Abstract
The genetic distance analysis for selection of suitable parents has been established and effectively used in many crops; however, there is dearth of conclusive report of relationship of genetic distance analysis with heterosis in sesame. In the present study, an attempt was made to estimate the associations of genetic distances using SSR (GDSSR), seed-storage protein profiling (GDSDS) and agro-morphological traits (GDMOR) with hybrid performance. Seven parents were selected from 60 exotic and Indian genotypes based on genetic distance from clustering pattern based on SSR, seed-storage protein, morphological traits and per se performance. For combining ability analysis, 7 parents and 21 crosses generated from 7 × 7 half diallel evaluated at two environments in a replicated field trial during pre-kharif season of 2013. Compared with the average parents yield (12.57 g plant−1), eight hybrids had a significant (P < 0.01) yield advantage across environments, with averages of 26.94 and 29.99% for better-parent heterosis (BPH) and mid-parent heterosis (MPH), respectively, across environments. Highly significant positive correlation was observed between specific combining ability (SCA) and per se performance (0.97), while positive non-significant correlation of BPH with GDSSR (0.048), and non-significant negative correlations with GDMOR (− 0.01) and GDSDS (− 0.256) were observed. The linear regressions of SCA on MPH, BPH and per se performance of F1s were significant with R2 value of 0.88, 0.84 and 0.95 respectively. The present findings revealed a weak association of GDSSR with F1’s performance; however, SCA has appeared as an important factor in the determination of heterosis and per se performance of the hybrids. The present findings also indicated that parental divergence in the intermediate group would likely produce high heterotic crosses in sesame.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sesame (Sesamum indicum L.) is an ancient oil-yielding crop in tropical and subtropical regions of Asia, Africa and South America producing the highest -quality of oil among the major oilseed crops including peanut, soybean and rapeseed (Bhat et al. 1999). Sesame seeds contain about 50–60% edible oil, which is consumed as a traditional health food for its specific antihypertensive effect and anti-oxidative activity (Jan et al. 2011). The consumption of vegetable oil is expected to touch almost 200 billion kilograms by 2030 resulting in huge demand of oil seed crops (Wang et al. 2014). To fill the gap between demand and supply of oil seed, it is necessary to further increase both yield level and total production by exploiting heterosis. Indian sesame collection represents wide diversity for morphological and agronomic characteristics over diverse eco-geographical regions (Bisht et al. 2004). In the present era, molecular techniques and biometrical methods have unlocked the several ways to evaluate germplasm regarding their suitability as parents. To achieve higher yield, it is required to identify elite parents that can produce exceptionally high yielding hybrids. The use of molecular markers for assessing the diversity amongst parental lines has been suggested for overcoming bottlenecks in hybrid development and selection (Ndhlela et al. 2015). The identification of best hybrid combinations basically relies on the combining ability of the parental genotypes and the gene effects that are associated to the expression of the traits of interest. For any hybrid breeding programme, the information on the effects of general combining ability (GCA) and specific combining ability (SCA) is crucial for the selection of parental genotypes. Best parental combinations are likely to produce heterotic F1 progeny. The successful breeding and utilization of elite lines in diverse heterotic groups have not only assisted in increasing the maize productivity by a big difference but also encouraged breeders to adopt this technique in other crops (Hallauer 1999). Very few studies have used genetic distance to predict hybrid performance in sesame. Some researchers reported association between marker-based genetic distance and heterosis (Banerjee and Kole 2010), whilst others have reported no association with heterosis (Dikshit and Swain 2000). The potential application of markers in determining the degree of heterosis in sesame is, therefore, inconclusive. In this context, the present study is an attempt to classify the accessions according to their relationships by means of the genetic distance based on SSR (GDSSR), Seed-storage protein profiling (GDSDS) and 37 agro-morphological traits (GDMOR) for their future use in hybrid breeding. The objectives of this study were as follows: (i) to classify sesame genotypes based on GDSSR, GDSDS and GDMOR, (ii) to estimate GCA, SCA and heterosis effects, and (iii) to correlate the estimated parental genetic diversity based on GDSSR, GDSDS and GDMOR with SCA and heterosis effects.
Materials and Methods
Experimental Material
In an earlier study reported by Pandey et al. (2015), a collection of 60 sesame genotypes including exotic collections, indigenous collections and landraces were classified based on morphological and marker analysis which served as a base population for selection of seven parents. Based on three clustering pattern and yield performance records, the seven parents namely, Gujarat Til-2 (Western India), TKG-22 (Central India), OSC-593 (South Eastern India), RT-348 (North Western India), TKG-352 (Central India), UMA (South Eastern India) and one indigenous collection, NIC-8316 (Eastern India) (Table 1) from five major sesame-growing states of India were selected for a 7 × 7 half-diallel mating design. Twenty-one F1s along with seven parents were again clustered based on GDSSR, GDSDS and GDMOR according to the methods described below.
Field Experiment
All the possible crosses were made except the reciprocal ones. All F1 hybrids with their parents were planted during pre-kharif season of 2013 using random complete block design with three replications in two environments: the first field site was in Nonaghata (latitude 23°42′ and longitude 88°44′) and the second was in Baruipur South 24-Paraganas (latitude 22°37′ and longitude 88°43′) of West Bengal, India. The soil characteristic was silty clay with pH 6.65 at Nonaghata and sandy loam type with pH 7.20 at Baruipur.
Agronomic Characteristics
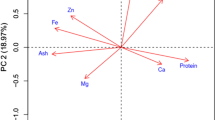
The observations of following nine agronomic characters namely plant height (PH), days to 50% flowering (DTF) (days), days to maturity (DTM) (days), number of primary branches/plant (BP), number of capsules/plant (CP), capsule length (CL) (cm), number of seeds/capsule (SC), 1000 seed weight (SW) (g) and seed yield/plant (SY) (g) were recorded for combining ability analysis. Additional 28 traits namely seed coat colour, stem shape, stem pubescence, leaf arrangement, leaf shape, leaf angle, petal colour, petal hairiness, flowers/axil, capsule shape, capsule hairiness, branching habit, shape of stem hair, stem branching, leaf hairiness, basal leaf profile, basal leaf margin, lobe incision of basal leaf, petiole colour, petiole hairiness, extra floral nectaries, extra floral nectaries colour, calyx hairs, interior corolla colour, interior corolla pigment, lower lip colour, capsule arrangement and capsule beak shape were also recorded along with nine agronomic traits for diversity analysis based on 37 morphological traits.
Molecular Marker Analyses
DNA Extraction
DNA extraction was done from apical young leaves of 10–12 days old. After grinding them in liquid nitrogen, the leaves were treated with CTAB buffer following the method of Saghai-maroof et al. (1984), and then the DNA was purified with RNaseA followed by phenol: chloroform. Purified DNA was quantified in Nanodrop Lite (Thermo Scientific, USA).
SSR Primers
Initially, 36 SSR and EST-SSR markers were used, but final genetic divergence was measured based on 11 highly polymorphic SSR markers (Bhattacharya et al. 2014). The details of 11 polymorphic markers are given in Supplementary Table 1.
PCR Amplification
DNA amplification was done in 25 μL reaction mixture that consists of 0.2 μmol L−1 SSR primers, 0.2 mM of each dNTPs, 2 mmol L−1 MgCl2, 1X PCR buffer and 0.5 unit Taq polymerase, and 50 ng sample DNA. The procedures for SSR and Est-SSR were performed on a DNA thermocycler kit (Eppendorf AG 6321, Germany) as described by Pandey et al. (2015). 3% agarose gel (Sigma USA) was used for separating the amplified PCR products. A 50 base-pair ladder marker (GeneRuler 50 bp DNA Ladder, Thermo Scientific, USA) was used to estimate PCR fragment size.
Seed-Storage Protein Extraction and Profiling
Seed-storage protein extraction and protein profiling were performed according to Lowry et al. (1953) and Laemmli (1970). Standard marker protein (Fermentes, PageRuler™ Pre-stained Protein Ladder—SM0671) was used for estimation of molecular weights of sample protein bands using Gel Documentation Unit (UVP, USA). Molecular weights (MWs) and relative mobility (Rm values) of each band were obtained using the Life Sciences Software loaded in gel documentation unit (UVP GelDoc) by characterizing the molecular weights of each band of the known marker protein. Genetic similarity (GS) coefficients were computed using SIMQUAL program. Dendrogram was constructed using Unweighted Pair Group Method with Arithmetic average to assess relationship among genotypes.
Genetic Distance Analysis Using SSR, Protein and Morphological Data
The clear and reproducible bands from both protein and DNA fragments were selected for data analysis. The presence or absence of bands was scored as ‘1’ or ‘0’, respectively, for all genotypes. Effective allele/locus was calculated following Weir (1990). To eliminate the effect of different measurements, the data were first standardized using the program STAND. The distance coefficient through DICE similarity index was then calculated, and dissimilarity coefficients between genotypes were worked out following Jaccard’s coefficient method. Genetic distances were calculated as 1—genetic similarity (GS). The morphological dissimilarity matrix was calculated using program SIMINT, whereas SIMQUAL was used for molecular dissimilarity matrix through NTSYS-pc (Rohlf 2005). Dendrogram was constructed using the NJ method based on dissimilarity matrix using DARwin 6.0.13 software program.
Statistical Analyses
Griffing’s Method 2 (no reciprocals) (Griffing 1956) was used to determine the estimates of general combining ability and specific combining ability of F1s, which are developed to study the yield of sesame. Combined and environment-wise analyses of variance of hybrid trial were performed using MIXED procedure of SAS 9.4 (SAS 2015; Zhang and Kang 1997) considering environment, hybrid and replication as fixed effects. Individual environment variances were modelled into combined analysis. F-test was used for testing the significance of fixed effect factor. The linear model for combined analysis of hybrids across environments for yield is
where µ is the overall mean; env i is ith environment, i = 1,2, rep(env) ki is the effect of k replication within ith environment; GCA j is jth parent general combining ability, j = 1 to p − 1; SCAjj′ is jj′th F1 hybrid specific combining ability, j′ = j + 1 to p; (GCA × env) ij is the interaction of GCA and environment; (SCA × env)ijj′ is the interaction of SCA and environment; and є ijk is the random error ~ NID(0, σ2e). The relative importance of GCA and SCA effects on yield was assessed using the formula (2σ 2GCA /(2σ 2GCA + σ 2SCA )) (Baker 1978; Lu and Myers 2011)—the closer the ratio to unity, greater is the predictability of a specific hybrid’s performance based on the GCA alone (Hung and Holland 2012).
Mid-parent heterosis (MPH) and best-parent heterosis (BPH) were calculated for yield. The MPH was calculated as follows: [(F1 − MP)/MP] × 100; where, F1 is the mean performance of the hybrid; MP is the mid-parent value given by (P1 + P2)/2; P1 and P2 are the means of parent 1 and parent 2, respectively. The BPH was calculated as [(F1 − BP)/BP] × 100: where BP = the mean of the best parent. Simple linear regression was computed to determine the relationships between SW, SY, SCA, BPH and MPH. The binary data from SSR scoring were used to compute pairwise similarity coefficients (Jaccard1908). The similarity matrix thus obtained was subjected to cluster analysis using the UPGMA algorithm using NTSYS-pc software (Rohlf 2000). Relationships among F1s and parents were visualized in dendrograms. Means per environment and across environments were used to calculate Pearson’s correlation coefficients (r) between genetic distances, F1SY, MPH, BPH and SCA using SAS (SASV9.4).
Result and Discussion
Genetic Distance and Groups of Parents
Grouping of original 60 genotypes were done based on GDSDS (range 1.98–12.44), GDMOR (range 3.29–13.65) and GDSSR (range 3.31–11.91). The original 60 genotypes were clearly clustered into three groups based on genetic distance (GDSSR) through SSR markers. GDSSR of the seven parents ranged from 0.05 to 0.55, with an average of 0.301, which was lower than that of the original parent population. To select seven diallel parents, two parents each from groups—GI and GII, and three parents from GIII were selected based on GDSSR. Based on GDSDS, the 60 genotypes clustered into two main groups GI and GII; Group GI was further subclustered into IA and IB, and GII subclustered into IIA, IIB and IIC. According to the grouping based on GDSDS, all the selected seven genotypes fall in the subgroups of GII—IIA, IIB and IIC. Following the same pattern of GDSDS, GDMOR also grouped the 60 genotypes into two main groups (GI and GII), both with three subclusters IA, IB and IC; and IIA, IIB and IIC respectively. As evident from the results, similar fashion of grouping was observed based on GDSDS and GDMOR, and both these grouping patterns were remarkably different from the clustering based on GDSSR.
These selected seven parents were further clustered into different subgroups based on genetic distances revealed by agro-morphological traits (GDMOR; range 0.002–0.041) (Fig. 1), seed-storage protein banding pattern (GDSDS; range 0.055–0.250) (Fig. 2); and SSR (GDSSR; range 0.05–0.55) (Fig. 3), and they maintained clustering pattern similar to their respective original cluster structure for 60 genotypes. Similar clustering pattern has been observed in a different study by Huang et al. (2015). Clusters originating from GDMOR, GDSSR and GDSDS grouped the seven parents in a different manner. In case of clustering based on GDSDS and GDMOR for parents, the grouping of genotypes was not in accordance with their geographic origin, as P2 (TKG-22) and P7 (TKG-352) were from same geographical region, occupying different clusters. On the contrary, cluster analysis-based SSR grouped P2 and P7 in same cluster during the clustering of parents.
Again, the 21 F1s along with seven parents were together classified into different clusters based on agro-morphological traits (GDMOR ranging from 0.0004 to 0.079), SDS-PAGE (GDSDS ranging from 0.056 to 0.769) and SSR (GDSSR ranging from 0.103 to 0.414). As shown in Fig. 1 and 2, the dendrograms constructed using GDMOR and GDSDS divided the seven parents and F1s into three major groups. Although the number of lines clustered in each major group was different for both the clustering systems, i.e. SDS-PAGE and SSR, there is a similarity in both grouping patterns based on GDSDS and GDMOR; four out of the seven parents were present in a separate group along with one or two F1 lines. The parental lines did not form distinct groups based on their origin, but the grouping followed the pedigree record. On the contrary, Cluster analysis based on SSR provided a fairly good resolution of the F1 lines from the parents. The F1 lines clustered into three groups, indicating existing pedigree records. The parents were distinctly separated among each other in the dendrograms, as expected, based on their genetic backgrounds (Fig. 3). Cluster analysis using the GDSSR matrix classified the 28 lines into three main groups (Fig. 3). Viewing these associations from the top of the dendrograms, the first group consists of two parents namely parent-3 (OSC-593) and parent-7 (TKG352) and 8 F1s and most of which having either one of the parents in their parentage. The second group has a mixture of seven F1s along with parent 2 (TKG22), parent 5 (UMA) and Parent 6 (NIC-8316). The third group consists of six F1s along with two parents, P1 (GT-2) and P4 (RT-348). Within each group, mostly the F1s bred with a common parentage cluster together (Figs. 1, 2, 3).
Combining Ability Analysis and Genetic Parameters
Results of ANOVA for two environments and pooled are given in Table 2. All main effects (environments and hybrids) were highly significant (P < 0.01), as were all possible two-way interactions between the main effects, but replication was insignificant for most of the traits except BP and DTF at P < 0.05 in pooled analysis. The significant genotype × environments interactions strongly suggested that SY and other traits of genotype depended on the environments in which they were grown. The GCA × environment interaction was significant at P < 0.05, and SCA × environment interaction was significant at P < 0.01 for SY; however, for few other traits like PH, DTF, CP and SC, both GCA × environment and SCA × environment were significant at P < 0.01. These results suggested that the GCA and SCA effects were environment specific and that a single-environment testing would be inadequate. Involvement of both additive and non-additive types of gene action was revealed by components of GCA and SCA mean sums of squares which were highly significant for all the traits (Table 2). Variance due to SCA (σ2s) was higher than the variance due to GCA (σ2g) (Table 3) for all the traits indicating the predominance of non-additive type of gene action in controlling the expression of these traits. This was further confirmed by low magnitude of GCA/SCA ratios, indicating the non-additive type of gene action controlling the expression of most of the traits and suggests exploitation of these non-additive genetic variation through hybrid breeding (Ramalingam et al. 1997). Predictability ratio calculated from GCA and SCA variances exhibits the extent to which character is transmitted to the progeny (Banerjee and Kole, 2009). The predictability ratio (Baker 1978) was high in case of the character plant height (0.52) which is higher than 0.50 indicating the importance of additive gene action (Table 3) (Saravanan and Nadarajan 2003). On the contrary, the ratios were lower than 0.50 for all other characters, indicating that both additive and non-additive gene actions influenced the performance of the hybrids (Table 3) (Banerjee and Kole 2009; Solanki et al. 2006).
General Combining Ability Effects of the Parents
Usually, GCA effects of individual lines are considered to be controlled by genes with additive effects, and these effects can be passed on to the next generation (Hallauer and Miranda Filho 1988; Kang 1994). Thus, GCA effects are a major criterion for evaluating lines for their potential application in hybrid development programmes (Fan et al. 2014; Yao et al. 2013). After comparing the nature of GCA effects of different groups, it was evident that relative magnitudes of the GCA effects of lines within each group were quite similar, with very few exceptions; similar results in pigeon pea have been reported by Saxena and Sawargaonkar (2014). Estimates of the GCA effects of the parents in F1 generation are shown in Table 4. Although significant GCA was observed in all the traits, no parent was found having significant GCA for all the traits studied. OSC-593 and UMA were indicated as the best general combiners because they showed highly positive significant GCA effects for CP, SC and SY, and negative GCA effects for DTM indicating early maturity. These genotypes can be exploited to transfer gene in crossing programme because of favourable expression for SY and SC. Crossing between these parents would likely produce wide genetic variability of fixable nature in segregating generation and thus would offer good scope to select desirable segregating lines with higher yield.
Specific Combining Ability (SCA) Effects
SCA effects of the crosses in F1 generation are given in Table 5. Maximum positive SCA effect for SY was observed in cross-combination of UMA × NIC-8316. Considering the SCA effects and per se performance, crosses RT-348 × TKG-352 and UMA × NIC-8316 were the top combinations. It was observed that on a pooled basis, the data of different cross-combinations for SY revealed that the crosses involved five types of parental combinations, viz. positive, significant gca effects × positive significant gca effects (H × H), positive significant gca effects × positive but insignificant gca effects (H × M or M × H), positive significant gca effects × negative gca effects (H × L or L × H), positive but insignificant gca effects × negative gca effects (M × L or L × M) and negative gca effects × negative gca effects (L × L). The H × H, type of combinations are desirable in self-pollinated crops like sesame as they involve additive and additive × additive type of interaction which is fixable in early generations and this kind of combination for SY was observed in cross-combinations UMA × NIC-8316 and UMA × OSC-593. Solanki and Gupta (2003) reported that crosses expressing high sca effects for SY and its components had parental combinations of H × L, M × L, L × M and L × L gca effects. No cross-combinations exhibited significantly positive sca effects for all the characters. It was observed that on a pooled basis, all the nine cross-combinations showing MPH showed significant and positive SCA effects for SY. Out of nine, two exhibited H × H combinations (UMA × NIC-8316 and UMA × OSC-593); four showed H × L or L × H type of combinations (TKG22 × OSC-593; GT-2 × OSC-593; OSC-593 × RT-348; RT-348 × NIC-8316); two showed H × M type of combinations (UMA × TKG-357; NIC-8316 × TKG-357); and one cross RT-348 × TKG-352 showed L × M type of combinations. Majority of the cross-combinations came under L × H, H × L type that represents at least one parent with high gca effect and thus additive effect was preponderant in the genetic control of these cross-combinations, and this would obviously lead to useful outcome of these combinations as desirable segregants; being additive in nature, they were early fixable and might lead to evolve high yielding varieties.
Analysis of Heterosis
The variation patterns of MPH and BPH over environments were very similar in nature. Environment was the major source of variation contributing to the total sum of squares followed by genotype, and G x E interaction factor. On comparing with the average parents yield (12.57 g plant−1), eight hybrids (38.09%) had a significant (P < 0.01) yield advantage over their parents in both the environments, with an average of 26.94% and a range of − 28.26–47.7% for BPH; and an average of 29.99% and a range of − 27.00 to 55.84% for MPH in both the environments. Comparatively high MPH and BPH were observed in the environments 1 (Supplementary Table 3 and 4). The inter-group hybrids had significantly (P < 0.001) higher yield and yield heterosis than the intra-group hybrids. Among the nine hybrids that showed significant high MPH and BPH (> 10% of the average yield), seven were from inter-group and two from intra-group crossing based on GDSSR. The hybrids in the G3 and G1 group had the highest SY plant−1, heterosis for SY and combining ability amongst the hybrid groups, followed by the G2 hybrid group. The parents involved in those nine heterotic hybrids were mainly from the groups of G3 (56.25%), G1 (25.0%) and G2 (18.75%). The parent UMA had the highest GCA for SY followed by OSC-593 and NIC-8316, and they belong to the group G3 (Table 6). Among the individual hybrid groups, the highest yielding hybrid group was an intra-cluster cross of UMA × NIC-8316 (G-IIIB × G-IIIA) which produced 18.95 g plant−1, significantly (P < 0.01) higher than the yields of other 20 hybrids. The G-IIB × G-IA hybrid group was the second highest yielding group which produced 16.50 g plant−1 (significant at P < 0.01).
Correlation Between Heterosis and Genetic Divergence
Parental genetic diversity and combining ability along with per se performance have been effectively exploited to develop higher frequencies of heterotic hybrids in several crops (Betran et al. 2003). Further, advances in genome researches have raised the interest in predicting heterotic groups using molecular markers (Krystkowiak et al. 2009). Heterosis in relation to genetic divergence had been studied earlier in many crops, although in sesame, information is inadequate. Highly significant positive correlations were observed between SCA and per se performance of hybrids (0.97), while showing positive non-significant correlation with GDSSR (0.048) but non-significant negative correlation with GDMOR (− 0.01) and GDSDS (− 0.256) (Supplementary Table 2). Results showed that positive non-significant correlation was found in GDSSR with SCA (0.154) and hybrid performance for SY (0.108). Similar trends were found for MPH and other traits except for GDMOR where positive non-significant correlation was observed (0.051).The linear regressions of GDSSR on SCA, BPH and MPH were non-significant with R2 value of 0.030, 0.0018 and 0.0031, respectively. Similar linear regression was observed for GDSDS on BPH (0.038), MPH (0.032), SCA (0.085) and GDMOR on BPH (0.049), MPH (0.057) and SCA (0.010). The mean performance of F1s was little influenced by GDMOR (0.089), GDSDS (0.014) and GDSSR (0.013) as evidenced from non-significant regression. The linear regressions of SCA on MPH, BPH and per se performance of F1s were significant with R2 value of 0.88, 0.84 and 0.95, respectively (Fig. 4a, d, e,). The MPH and BPH also established significant positive associations as well as linear regressions with per se performance of the hybrids along with R2 value of 0.96 and 0.94, respectively (Fig. 4b, c). The present study strongly indicates that SCA is a main determinant of heterosis, as well as of F1 performance and can be used consistently in the selection of parents (Hallauer and Miranda Filho 1988). Non-significant linear regression of heterosis for grain dry weight with mean performance of hybrids on GD has been reported by Shieh and Thseng (2002). The extents of correlation coefficients found between GDSSR with BPH and MPH in the present study were not large enough for the prediction of hybrid performance in sesame as earlier reported by Banerjee and Kole (2010). Similar results with weak correlation have been reported in many crops (Oliveira et al. 2004). There can be many plausible reasons behind the weak correlation of GDSSR with hybrid performance and heterosis; Some of the reasons could be lack of linkage between genes controlling the traits under study, inadequate genome coverage, and random marker distribution and diversified effect of dominance (Bernardo 1992). Bernardo (1992) suggested that prediction of hybrid through markers would be possible only if a significant number of markers were linked with QTL. In the present study, SSR grouping of the hybrids into different clusters are in agreement with their pedigree records signifying the efficiency of SSR marker for diversity analysis and clustering analysis. The cross-combinations from parents with intermediate genetic diversity group were more often heterotic than those obtained from parents with high levels of genetic divergence between them (Supplementary Table 2). The present investigation suggests that parental divergence in the intermediate group, i.e., being neither low nor high, would likely generate high heterotic crosses for SY in sesame irrespective of GDSSR, GDMOR and GDSDS. The extent of parental divergence as predictive estimates of heterosis was studied earlier by many researchers in different crops like chilli (Geleta et al. 2004); maize (Ndhlela et al. 2015); wheat (Krystkowiak et al. 2009); and groundnut (Arunachalam et al. 1984).
Conclusion
Based on the present findings, we conclude that genetic distances, based on SSR marker, agro-morphological traits or seed-storage profile, were not efficient for the prediction of heterosis in sesame. Nevertheless, SSR-based clusters are in accordance with their pedigree records signifying the efficacy of SSR marker for diversity analysis and clustering analysis. SCA, on the other hand, has appeared as the utmost important factor in the determination of heterosis and per se performance of the hybrids in sesame. In accordance with the earlier reports, weak correlation was observed in the present study which is not useful for predicting hybrid performance in sesame (Zhang et al. 1995; Ndhlela et al. 2015). However, this study also suggests that parental divergence in the intermediate group would like to generate high heterotic crosses for seed yield/plant in sesame as reported in other crops (Geleta et al. 2004).
References
Arunachalam V, Bandyopadhyay A, Nigam SN, Gibbons RW (1984) Heterosis in relation to genetic divergence and specific combining ability in groundnut (Arachishypogaea L.). Euphytica. https://doi.org/10.1007/bf00022747
Baker RJ (1978) Issues in diallel analysis. Crop Sci 18:533–536. https://doi.org/10.2135/cropsci1978.0011183X001800040001x
Banerjee PP, Kole PC (2009) Analysis of genetic architecture for some physiological characters in sesame (Sesamum indicum L.). Euphytica 168(1):11–22
Banerjee P, Kole P (2010) Heterosis, inbreeding depression and their relationship with genetic divergence in sesame (Sesamum indicum L.). Acta Agronomica Hungarica. https://doi.org/10.1556/AAgr.58.2010.3.15
Bernardo R (1992) Relationship between single-cross performance and molecular marker heterozygosity. Theor Appl Genet 83(5):628–634
Betran FJ, Ribaut JM, Beck D, De Leon DG (2003) Genetic diversity, specific combining ability, and heterosis in tropical maize under stress and nonstress environments. Crop Sci 43(3):797–806
Bhat KV, Babrekar PP, Lakhanpaul S (1999) Study of genetic diversity in Indian and exotic sesame (Sesamum indicum L.) germplasm using random amplified polymorphic DNA (RAPD) markers. Euphytica 110(1):21–34
Bhattacharya U, Pandey SK, Dasgupta T (2014) Identification of EST-SSRs and FDM in sesame (Sesamum indicum L.) through data mining. Sch J Agric Sci 4:60–69
Bisht IS, Bhat KV, Lakhanpaul S, Biswas BK, Pandiyan M, Hanchinal RR (2004) Broadening the genetic base of sesame (Sesamum indicum L.) through germplasm enhancement. Plant Genet Resour 2(03):143–151
Dikshit UN, Swain D (2000) Genetic divergence and heterosis in sesame. Indian J Genet Pl Br 60(2):201–212
Fan XM, Zhang YD, Yao WH, Bi YQ, Liu L, Chen HM, Kang MS (2014) Reciprocal diallel crosses impact combining ability, variance estimation, and heterotic group classification. Crop Sci 54:89–97. https://doi.org/10.2135/cropsci2013.06.0393
Geleta LF, Labuschagne MT, Viljoen CD (2004) Relationship between heterosis and genetic distance based on morphological traits and AFLP markers in pepper. Plant Breed 123(5):467–473
Griffing B (1956) Concept of general and specific combining ability in relation to diallel crossing system. Aust J Biol Sci 9:463–493
Hallauer AR (1999) Temperate maize and heterosis. In: Coors JG, Pandey S (eds) The genetics and exploitation of heterosis in crops. American Society of Agronomy, Crop Science Society of America, Madison, pp 353–361
Hallauer AR, Miranda Filho JB (1988) Quantitative genetics in maize breeding, 2nd edn. Iowa State University Press, Ames, IA
Huang M, Chen LY, Chen ZQ (2015) Diallel analysis of combining ability and heterosis for yield and yield components in rice by using positive loci. Euphytica 205(1):37–50
Hung HY, Holland JB (2012) Diallel analysis of resistance to fusarium ear rot and fumonisin contamination in maize. Crop Sci 52(5):2173–2181
Jaccard P (1908) Nouvellesrecherche ´s sur la distribution florale. Bull Soc Vaud Sci Nat 44:223–270
Jan HU, Rabbani MA, Shinwari ZK (2011) Assessment of genetic diversity of indigenous turmeric (Curcuma longa L.) germplasm from Pakistan using RAPD markers. J. Med. Plants Res. 5:823–830
Kang MS (1994) Applied quantitative genetics. M.S. Kang, Baton Rouge
Krystkowiak K, Adamski T, Surma M, Kaczmarek Z (2009) Relationship between phenotypic and genetic diversity of parental genotypes and the specific combining ability and heterosis effects in wheat (Triticumaestivum L.). Euphytica 165(3):419–434
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. https://doi.org/10.1038/227680a
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1953) Protein measurement with the folin-phenols reagent. Jr Biol Chem 193:265–275
Lu H, Myers GO (2011) Combining abilities and inheritance of yield components in influential upland cotton varieties. Aust J Crop Sci 5:384–390
Ndhlela T, Herselman L, Semagn K, Magorokosho C, Mutimaamba C, Labuschagne MT (2015) Relationships between heterosis, genetic distances and specific combining ability among CIMMYT and Zimbabwe developed maize inbred lines under stress and optimal conditions. Euphytica 204(3):635–647
Oliveira KM, Laborda PR, Garcia AAF, Zagatto-Paterniani MEAG, Souza AP (2004) Evaluating genetic relationships between tropical maize inbred lines by means of AFLP profiling. Hereditas 140(1):24–33
Pandey SK, Das A, Rai P, Dasgupta T (2015) Morphological and genetic diversity assessment of sesame (Sesamum indicum L.) accessions differing in origin. Physiol Mol Bio Plants 21(4):519–529
Ramalingam J, Nadarajan N, Vanniyarajan C, Rangasamy P (1997) Combining ability studies involving CMS lines in rice. Oryza 34:4–7
Rohlf FJ (2000) NTSYS-pc ver 2.11, Exter Software, Setauket, New York
Rohlf FJ (2005) NTSYS-pc: numerical taxonomy and multivariate analysis system, version 2.20, applied biostatistics, New York
Saghai-Maroof MA, Soliman KM, Jorgensen RA, Allard RW (1984) Ribosomal DNA spacer-length polymorphisms in barley: Mendelian inheritance, chromosomal location, and population dynamics. Proc Natl Acad Sci 81:8014–8018
Saravanan S, Nadarajan N (2003) Combining ability studies in sesame. Crop Res 25:319–324
SAS Institute Inc. 2015. SAS/STAT® 14.1 user’s guide. Cary, NC
Saxena KB, Sawargaonkar SL (2014) First information on heterotic groups in pigeonpea [Cajanuscajan (L.) Millsp.]. Euphytica 200(2):187–196
Shieh GJ, Thseng FS (2002) Genetic diversity of Tainan-white maize inbred lines and prediction of single cross hybrid performances using RAPD markers. Euphytica 124:307–313
Solanki ZS, Gupta D (2003) Variability and character association among quantitative characters of sesame. J Oilseeds Res 20:276–277
Solanki ZS, Singh I, Rajpurohit TS, Ahuja DB (2006) Combining ability and heterosis for stem and root rot and leaf webber/capsule borer in sesame. Indian J Agric Sci 1(1–2):171–174
Wang L, Yu S, Tong C, Zhao Y, Liu Y, Song C, Li D (2014) Genome sequencing of the high oil crop sesame provides insight into oil biosynthesis. Genome Biol 15(2):1
Weir BS (1990) Genetic data analysis. Methods for discrete population genetic data. Sinauer Associates, Inc., Sunderland
Yao WH, Zhang YD, Kang MS, Chen HM, Liu L, Yu LJ, Fan XM (2013) Diallel analysis models: a comparison of certain genetic statistics. Crop Sci 53(4):1481–1490
Zhang Q, Gao YJ, Maroof MS, Yang SH, Li JX (1995) Molecular divergence and hybrid performance in rice. Mol Breed 1(2):133–142
Zhang Y, Kang MS (1997) DIALLEL-SAS: A SAS program for Griffing’s diallel analyses. Agron J 89(2):176–182
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pandey, S.K., Dasgupta, T., Rathore, A. et al. Relationship of Parental Genetic Distance with Heterosis and Specific Combining Ability in Sesame (Sesamum indicum L.) Based on Phenotypic and Molecular Marker Analysis. Biochem Genet 56, 188–209 (2018). https://doi.org/10.1007/s10528-017-9837-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10528-017-9837-2