationship among SnRK2s in different species, we constructed a SnRK2 phylogenetic tree, which comprised four species (tobacco, maize, rice, and Arabidopsis). Similar to that in previous studies, NtSnRK2 proteins were divided into three groups (Umezawa et al. 2010). Homologous SnRK2 proteins from maize and rice showed high homology, and tobacco SnRK2 proteins showed the highest similarity to their counterparts from Arabidopsis (Fig. 2). These data implied that the differentiation of SnRK2 family genes occurred after the separation of monocots and dicots.
Protein kinases localize to certain cell compartments to perform their proper function, and scanning sequences often specify their intracellular locations. It has been reported that the NtSnRK2 proteins in Group 1 and 2 are present in the cell membrane, cytoplasm, and nucleus (Zhang et al. 2014a, b). All NtSnRK2 proteins in Group 3 also contained a conserved transmembrane-spanning motif (DVWSCGVTLYVMLVGAYPF) and a potential N-myristoylation site (SGVSY/FCH). The N-terminal myristoylation and transmembrane-spanning motifs are crucial for proteins to function in mediating membrane targeting and signal transduction in plant responses to environmental stresses (Ishitani et al. 2000; Podell and Gribskov 2004). To study the cellular localization of the NtSnRK2s proteins in Group 3, the NtSnRK2s::GFP construct driven by the CaMV 35S promoter was transiently expressed in tobacco protoplasts. As predicted, the NtSnRK2s proteins in Group 3 were all localized to the cell membrane, cytoplasm, and nucleus (Fig. 4).
Gene expression patterns can be a direct indication of a gene’s function in plant growth and development. A study regarding stress resistance in maize indicated that the expression levels of ZmSnRK2.2, 2.4, 2.5, 2.7, and 2.10 were enhanced after ABA treatment; the expression levels of ZmSnRK2.5 and ZmSnRK2.6 were obviously induced by NaCl treatment; ZmSnRK2.3 and ZmSnRK2.7 expression was significantly increased under low temperatures, while the expression levels of ZmSnRK2.5, 2.6, and ZmSnRK2.9 decreased under high-temperature conditions (Huai et al. 2008). In Arabidopsis, SnRK2 subfamily II and III members participate in ABA-dependent signal transduction (Boudsocq et al. 2004; Zheng et al. 2010). In our study, the expression of Group 3 NtSnRKs was strongly induced by ABA treatment; the expression levels of Group 2 NtSnRKs were also responsive to ABA, but the response was weak; and the expression of Group 1 NtSnRKs was not activated by ABA treatment (Fig. 5). Similar results were observed in rice, maize, and wheat in previous studies (Huai et al. 2008; Kobayashi et al. 2004; Zhang et al. 2014a).
All NtSnRK2 members were activated by NaCl treatment (Kobayashi et al. 2004), similar to the results of OsSAPK in rice (Diédhiou et al. 2008), but genes of Groups 2 and 3 were more sensitive to salt stress and strongly induced transcription 3 and 6 h after exposure (Fig. 5), respectively. All NtSnRK2 members were activated by low temperature or drought; among them, Group 2 genes were highly sensitive to low-temperature stress and expressed strongly after exposure to low temperature for 1 h (Fig. 5)—this result differs from that of other similar studies (Zhang et al. 2014b).
The present study focused on the identification of evolved and conserved motifs of SnRK2 gene family members from Nicotiana tabacum, and the expression of each NtSnRK2 family member was analyzed under a variety of abiotic stresses. Together, our results can provide an important theoretical basis for improving the stress resistance of tobacco.
Data Availability
The data sets supporting the results of this article are included within the article and its additional file.
References
Abel S, Theologis A (1994) Transient transformation of Arabidopsis leaf protoplasts: a versatile experimental system to study gene expression. Plant J 5:421–427
Anderberg RJ, Walker-Simmons MK (1992) Isolation of a wheat cDNA clone for an abscisic acid-inducible transcript with homology to protein kinases. Proc Natl Acad Sci USA 89:10183–10187. https://doi.org/10.1073/pnas.89.21.10183
Bailey-Serres J, Voesenek LACJ (2008) Flooding stress: acclimations and genetic diversity. Annu Rev Plant Biol 59:313–339. https://doi.org/10.1146/annurev.arplant.59.032607.092752
Blum A (2017) Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ 40:4–10. https://doi.org/10.1111/pce.12800
Boudsocq M, Barbier-Brygoo H, Lauriere C (2004) Identification of nine sucrose nonfermenting 1-related protein kinases 2 activated by hyperosmotic and saline stresses in Arabidopsis thaliana. J Biol Chem 279:41758–41766. https://doi.org/10.1074/jbc.M405259200
Chaves MM, Maroco JP, Pereira JS (2003) Understanding plant responses to drought—from genes to the whole plant. Funct Plant Biol 30:239–264. https://doi.org/10.1071/FP02076
Chen W, Yu K, Bin L, Lan Y, Sun R, Li Q, He F, Pan Q, Duan C, Wang J (2017) Comparison of transcriptional expression patterns of carotenoid metabolism in ‘Cabernet Sauvignon’ grapes from two regions with distinct climate. J Plant Physiol 213:75–86. https://doi.org/10.1016/j.jplph.2017.03.00
Diédhiou C, Popova OV, Dietz KJ, Golldack D (2008) The SNF1-type serine–threonine protein kinase SAPK4 regulates stress-responsive gene expression in rice. BMC Plant Biol 8:49. https://doi.org/10.1186/1471-2229-8-49
Finn RD, Coggill P, Eberhardt RY, Eddy SR, Mistry J, Mitchell AL, Potter SC, Punta M, Qureshi M, Sangrador-Vegas A et al (2016) The Pfam protein families database: towards a more sustainable future. Nucleic Acids Res 44:D279–D285. https://doi.org/10.1093/nar/gkv1344
Gregor WS, Delaney SK (2010) Stable internal reference genes for normalization of real-time RT-PCR in tobacco (Nicotiana tabacum) during development and abiotic stress. Mol Genet Genom 283:233–241. https://doi.org/10.1007/s00438-010-0511-1
Hu W, Hu G, Han B (2009) Genome-wide survey and expression profiling of heat shock proteins and heat shock factors revealed overlapped and stress specific response under abiotic stresses in rice. J Plant Sci 176:583–590. https://doi.org/10.1016/j.plantsci.2009.01.016
Huai J, Wang M, He J, Zheng J, Dong Z, Lv H, Zhao J, Wang G (2008) Cloning and characterization of the SnRK2 gene family from Zea mays. Plant Cell Rep 27:1861–1868. https://doi.org/10.1007/s00299-008-0608-8
Ishitani M, Liu J, Halfter U, Kim CS, Shi W, Zhu J (2000) SOS3 function in plant salt tolerance requires N-myristoylation and calcium binding. Plant Cell 12:1667–1678. https://doi.org/10.2307/3871181
Johnson RR, Shin M, Shen JQJPMB (2008) The wheat PKABA1-interacting factor TaABF1 mediates both abscisic acid-suppressed and abscisic acid-induced gene expression in bombarded aleurone cells. Plant Mol Biol 68:93–103. https://doi.org/10.1007/s11103-008-9354-0
Kobayashi Y, Yamamoto S, Minami H, Kagaya Y, Hattori T (2004) Differential activation of the rice sucrose nonfermenting1-related protein kinase2 family by hyperosmotic stress and abscisic acid. Plant Cell 16:1163–1177. https://doi.org/10.2307/3872079
Krasensky J, Jonak C (2012) Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J Exp Bot 63:1593–1608. https://doi.org/10.1093/jxb/err460
Leitch IJ, Hanson L, Lim KY, Kovarik A, Chase MW, Clarkson JJ, Leitch AR (2008) The ups and downs of genome size evolution in polyploid species of Nicotiana (Solanaceae). Ann Bot—London 101:805–814. https://doi.org/10.1093/aob/mcm326
Li J, Assmann SM (1996) An abscisic acid-activated and calcium-independent protein kinase from guard cells of fava bean. Plant Cell 8:2359–2368. https://doi.org/10.2307/3870474
Mahdieh M, Mostajeran A (2009) Abscisic acid regulates root hydraulic conductance via aquaporin expression modulation in Nicotiana tabacum. J Plant Physiol 166:1993–2003
Mao X, Zhang H, Tian S, Chang X, Jing R (2010) TaSnRK2.4, an SNF1-type serine/threonine protein kinase of wheat (Triticum aestivum L.), confers enhanced multistress tolerance in Arabidopsis. J Exp Bot 61:683–696. https://doi.org/10.1093/jxb/erp331
Podell S, Gribskov M (2004) Predicting N-terminal myristoylation sites in plant proteins. BMC Genom 5:37. https://doi.org/10.1186/1471-2164-5-37
Schmittgen TD (2001) Real-time quantitative PCR. Methods 25:383–385. https://doi.org/10.1101/gr.6.10.986
Umezawa T, Sugiyama N, Mizoguchi M, Hayashi S, Myouga F, Yamaguchis-Shinozaki K, Ishihama Y, Hirayama T, Shinozaki K (2009) Type 2C protein phosphatases directly regulate abscisic acid-activated protein kinases in Arabidopsis. Proc Natl Acad Sci USA 106:17588–17593. https://doi.org/10.1073/pnas.0907095106
Umezawa T, Nakashima K, Miyakawa T, Kuromori T, Tanokura M, Shinozaki K, Yamaguchi-Shinozaki K (2010) Molecular basis of the core regulatory network in ABA responses: sensing, signaling and transport. Plant Cell Physiol 51:1821–1839. https://doi.org/10.1093/pcp/pcq156
Wang W, Vinocur B, Shoseyov O, Altman A (2004) Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci 9:244–252. https://doi.org/10.1016/j.tplants.2004.03.006
Wang Y, Tang H, Debarry JD, Tan X, Li J, Wang X, Lee T, Jin H, Marler B, Guo H et al (2012) MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. https://doi.org/10.1093/nar/gkr1293
Yang Z, Zhou Y, Wang X, Gu S, Yu J, Liang G, Yan C, Xu C (2008) Genomewide comparative phylogenetic and molecular evolutionary analysis of tubby-like protein family in Arabidopsis, rice, and poplar. J Genom 92:246–253. https://doi.org/10.1016/j.ygeno.2008.06.001
Ying S, Zhang D, Li H, Liu Y, Shi Y, Song Y, Wang T, Yu L (2011) Cloning and characterization of a maize SnRK2 protein kinase gene confers enhanced salt tolerance in transgenic Arabidopsis. Plant Cell Rep 30:1683–1699. https://doi.org/10.1007/s00299-011-1077-z
Zhang H, Jia H, Liu G, Yang S, Zhang S, Yang Y, Cui H (2014a) Cloning and characterization of NtSnRK2.7 and NtSnRK2.8 genes involved in abiotic stress responses from Nicotiana tabacum. Acta Physiol Plant 36:1673–1682. https://doi.org/10.1007/s11738-014-1542-8
Zhang H, Jia H, Liu G, Yang S, Zhang S, Yang Y, Yang P, Cui H (2014b) Cloning and characterization of SnRK2 subfamily II genes from Nicotiana tabacum. Mol Biol Rep 41:5701. https://doi.org/10.1007/s11033-014-3440-y
Zheng Z, Xu X, Crosley RA, Greenwalt SA, Sun Y, Blakeslee B, Wang L, Ni W, Sopko MS, Yao C et al (2010) The protein kinase SnRK2.6 mediates the regulation of sucrose metabolism and plant growth in Arabidopsis. Plant Physiol 153:99–113. https://doi.org/10.2307/25680832
Acknowledgements
We thank Nanping Branch of Fujian Tobacco Company, Chongqing Branch of China National Tobacco Corporation, and Henan Agricultural University for the financial support and technical assistance. We also appreciate the reviewers and editors for their patience with regard to this work.
Funding
This work was supported by Chongqing Branch of China National Tobacco Corporation (A2020NY01-1303-1) and Fujian Branch of China National Tobacco Corporation (2021350000240019).
Author information
Authors and Affiliations
Contributions
FH and JHL conceived and designed the experiments. JYS, JTM, JWL, and CJL performed the experiments and participated in data analysis. XWZ and JCL performed the qRT-PCR experiments. YC revised the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, J., Song, J., Li, C. et al. Genome-Wide Identification and Expression Profile Analysis of the SnRK2 Gene Family in Nicotiana tabacum. Biochem Genet 60, 1511–1526 (2022). https://doi.org/10.1007/s10528-021-10170-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10528-021-10170-8